INTRODUCTION
Cryoconite holes are common features found in cold and polythermal glaciers of polar regions and higher altitudes (Säwström and others, Reference Säwström, Mumford, Marshall, Hodson and Laybourn-Parry2002; Takeuchi, Reference Takeuchi2002; Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; Anesio and others, Reference Anesio, Mindl, Laybourn-Parry, Hodson and Sattler2007; Edwards and others, Reference Edwards2011). They are found in the ablation zones of glaciers worldwide (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; Hodson and others, Reference Hodson, Paterson, Westwood, Cameron and Laybourn-Parry2013) and also in the temperate regions with low melt rates and deficient runoffs incapable of washing the sediments off the glacier surface (Anesio and others, Reference Anesio2010). The cryoconite holes are formed when windblown dust and organic matter accumulate on the snow surface leading to the melting of ice beneath it, due to the lower albedo of dust and organic matter (McIntyre, Reference McIntyr1984; Podgorny and Grenfell, Reference Podgorny and Grenfell1996). The depth and diameter of these holes can vary from a few centimetres to >1 m (McIntyre, Reference McIntyr1984; Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; Tranter and others, Reference Tranter2004). Due to the abundance of microorganisms inoculated by the sediments forming the cryoconite holes, the cryoconite holes are sites for biogeochemical cycling of carbon, nitrogen and other nutrients (Säwström and others, Reference Säwström, Mumford, Marshall, Hodson and Laybourn-Parry2002; Fountain and Tranter, Reference Fountain and Tranter2008; Stibal and others, Reference Stibal, Tranter, Benning and Rěhák2008; Anesio and others, Reference Anesio, Hodson, Fritz, Psenner and Sattler2009; Hodson and others, Reference Hodson2010; Telling and others, Reference Telling2014; Cook, Reference Cook2016) on otherwise relatively passive glaciers and ice sheets.
Depending on the air temperature, cryoconite holes can stay open to the atmosphere favouring air and water exchange (Stibal and Tranter, Reference Stibal and Tranter2007) or can develop an ice lid and remain isolated to atmospheric exchange processes (Foreman and others, Reference Foreman, Sattler, Mikucki, Porazinska and Priscu2007). Open and closed cryoconite holes may have different chemical and biological characteristics associated with them (Mueller and Pollard, Reference Mueller and Pollard2004; Stibal and Tranter, Reference Stibal and Tranter2007; Anesio and others, Reference Anesio2010; Bagshaw and others, Reference Bagshaw2013; Webster-Brown and others, Reference Webster-Brown, Hawes, Jungblut, Wood and Christenson2015), due to the fact that open holes allow for greater exchange of gases and in wash of microbes, nutrients and water into the cryoconite hole, while closed holes have restricted exchange of materials between a cryoconite hole and surrounding glacier surface (Hodson and others, Reference Hodson2008). These open/closed cryoconite holes can further be differentiated into hydrologically connected and isolated holes (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; MacDonnell and Fitzsimons, Reference MacDonnell and Fitzsimons2012). This demarcation is based on the exchange of the cryoconite hole water with nearby streams, channels below the ice surface or other cryoconite holes. In the hydrologically connected cryoconite holes, there is a significant exchange of water, microbes and chemical components with each other and the surrounding area (MacDonnell and Fitzsimons, Reference MacDonnell and Fitzsimons2012). Contrastingly, there is an accumulation of chemical constituents due to the dissolution of debris, as well as, due to the photo-chemically and biologically driven reactions in the hydrologically isolated cryoconite holes (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; Telling and others, Reference Telling2014). The ultimate decay of cryoconite holes can either occur via shrinkage caused by the accumulation of ice on the walls of the cryoconite hole or via breaching of water through the walls by the growing supraglacial drainage (McIntyre, Reference McIntyr1984). Thus, cryoconite holes can provide a mechanism for the storage of chemical and microbial constituents on the glacier surface and can significantly affect the rate of their transfer to the supraglacial or subglacial drainage systems. This could potentially impact the ecological balance in downstream ecosystems. Given their significance in biogeochemical cycling (Anesio and others, Reference Anesio, Hodson, Fritz, Psenner and Sattler2009) and potential to impact downstream ecosystems through the exchange of carbon and nutrients (Foreman and others, Reference Foreman, Wolf and Priscu2004; Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004; Tranter and others, Reference Tranter, Fountain, Lyons, Nylen and Welch2005), characterizing the chemical composition of cryoconite holes and their hydrological connectivity is of importance.
The present study reports the chemical composition (major ions, total organic carbon (TOC) and dissolved inorganic carbon (DIC)) of open and hydrologically connected, as well as, closed and hydrologically connected/isolated holes from three geographically distinct regions in East Antarctica, namely Larsemann Hills (LHS), Amery Ice Shelf (AIS) and central Dronning Maud Land (cDML) in order to understand the characteristics of hydrologically isolated and connected cryoconite holes within Antarctica.
