Significant outcomes
-
Cannabis withdrawal can induce psychotic symptoms in vulnerable individuals.
-
This study raises awareness for public health given the increasing cannabis use legalisation trends.
Limitations
-
This study limitations are mainly related to its retrospective observational design.
-
The information is reliant on medical records, which affected accurate determination cannabis consumption duration and presence of other psychoactive substances in some cases.
Introduction
The relationship between cannabis use and psychotic symptoms or disorders is well studied (Volkow et al., Reference Volkow, Swanson, Evins, DeLisi, Meier, Gonzalez, Bloomfield, Curran and Baler2016). High-potency cannabis daily use and consumption started in adolescence are associated with earlier psychotic episodes (Di Forti et al., Reference Di Forti, Sallis, Allegri, Trotta, Ferraro, Stilo, Marconi, La Cascia, Reis Marques, Pariante, Dazzan, Mondelli, Paparelli, Kolliakou, Prata, Gaughran, David, Morgan, Stahl, Khondoker, MacCabe and Murray2014).
However, the relation between cannabis withdrawal and psychotic symptoms is less known. Cannabis withdrawal syndrome (CWS) is a DSM5 diagnosis, and it is well characterised: symptoms begin 24 hours after cessation, peak within 7 days and dissipate following 28 days of abstinence. Its prevalence among cannabis users after cessation is estimated to range from 47 to 52% (Bahji et al., Reference Bahji, Stephenson, Tyo, Hawken and Seitz2020). CWS consists mainly of irritability, anxiety, sleep difficulty, decreased appetite, restlessness and/or dysphoria occurring approximately after a period of 1 week (Budney, Reference Budney2004). The DSM5 did not incorporate psychotic symptoms as a recognised manifestation within the context of cannabis withdrawal (American Psychiatric Association, 2013), nor did its recent revision (DSM5-TR) (American Psychiatric Association, 2022). Substance-induced psychotic disorder is also a DSM5 diagnosis. It consists of hallucinations or delusions developing during or soon after substance intoxication or withdrawal and not persisting for a substantial period of time (American Psychiatric Association, 2013).
To date, few individual cases of cannabis-induced psychotic disorder with onset during withdrawal have been reported (Joseph et al., Reference Joseph, Ojo, Popoola, Azizi, Khan, Pramanik, Kahn, Chaudhry, Rimawi, Singh, Mallick, Paul, Ojimba and Jolayemi2018; Marín et al., Reference Marín, Pérez de Mendiola, Fernández and Chart2021; Shakya and Upadhaya, Reference Shakya and Upadhaya2021; Kung et al., Reference Kung, Lin, Tai, Chang, Chiao, Huang and Tzeng2022; Ramos et al., Reference Ramos, Santos Martins and Lima Osório2022). Overall, previous reports described the onset of a psychotic episode in the context of cannabis withdrawal in a group of five young subjects (mean age [SD] = 25.8 [4.8] years; gender [male/female] = 3/2). Taken together, these cases are characterised by a prolonged daily cannabis consumption that was abruptly interrupted. Moreover, all cases were resolved with antipsychotic medication within a timeframe ranging from 36 h to 1 week.
It is accepted that the component most associated with psychotic-related symptomatology is the delta-9-tetrahydrocannabinol (THC) (D’Souza et al., Reference D’Souza, Perry, MacDougall, Ammerman, Cooper, Wu, Braley, Gueorguieva and Krystal2004) and, in turn, recreational marijuana has witnessed increasing percentages of THC over the last decades (Freeman et al., Reference Freeman, Craft, Wilson, Stylianou, ElSohly, Di Forti and Lynskey2021). The effects of daily cannabis consumption are diverse and can have a significant impact on users’ functionality. The development of psychotic symptoms associated with the cessation of cannabis consumption has been sparsely described to date. This study is the first standardised report of cannabis-induced psychotic disorders with onset during withdrawal.