MATERIALS AND METHODS
Study area and sampling
In the present study, cryoconite holes were studied from three geographically distinct sites located in coastal Antarctica, namely Larsemann Hills (LHS), Amery Ice Shelf (AIS) and central Dronning Maud Land (cDML) (Fig. 1). The sampling site at LHS is located in a coastal valley surrounded by hills at the northern and southern region, an ice wall on the eastern side and the Thala fjord on the western side. The open holes in this region seem to be hydrologically connected with supraglacial streams flowing at the study site. The frozen cryoconite holes at cDML are located in a blue ice region immediately to the north of Schirmacher Oasis that is nearly 100 km inland from the sea and is surrounded by many nunataks. Although the cryoconite holes were frozen at the time of sampling in March, they are affected by the surface melting that occurs every year during the summer season. At AIS, frozen cryoconite holes were located 110 km away from the coast in a blue ice region near a prominent rocky promontory.
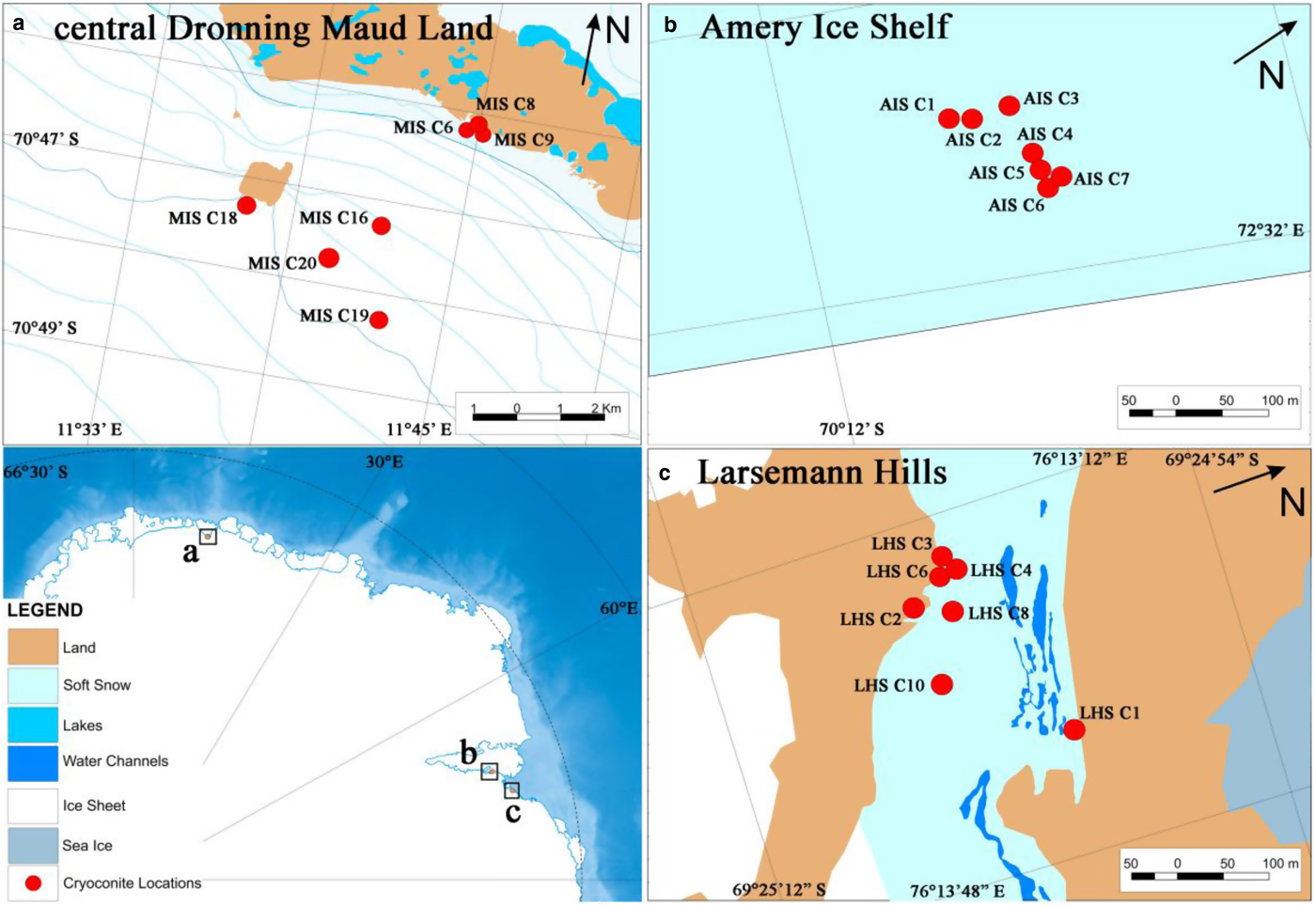
Fig. 1. Map with the inset showing locations of Cryoconite hole sampling in (a) central Dronning Maud Land, (b) Amery Ice Shelf and c) Larsemann Hills.
Seven cryoconite holes each from LHS, AIS and cDML were sampled. Meltwater from the open cryoconite holes at LHS were collected in sterile Whirlpak bags using sterile syringes. The samples for organic carbon analysis were collected in glass bottles which were washed (0.5% HNO3) and combusted (450°C, 4 h) just a few hours prior to sampling. The frozen cryoconite holes at AIS and cDML were drilled using a KOVACS Mark IV coring device and collected in clean high-density polyethelene bags. All the samples were stored and transported in frozen state in expanded polypropylene boxes. Samples were processed in a cold room (−15°C) and melted in a Class-100 clean room prior to analysis.
Cryoconite hole water
Ionic chemistry
Inorganic anions (Cl−, SO42−, NO3− and F−) and carboxylate ions (acetate (Ac−), formate (Fo−), lactate (Lc−) and oxalate (Oxy2−)) were measured using a Dionex ICS 2000 Ion chromatography system with a CD25 electrical conductivity detector. The system is equipped with IonPac AS11-HC (4 mm) column and AG11-HC (4 mm) guard column with an ASRS-Ultra Anion Self Regenerating Suppressor. Potassium hydroxide (KOH) was used as the eluent and the sample injection volume was 1 mL. Cations (Na+, K+, Mg2+ and Ca2+) were measured using a Dionex DX-2500 ion chromatography system (DS 6 Conductivity Detector) with IonPac CS17 (4 mm) column and an IonPac CG17 Guard column (4 mm) with a CSRS-ULTRA Cation Self Regenerating Suppressor. Methanesulfonic acid (CH3SO3H) was used as the eluent and the sample injection volume was 0.1 mL. Calibration of all the ions was carried out using high-purity standard solutions from Inorganic Ventures. Detection limits achieved were 3 µg L−1 (Na+, K+, Ca2+ and Mg2+), 3–5 µg L−1 (SO42−, NO3−, Cl− and F−), 0.1 µg L−1 (Ac− and Fo−), 1 µg L−1 (Oxy2−) and 2 µg L−1 (Lc−). The analytical error of the measurements was <10%.
Total organic carbon
TOC analysis was carried out by the non-purgeable organic carbon method using high sensitivity TOC analyzer (Shimadzu TOC-VCPH) as described in Antony and others (Reference Antony, Mahalinganathan, Thamban and Nair2011). The sample injection volume was 2 mL. Minimum of three measurements were taken for each sample to ensure repeatability. The instrument was calibrated using reagent grade potassium hydrogen phthalate as the organic carbon calibration standard. The detection limit for TOC concentration was 8.0 µg L−1. The instrumental precision based on replicate injections of the standard was better than 10%.
Cell abundance
In order to determine bacterial abundance in the cryoconite hole water, cell counts were determined by filtering 5 mL sample (stained with 4′,6-Diamidino-2-phenylindole, final concentration – 5 µg mL−1) through a brown 0.22 µm isopore polycarbonate track-etched membrane filter (Millipore) and counting by epifluorescence microscopy at 1000 × magnification (Nikon Ti-U Eclipse). The number of cells in 20 random fields was counted and bacterial abundance in controls comprising filtered ultrapure water was also determined in a similar manner as the samples.
Cryoconite hole sediments
Organic carbon in the sediments collected from Larsemann Hills was analyzed in a TOC Analyzer (TOC-V series SSM-5000A from Shimadzu) with a Non-dispersive Infrared detector (NDIR) and for calibration, glucose was used as the carbon standard. Prior to the organic carbon measurement, each sediment sample was dried, powdered and treated with trace metal grade 2N HCl in order to remove inorganic carbon from the sample. Hundred milligrams of treated sample was placed in a clean ceramic sample boat, which was transferred to a 900°C catalytic combustion chamber inside the analyzer and oxidized to CO2 in the presence of O2. Carbon dioxide was then detected by the NDIR detector. The analytical precision of the TOC measurements was <5%. Qualitative analysis of minerals in the sediment samples was carried out by using Rigaku UltimaIV powder diffractometer with CuKα radiation.