Methods
Patient registrations to the emergency department (ED) in a major university hospital specialised in psychiatry in Canada were screened (6,000 registrations per year) between January 2020 and September 2023. In this psychiatric ED, all patients are screened for substance use during nurse triage, and subsequently, they undergo a psychiatric interview. The clinical information obtained is entered into the patient’s electronic record. This record includes previous psychiatric history in the institution and affiliated health centres within the same geographical area.
Cases whose primary reason for consultation was related to the presence of psychotic symptoms and that stopped cannabis in the month prior were reviewed. Cases with substance consumption (stimulants, hallucinogens, inhalants, alcohol, opioids) that could explain the psychotic symptoms were excluded. Cases in which psychotic symptoms could be attributed to cessation of psychotropic medication were excluded.
Following clinical records review, a retrospectively administered a Positive And Negative Syndrome Scale (PANSS) positive subscale was applied to standardise ED presentations. Each of the seven sub-scale items was determined by a senior medical doctor specialised in psychiatry, expert in addictions and chief of the ED (JC).
Results
A total of eight cases presenting with an acute psychotic syndrome following cannabis cessation were identified. Main sample characteristics are described in Table 1.
Table 1. Sample characteristics
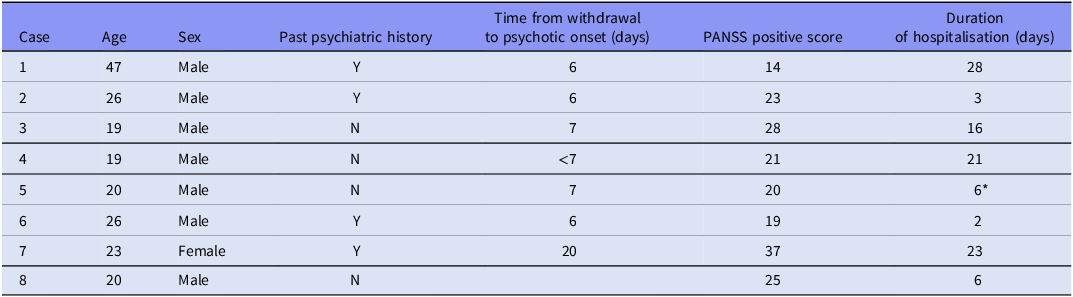
The table summarises sociodemographic and clinical characteristics of each case. Past psychiatric history refers to any previous psychiatric treatment or diagnosis registered in medical records. Time from withdrawal to psychotic onset considers the number of days between the cessation of cannabis use and the development of psychotic symptoms. PANSS positive scores reflect the severity of positive psychotic symptoms at admission.
* The patient was transferred to a hospital closer to his place 6 days after admission. He had not reached remission at the time of being transferred.
The first patient was an unemployed man who presented with paranoid and persecutory delusions together with suicidal ideation with a plan. Six days earlier, he had started with symptoms of anxiety for which he requested his mother for help. He had smoked approximately 1 g of cannabis per day for seven years, he continued smoking one pack of cigarettes a day. In addition, He had a psychostimulant-induced psychotic episode (paranoid delusions), with onset during intoxication 14 years earlier. Quetiapine was initially prescribed, and aripiprazole was added after 1 week.
The second patient was an employed male, brought by his parents being self-talkative and wandering. His presentation was characterised by alternating periods of logorrhoea and mutism, having access to posturing and displaying perplexity. Six days after cessation, he developed insomnia and psychomotor acceleration, 9 days later he absconded from home. This patient had previously been seen twice in the ED, due to a previous psychotic episode two years earlier and a suicide attempt related to cannabis withdrawal. Olanzapine 10 mg/day was introduced.
The third patient was a university student without past psychiatric history. The patient was brought to the ED by ambulance with disorganised behaviour, affective lability and decreased need for sleep 1 week after cannabis cessation. This presentation began with increasing activity and anxiety symptoms after which he developed grandiosity and paranoid ideation with auditory hallucinations. In this case, the patient only has occasional alcohol consumption. Ziprazidone was initially prescribed and switched a few days later to olanzapine. Olanzapine was gradually increased to 25 mg/day.