RESULTS
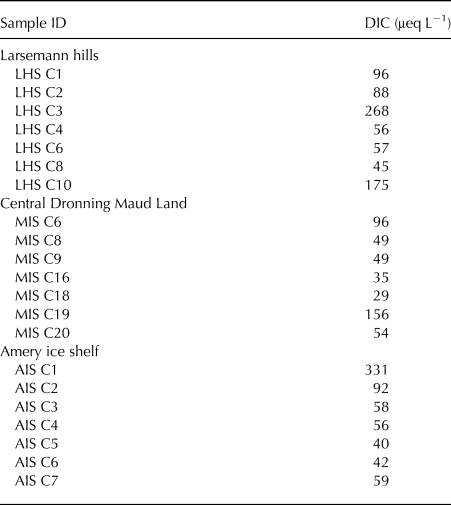
Concentrations of inorganic ions (Na+, K+, Mg2+, Ca2+, Cl−, SO42−, NO3− and F−) detected in the cryoconite holes from the three study regions are given in Table 1. Mean concentration of all measured carboxylate ions (Ac−, Fo−, Oxy2− and Lc−) in LHS, cDML and AIS were 54.64 µg L−1, 44.12 µg L−1 and 40.46 µg L−1, respectively. Details of carboxylate ion concentrations are provided in Sanyal and others (Reference Sanyal, Antony, Samui and Thamban2018). DIC in the cryoconite hole water samples was estimated using the charge balance equation as HCO3− (μeq L−1) = (Na+ + Ca2+ + Mg2+ + K+)−(Cl− + SO42− + NO3− + F− + Ac− + Fo− + Lc− + Oxy2−). Ionic concentrations used in the ion balance equation were in μeq L−1. Mean DIC concentration was 112 µeq L−1, 67 µeq L−1 and 97 µeq L−1 for LHS, cDML and AIS, respectively (Table 2), while mean TOC concentrations were 62.3 µg L−1, 498.7 µg L−1 and 581.4 µg L−1 for LHS, cDML and AIS, respectively (Sanyal and others, Reference Sanyal, Antony, Samui and Thamban2018).
Table 1. Major ion (Na+, K+, Mg2+, Ca2+, Cl−, SO42−, NO3− and F−) concentrations in the cryoconite holes at Larsemann Hills, central Dronning Maud Land and Amery Ice Shelf

* Below Detection Limit.
Table 2. Dissolved inorganic carbon (DIC) concentration in the cryoconite holes
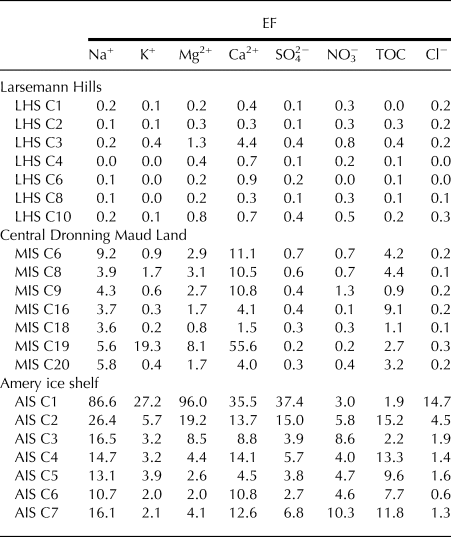
Within the cryoconite holes, the ionic concentration of the water can get significantly affected by microbial activity and dissolution of minerals in the underlying sediment (Tranter and others, Reference Tranter2004; Bagshaw and others, Reference Bagshaw2016). To understand the sources/sinks of ions and TOC present in the cryoconite holes, Enrichment Factor (EF) of the ion and TOC was estimated as EF(i) = (C i)cryoconite hole/(C i)snow, where (C i)cryoconite hole and (C i)snow are the ion or TOC concentrations in a cryoconite hole (Table 1) and surface snow, respectively. Data for ionic and TOC concentrations in surface snow ((C i)snow) are from this study and from previous studies from the same region. Values of (C i)snow are: Na+ (16 976 µg L−1, Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011), K+ (751 µg L−1), Mg2+ (537 µg L−1), Ca2+ (382 µg L−1), Cl− (5878 µg L−1), SO42− (1200 µg L−1), NO3− (186 µg L−1) and TOC (420 µg L−1, Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011) for LHS; Na+ (199 µg L−1, Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011), K+ (22 µg L−1), Mg2+ (14 µg L−1), Ca2+ (34 µg L−1), Cl− (418 µg L−1), SO42− (182 µg L−1), NO3− (145 µg L−1) and TOC (134 µg L−1, Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011) for cDML; Na+ (77 µg L−1), K+ (7 µg L−1), Mg2+ (11 µg L−1), Ca2+ (17 µg L−1), Cl− (161 µg L−1), SO42− (25 µg L−1), NO3− (9 µg L−1) and TOC (65 µg L−1) for AIS (Samui and others, Reference Samui, Antony, Mahalinganathan and Thamban2017). EF of the ions in the cryoconite hole samples from the three study regions are given in Table 3, where, EF(i)>1 represents the enrichment of ion i and EF(i)<1 indicates the depletion of the ion (Telling and others, Reference Telling2014). Enrichment of the conservative tracer Cl− is used to determine the hydrological connectivity and isolation of the cryoconite holes (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004). Range of EF(Cl−) in the three regions were: LHS, 0.0–0.3 (mean, 0.1); cDML, 0.1–0.3 (mean, 0.2) and AIS, 0.6–14.7 (mean, 3.7) (Table 3). EF of Cl−>1 represents the accumulation of Cl− during isolation and aging of the hole, while EF(Cl−)<1 indicates water exchange with other meltwater implying that the cryoconite hole is hydrologically connected. Previous studies on snow chemistry in the LHS (Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011), AIS (Samui and others, Reference Samui, Antony, Mahalinganathan and Thamban2017) and cDML (Mahalinganathan and others, Reference Mahalinganathan, Thamban, Laluraj and Redkar2012) region have shown that surface snow concentrations of Cl− in these regions are heavily influenced by marine inputs, with sea-spray contributing to at least 80% of the Cl− in these regions.
Table 3. Enrichment factor of ions (Na+, K+, Mg2+, Ca2+, SO42−, NO3− and Cl−) and Total Organic Carbon (TOC) detected in the cryoconite hole water samples
Total cell counts in the cryoconite hole water ranged from 0.47 × 104 to 11.8 × 104 cells mL−1 (LHS); 0.07 × 104–1.62 × 104 cells mL−1 (cDML) and 0.13 × 104–9.57 × 104 cells mL−1 (AIS) and include diverse heterotrophic bacteria and eukarya (Sanyal and others, Reference Sanyal, Antony, Samui and Thamban2018). X-Ray Diffraction analysis carried out on cryoconite sediments from LHS showed the presence of quartz, orthoclase, plagioclase feldspar, biotite, spinel and magnetite as the major minerals. TOC in these sediments ranged from 0.4 to 1.8 mg C g−1. Sediment samples were not retrieved from cryoconite holes in the cDML and AIS regions due to the frozen state of the holes during sampling. The composition of cryoconite hole sediments and TOC concentration from these regions could therefore not be determined.
DISCUSSION
Hydrological connectivity
Chloride ion (Cl−) behaves as a relatively conservative tracer ion in nearly all hydrological streams with the high sea salt input of Cl− (Zellweger, Reference Zellweger1994; Gooseff and others, Reference Gooseff, McKnight and Runkel2004; Svensson and others, Reference Svensson, Lovett and Likens2012). Similarly, Cl− in cryoconite holes, which is primarily sourced from sea salt aerosols present in the snowmelt, is considered to behave conservatively as long as they are hydrologically isolated and is only affected by the deepening and aging of the holes (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004). In the present study, the calculated EFs indicate a depletion of Cl− in LHS (mean EF, 0.1) and cDML (mean EF, 0.2), suggesting that the cryoconite holes in both the study regions are hydrologically connected, resulting in the discharge of solutes from the holes. The elapsed time or age (Δt) of a cryoconite hole was also estimated to confirm the hydrological connectivity of the cryoconite holes at LHS using the equation Δt = [(Mt /(a × i))–h]/(dz /dt) (Fountain and others, Reference Fountain, Tranter, Nylen, Lewis and Mueller2004), where M t is the amount of Cl− present in the hole at a particular time t, a is the cross-sectional area of the hole, i is the Cl− concentration in the surrounding ice, h represents the depth of the hole and dz /dt represents the melt rate of the ice which is assumed to be equal to the ablation rate of the ice surface. We used an ablation rate value of 1.8 cm a−1 which is based on the average value determined for this region (personal communication from Mohd. Yunus Shah). The Δt<0 observed at LHS indicate hydrological connectivity of the holes with the nearby streams. The hydrological connectivity of the holes at LHS is corroborated by the depletion of other ions (Na+, Ca2+, Mg2+, K+, SO42− and NO3−) (Table 3) and also by the observed exchange of water with the nearby flowing stream during the sampling. The TOC concentration in the LHS cryoconite holes also showed depletion in concentration (Table 3), further indicating that hydrological connectivity could result in the flushing out of the organic carbon and microbes.
While no obvious conduits were visible at the AIS and cDML cryoconite hole sites at the time of sampling, hydrological connectivity between the frozen holes in these sites via fractures within the ice or via channels below the ice surface cannot be ruled out. At cDML, the surface melting occurs annually during the summer season (Bøggild and others, Reference Bøggild, Winther, Sand and Elvehøy1995). While the EF (Cl−) in cDML was depleted indicating dilution of cryoconite hole water with surrounding meltwater, the observed enrichment of Na+ (EF: 3.6–9.2, mean 5.2), K+ (EF: 0.2–19.3, mean 3.4), Mg2+ (EF: 0.8–8.1, mean 3.0) and Ca2+ (EF: 1.5–55.6, mean 13.9), indicate that there is interaction of the cryoconite water with the underlying sediment. This suggests limited hydrological connectivity or a slower rate of exchange of water with the surface meltwater pool allowing the sediment to affect the ion concentration of water within the holes. The high EF (Cl−) at AIS (mean, 3.7) indicates that the holes in this region are isolated. In addition, higher EFs observed for Na+, K+, Mg2+, Ca2+ and SO42− as compared with EF(Cl−) suggest dissolution of minerals containing these ions from the cryoconite sediments at AIS. Presence of minerals comprising Na+, K+, Mg2+, Ca2+ and SO42− is further supported by the significant correlation (p < 0.001) observed among these ions.