The fourth patient was a college student who had been smoking around 1 g of cannabis per day for 1 year. He had no previous history of tobacco or other psychoctive drug use. A few days after withdrawal, he started being self-talkative and displayed paranoid and self-referential ideas. Later, he became disorganised, which was represented by marked difficulties in maintaining simple hygiene and eating routines. It was known that the patient had also presented hetero-aggressive behaviours against family members in the context of agitation. His family called the police, and the patient was brought to the ED. Upon admission, he was distractible, perplexed, distrustful and displayed thought blocking. Aripiprazole 5 mg/day was introduced.
The fifth patient was a college student that had been smoking 1 to 2 g of cannabis for more than six months, which he interrupted. One week later, he developed a paranoid delusion with persecutory ideas. He was brought to the ED by his family members, manifesting paranoid ideas against his neighbours. He also displayed thought disorganisation, mainly characterised by thought blocking and irritability. Urine drug tests were positive only for cannabis. He was treated with olanzapine 10 mg/day.
The sixth patient was an unemployed man who had been admitted twice to the ER due to brief psychotic episodes that lasted 48 h each. The first one was also related to cannabis withdrawal and treated with risperidone 2 mg/day. He received treatment in a first-episode psychosis outpatient programme on two previous occasions. The third admission occurred after interrupting cannabis use. He had been smoking 4 g of cannabis per day for 4 years, which he discontinued by his own decision. He began to exhibit disorganisation in his behaviour and speech. In the context of psychotic symptoms, he displayed heteroaggression and psychomotor agitation. Law enforcement personnel brought the patient to the ED who required physical restraint and injectable medication. Symptoms were treated with olanzapine 5 mg/day.
The seventh patient was an unemployed woman. She had been admitted 6 years before for depression and self-harm behaviours and treated with sertraline. She had been smoking cannabis daily for several months. She voluntarily ceased cannabis use 3 weeks prior to admission due to self-perceived anxiety symptoms. The day prior to ED admission, her mental state changed abruptly starting with disorganised behaviour, heteroaggression, auditory hallucinations and grandiose delusions. Olanzapine 15 mg was introduced and posteriorly increased to 20 mg/day.
The eighth patient was a college student who had been smoking cannabis for several months, which he interrupted 3 weeks before admission. The patient manifested hearing mandatory voices. He called the police himself in the context of paranoid delusions and suicidal thoughts. During the ED consultation, he was reluctant, paranoid, scared and aggressive. Urine drug tested negative for all drugs. Risperidone 3 mg/day was introduced and then switched to paliperidone 150 mg/month intramuscular before discharge.
Discussion
ED admissions between January 2020 and September 2023 were reviewed. Eight cases representing less than 1% of all admissions were identified. Most cases were male (one female). The average age [SD] observed was 24.7 [9.3] years old, ranging from 19 to 47 years old. All cases were diagnosed with a psychotic episode. Three patients (cases 1, 2 and 6) had presented previous substance-induced psychotic episodes. All patients were successfully treated with antipsychotics (risperidone, aripiprazole, olanzapine or quetiapine). The length of hospitalisation was registered for seven out of eight participants, with a mean [SD] duration of 14,4 [10.5] days. The mean [SD] PANSS positive subscale score at admission was 23.4 [6.9].
Cannabis-induced psychotic episodes with onset during withdrawal are scarcely described in medical literature (Joseph et al., Reference Joseph, Ojo, Popoola, Azizi, Khan, Pramanik, Kahn, Chaudhry, Rimawi, Singh, Mallick, Paul, Ojimba and Jolayemi2018; Marín et al., Reference Marín, Pérez de Mendiola, Fernández and Chart2021; Shakya and Upadhaya, Reference Shakya and Upadhaya2021; Kung et al., Reference Kung, Lin, Tai, Chang, Chiao, Huang and Tzeng2022; Ramos et al., Reference Ramos, Santos Martins and Lima Osório2022), and very little understood. Currently, the DSM does not consider psychotic symptoms as part of CWS (American Psychiatric Association, 2013). However, psychotic symptoms that emerge following the discontinuation of cannabis use, may be classified as cannabis-induced psychotic episodes with onset during withdrawal. Our cases, as well as those previously reported, fall within this category, given their documented cannabis use discontinuation.