Additionally, previous studies show EF(Cl−)<1 in open cryoconite holes (Hodson and others, Reference Hodson2008; Bagshaw and others, Reference Bagshaw2013) and EF(Cl−)>1 in closed cryoconite holes (Telling and others, Reference Telling2014). In this study, open cryoconite holes in LHS exhibited EF(Cl−)<1, consistent with earlier studies. Closed and frozen cryoconite holes in cDML, however, also exhibited EF(Cl−)<1. This discrepancy in EF values in the closed cryoconite holes in cDML is attributed to the hydrological connectivity of these holes as explained above.
TOC values recorded in the cryoconite hole water in cDML and AIS were 1–15 times (mean EF, 6) higher than that found in the surface snow (Table 3). This observation is consistent with that of other studies, which reports significantly higher concentrations of organic carbon in the cryoconite hole water than in the surrounding glacier ice (Bagshaw and others, Reference Bagshaw2007, Reference Bagshaw2013). This could be due to the dissolution of organic matter from the cryoconite debris in the closed cryoconite hole, which results in organic carbon concentrations higher than those found in the glacier ice. Thus, higher TOC concentration in the cryoconite hole water at AIS and cDML compared to LHS could be attributed to the interaction of organic-rich sediment (0.4 to 1.8 mg C g−1) with the overlying water. However, apart from sediment dissolution, microbial synthesis of organic matter could also contribute to the higher TOC concentration in cryoconite holes, as observed in previous studies (Anesio and others, Reference Anesio, Hodson, Fritz, Psenner and Sattler2009, Reference Anesio2010). Further, Bagshaw and others (Reference Bagshaw2013) suggested that lower photolytic degradation of organic matter occurs in closed holes due to the limited amount of solar radiation reaching the water through the lids. This observation is consistent with the high EF(TOC)>1 in the closed holes at AIS and cDML compared with the melted holes at LHS.
Ionic trend and sources
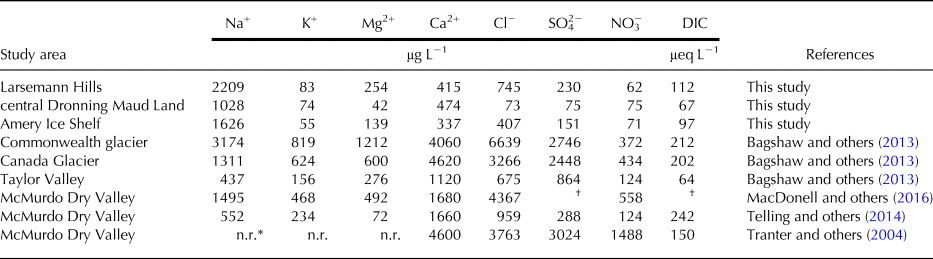
In addition to snowmelt, various minerals present in the cryoconite sediments can significantly influence the ionic composition in the overlying cryoconite water through sediment dissolution. For instance, mineral dissolution occurring in cryoconite sediments has been observed to significantly enrich certain ions over Cl− in the overlying water (Tranter and others, Reference Tranter2004; Telling and others, Reference Telling2014). However, the extent of connectivity of any cryoconite hole with the hydrological streams also determines the enrichment and depletion of ionic constituents. Ion concentrations obtained in the cryoconite holes from the three study regions are consistent with previous studies carried out in Antarctica (Table 4).
Table 4. Mean concentration of various inorganic ions in cryoconite holes from Larsemann Hills (LHS), central Dronning Maud Land (cDML) and Amery Ice Shelf (AIS) observed in this study and from other regions of Antarctica
* Not reported is denoted as n.r..
† SO42− + HCO3− = 69 µeq L−1 (MacDonell and others, Reference MacDonell, Sharp and Fitzsimons2016).