Noteworthy, in accordance with previous reports, our cases exhibited differences when compared to other primary psychotic disorders. Firstly, the remission period was mostly brief (within 2 weeks). This contrasts with psychotic episodes developed in primary psychiatric disorders like schizophrenia, in which symptomatic improvement tends to be slower. For example, one study reported a 20% reduction in PANSS scores within 2 weeks for nearly half of the cases (Emsley et al., Reference Emsley, Chiliza and Asmal2013), with longer timelines for the remaining. Others have reported periods of up to 16 weeks for 40% of patients to meet response criteria (Carbon and Correll, Reference Carbon and Correll2014). The remission periods are even more challenging to determine, with extended yet uncertain durations (Emsley et al., Reference Emsley, Chiliza and Asmal2013; Carbon and Correll, Reference Carbon and Correll2014).
The onset of psychotic symptoms following cannabis discontinuation could be explained by an imbalance of the interaction between the endocannabinoid system (ECS) and the dopamine system directly related to cannabis use timeline (Covey et al., Reference Covey, Mateo, Sulzer, Cheer and Lovinger2017).
The ECS acts as a gatekeeper, shaping how incoming inputs arrive onto dopaminergic neurons (Covey et al., Reference Covey, Mateo, Sulzer, Cheer and Lovinger2017) and is positioned at an intersection of three major neurotransmitter systems (glutamate/GABA and dopamine) thought to contribute to psychotic disorder neuropathology (Lewis et al., Reference Lewis, Hashimoto and Volk2005).
Despite widespread and well-replicated evidence for disturbances of the ECS in schizophrenia and psychotic disorders, the nature of the relationship between ECS function and psychotic disorders remains largely unexplored (Minichino et al., Reference Minichino, Senior, Brondino, Zhang, Godlewska, Burnet, Cipriani and Lennox2019; Garani et al., Reference Garani, Watts and Mizrahi2021). Furthermore, the alterations in the ECS in primary psychosis may not be applicable to the effects produced by exocannabinoids used. Under normal physiological conditions, activation of CB1 receptors would suppress neurotransmitter release only from the terminals that synapse onto activated neurons, whose excitation stimulates the ECS (Volk and Lewis, Reference Volk and Lewis2016). Conversely, exocannabinoids would not follow this spatiotemporal selectivity, hence affecting all terminals containing CB1 receptors. It has been suggested that this exocannabinoid indiscriminate activation could be causing dissimilar consequences when the ECS is previously altered. In other words, unlike endogenous ligands, exocannabinoids do not act in a localised or selective manner. Moreover, the effects they produce will depend to a large extent on other factors such as the density of CB1 receptors and the types of neuronal circuits involved. (Volk and Lewis, Reference Volk and Lewis2016).
Regular cannabis intake induces a desensitisation and downregulation of human brain CB1 receptors, which begins to reverse within the first 2 days of abstinence and return to normal levels within 4 weeks of abstinence (Bonnet and Preuss, Reference Bonnet and Preuss2017). At the same time, the Fatty Acid Amide Hydrolase (FAAH), which metabolises anandamide, has low levels in brain of cannabis users during early abstinence (within 24 h) (Jacobson et al., Reference Jacobson, Watts, Da Silva, Tyndale, Rusjan, Houle, Wilson, Ross, Boileau and Mizrahi2021). Lower FAAH levels were associated with positive psychotic symptoms in patients with psychosis (Watts et al., Reference Watts, Jacobson, Lalang, Boileau, Tyndale, Kiang, Ross, Houle, Wilson, Rusjan and Mizrahi2020). All these neurobiological changes where the ECS is involved contribute to loss of neurotransmitter homeostasis (Bonnet and Preuss, Reference Bonnet and Preuss2017). During withdrawal, the perturbation induced through the restoration of CB1 levels can deregulate presynaptic inhibition control of neurotransmitter release fine-tuned by the ECS. Recently, a randomised control trial showed that a FAAH inhibitor (PF-04457845), a drug that prevents anandamide degradation (thereby increasing its levels), reduced cannabis withdrawal symptoms following abstinence compared to a placebo group (D’Souza et al., Reference D’Souza, Cortes-Briones, Creatura, Bluez, Thurnauer, Deaso, Bielen, Surti, Radhakrishnan, Gupta, Gupta, Cahill, Sherif, Makriyannis, Morgan, Ranganathan and Skosnik2019). Together, this suggests that reduced ECS may underlie common cannabis withdrawal symptoms in humans.