Ionic concentration in the cryoconite hole samples from all the three study regions was dominated by Na+. At LHS, the dominance of Na+ in cryoconite holes is similar to the trend shown in surface snow samples at the coast (Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011). The observed similarity between cryoconite hole and surface snow samples could be due to the hydrological connectivity of the holes preventing longer interaction between cryoconite water and sediment within the holes which further leads to Na+ depletion in the holes (Table 3). In contrast, at cDML and AIS, the dominance of Na+ over Cl− in the cryoconite holes is contradictory to the trend observed in surface snow samples from these two regions. This dominance is most likely a result of cryoconite sediment dissolution which is corroborated by the higher enrichment of Na+ (cDML: mean EF, 5.2; AIS: mean EF, 26.3) compared to Cl− enrichment (cDML: mean EF, 0.2; AIS: mean EF, 3.7) in the cryoconite holes (Table 3). EF (Ca2+) in cryoconite holes at cDML (mean EF, 13.9) and AIS (mean EF, 14.3) exhibited a similar trend as that of Na+ (Table 3), suggesting possible mineral dissolution. Minerals containing Na+ and Ca2+ such as plagioclase feldspar are commonly found in the study regions at cDML (Ravich and Kamenev, Reference Ravich and Kamenev1975; Sengupta, Reference Sengupta1986; Joshi and Pant, Reference Joshi, Pant, Yoshida and Santosh1995) and AIS (Manton and others, Reference Manton, Grew, Hofmann, Sheraton, Yoshida, Kaminuma and Shiraishi1992; Mikhalsky and others, Reference Mikhalsky2001). Weathering of rocks containing such minerals and subsequent wind borne deposition could contribute to the sediment composition in the cryoconite holes in the study regions and lead to the enrichment of Na+ and Ca2+ over Cl− in the overlying water through sediment dissolution (Table 3). In addition to plagioclase feldspar, NaCl and CaCO3 in the sediments can also influence the Na+ and Ca2+ concentration in the overlying water. The cDML and AIS regions, which receive high sea salt inputs from the marine sea spray (Antony and others, Reference Antony, Mahalinganathan, Thamban and Nair2011; Samui and others, Reference Samui, Antony, Mahalinganathan and Thamban2017), could have significant contributions of NaCl in the cryoconite sediments, thereby resulting in an enrichment of Na+ in the cryoconite water. Similarly, the presence of CaCO3 in the promontories at cDML (Sengupta, Reference Sengupta1986; Bauer and Fitzner, Reference Bauer and Fitzner2003) and AIS (Manton and others, Reference Manton, Grew, Hofmann, Sheraton, Yoshida, Kaminuma and Shiraishi1992) supports its possible presence in the cryoconite sediments, thereby contributing to the enrichment of Ca2+ in the overlying water. Possible presence of minerals like gypsum-containing Ca2+ and SO42− at AIS is also indicated by a significant correlation (p < 0.001) between these ions. The presence of such minerals could also contribute to the enrichment of Ca2+ in the cryoconite hole. Dissolution of minerals like calcite, gypsum and the evaporites also influence the concentration of HCO3− and SO42−, respectively. This is supported by the high concentration of HCO3− observed in cryoconite holes in cDML and AIS, as well as, the significant enrichment of SO42− (mean EF, 10.8) in AIS. Enrichment of SO42− due to the dissolution of gypsum is also supported by previous studies where evaporite minerals have been found to enrich SO42− over Cl− in Antarctic cryoconite holes and lakes (Lyons and others, Reference Lyons2003; Tranter and others, Reference Tranter2004; Bagshaw and others, Reference Bagshaw2013). In addition to the dissolution of gypsum, oxidation of sulphur rich minerals during the isolation period can also result in the enrichment of SO42− in AIS. Unlike AIS, SO42− in cDML was found to be depleted. Depletion of SO42− was also accompanied by depletion in Cl− indicating the possibility of dilution during the melt season. The observed depletion in SO42− concentrations in cDML could also be a result of the lower initial concentration of minerals rich in sulphur within the cryoconite sediment (Bagshaw and others, Reference Bagshaw2007). This is supported by a weak correlation observed between SO42− and the cations (Na+, K+, Mg2+ and Ca2+). However, limited information on sediment composition of the cryoconite holes at this site precludes any inferences on the depletion of SO42−. Similar to Na+ and Ca2+, significant enrichment of Mg2+ and K+ were observed at both AIS and cDML. This can be attributed to the high sea spray influence in these regions. In addition to the sea spray, dissolution of Mg2+ and K+ containing minerals like biotite, feldspar and corderite commonly present in the AIS (Manton and others, Reference Manton, Grew, Hofmann, Sheraton, Yoshida, Kaminuma and Shiraishi1992; Mikhalsky and others, Reference Mikhalsky2001) and cDML region (Sengupta, Reference Sengupta1986; Musta and Tahir, Reference Musta and Tahir2012) could also contribute to the enrichment.
The EF of TOC and ions such as Na+, K+, Mg2+, Ca2+, Cl−, SO42− and NO3− in the open cryoconite holes in LHS were low (<1), which is consistent with previous observations from open holes (Bagshaw and others, Reference Bagshaw2013). Chemistry of open holes in LHS also reflects the continuous addition of water due to ice melting in summer, the shorter residence time of meltwater within the cryoconite holes and associated water exchange due to hydrological connectivity as previously reported (Hodson and others, Reference Hodson2008). EF>1 of Na+, K+, Mg2+, Ca2+, Cl−, SO42−, NO3− and TOC in cryoconite holes at AIS is consistent with the closed state of these holes. However, at cDML, few ions (Na+, Mg2+ and Ca2+) and TOC showed EF>1, while rest of the ions (K+, Cl−, SO42− and NO3−) showed EF<1. This complex trend in the closed cryoconite holes in cDML is attributed to the hydrological connectivity of the cryoconite holes with the surrounding glacier during the melt season.