Accordingly, therapeutic approaches aimed at normalising low endocannabinoid levels following abstinence, when withdrawal symptoms emerge, may be essential not only to prevent relapse and treating cannabis use disorders but also perhaps to treat/avoid cannabis-induced psychotic symptoms during withdrawal in vulnerable individuals. Consistent with previous reports (Joseph et al., Reference Joseph, Ojo, Popoola, Azizi, Khan, Pramanik, Kahn, Chaudhry, Rimawi, Singh, Mallick, Paul, Ojimba and Jolayemi2018; Marín et al., Reference Marín, Pérez de Mendiola, Fernández and Chart2021; Shakya and Upadhaya, Reference Shakya and Upadhaya2021; Kung et al., Reference Kung, Lin, Tai, Chang, Chiao, Huang and Tzeng2022; Ramos et al., Reference Ramos, Santos Martins and Lima Osório2022), the cases described in this article showed improvement after administration of dopamine D2 blockers. Nonetheless, alternative treatments other than targeting the dopaminergic system may be warranted.
Limitations
This study has several limitations related to its retrospective observational design. Firstly, the information is reliant on medical records. This was an obstacle to accurately determine the amount and duration of cannabis consumption for several cases. In contrast, our service uses standardised screening methods and triage protocols, actively inquiring about current and past drug use and psychiatric symptoms.
Secondly, other substances were not tested using blood or urine samples. However, a thorough review of medical records was conducted to identify history of other drug use, which was described for each case. Additionally, the physical examination of patients revealed no signs or symptoms consistent with other drug consumption. While recognising this as a significant limitation in interpreting the results, we believe it reflects the conditions prevalent in most healthcare centres.
Third, the PANSS scale score only considers the positive symptom domain and was conducted retrospectively. The current DSM only considers the presence of delusional or hallucinatory symptoms as part of cannabis-induced psychotic episodes. The method of symptom recording in the medical history limits the capture of other symptom domains (negative symptoms, disorganisation, or general symptoms) for a comprehensive PANSS scoring. PANSS scale score was utilised as a standardised method to allow comparability across cases. Nevertheless, the sample size and the absence of other subscales limit its interpretation.
Finally, the concentration of THC vs cannabidiol used by the patients is unknown, hence, it is unclear how it could have affected these clinical presentations.
Conclusion
Cannabis withdrawal can induce psychotic symptoms in vulnerable individuals. Although rare (corresponding to less than 0.05% of registrations in our psychiatric ER), this clinical situation does occur and has been described previously.
This case series also raises awareness for public health given the increasing number of individuals presenting to the ED with psychotic symptoms in the context of cannabis use, which is poised to increase given increasing cannabis use legalisation trends around the world.
Author contribution
All authors discussed and elaborated the ideas, wrote the first draft and corrected it until the final version was obtained. JC performed the patient databases analysis; JC, HP and MBB coordinated and produced the review. RM supervised. All authors have written and approved the manuscripts. REB approved the case series submission for publication.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. Permission was sought from the REB of the institute for this retrospective case reviews. The identity of the patients has been kept confidential.
Financial support
MBB received funding from the Dolanzky Foundation. JC, HP and RM received no specific grant from any funding agency, commercial or not-for-profit sectors.
Competing interests
HP is a salaried employee of the pvt company Benephyt. RM participated in an SAB for Boehringer-Ingelheim in 2021. JC and MBB declare no conflict of interest.