Influence of microbial activity
Estimated DIC concentrations in the three study regions ranged from 44 to 268 µeq L−1 (LHS, mean 112 µeq L−1); 29–156 µeq L−1 (cDML, mean 67 µeq L−1) and 40–331 µeq L−1 (AIS, mean 97 µeq L−1) (Table 2). In a previous study, a significant fraction of carbon (60–76%) in the cryoconite holes was found to be present as DIC (Bagshaw and others, Reference Bagshaw2013). Likewise, in the present study, the concentration of DIC (HCO3−; 1742–20 197 µg L−1) is significantly higher compared with the TOC concentration (7–1213 µg L−1) in all the samples.
Inspite of a considerable difference in the ion concentration in the three study regions, DIC concentration was found to be comparable between the sites. A recent study has shown that these samples harbour diverse heterotrophic bacteria and eukarya that are metabolically active, thereby significantly impacting the chemistry of these environments (Sanyal and others, Reference Sanyal, Antony, Samui and Thamban2018). Several other studies from the Arctic (Anesio and others, Reference Anesio2010; Hodson and others, Reference Hodson2010), Antarctic (Foreman and others, Reference Foreman, Sattler, Mikucki, Porazinska and Priscu2007; Anesio and others, Reference Anesio2010; Telling and others, Reference Telling2014) and Alpine region (Anesio and others, Reference Anesio2010) have also reported microbial activity within the cryoconite holes.
Microbial activity in the cryoconite holes produces CO2, which, in turn, replenishes the HCO3− in the cryoconite hole water (Tranter and others, Reference Tranter2004). Carbon dioxide produced by heterotrophic activity in cryoconite holes results into weathering of carbonate and silicate minerals thereby increasing the concentration of certain ions like K+, Mg2+ and Ca2+ (Bagshaw and others, Reference Bagshaw2016). These findings are corroborated by other studies which show that microbial activity in cryoconite holes can appreciably affect the nutrient cycling within the holes (Hodson and others, Reference Hodson, Mumford, Kohler and Wynn2005; Bagshaw and others, Reference Bagshaw2016). Thus, in-spite of isolation and resulting accumulation of the ions in an isolated hole, charge balance is most likely preserved due to the continued heterotrophic activity within the holes. This is supported by the presence of diverse and active microbial communities in these samples (Sanyal and others, Reference Sanyal, Antony, Samui and Thamban2018), with differences in bacterial abundance, activity, doubling times and diversity being detected between open and closed holes (Mueller and Pollard, Reference Mueller and Pollard2004; Anesio and others, Reference Anesio2010; Sanyal and others, Reference Sanyal, Antony, Samui and Thamban2018) owing to open and closed systems exerting different environmental pressures on the microbial communities.
CONCLUSIONS
Considering EF (Cl−), cryoconite holes at LHS were found to be hydrologically well connected and open and at AIS they were isolated and closed. In cDML, cryoconite holes, EF (Cl−) suggested that the ice-lidded cryoconite holes had limited hydrological connectivity. Major ions (Na+, K+, Mg2+, Ca2+ and SO42−) in the closed isolated holes at AIS were enriched by 7–26 times, while the conservative tracer Cl− was enriched by 4 times. The higher enrichment of the ions at AIS is attributed to the leaching of ions from the underlying sediment during the isolation period. These cryoconite holes can therefore be considered an important store of inorganic nutrients on the glacier surface. In contrast, the open and hydrologically connected cryoconite holes at LHS showed that all ions were depleted due to the flushing of the ions from the cryoconite holes via the interconnected streams and meltwater channels. Ion EFs in the cryoconite holes at cDML indicate limited hydrological connectivity. Thus, constraints in hydrological connectivity, as well as differences between open and closed cryoconite holes, can affect the extent of ion and organic matter accumulation within the holes. This study also shows that closed and isolated holes store an abundance of nutrients and carbon compared with open and connected holes which may further affect the extent of microbial activity within the holes and also their impact on nutrient and carbon transport to the downstream environments.
ACKNOWLEDGEMENTS
We thank the Director, National Centre for Polar and Ocean Research for support and encouragement. We are grateful to the Ministry of Earth Sciences for financial support through the project ‘Cryosphere and Climate’. This work forms part of the doctoral research being undertaken at the Department of Marine Sciences of the Goa University. Gautami Samui was supported by a fellowship from the Council of Scientific and Industrial Research, India. We thank the logistic and crew members of the 33rd Indian Scientific Expedition to Antarctica for field support. Many thanks to Ashish Painginkar for the laboratory assistance. Norwegian Polar Institute is acknowledged for the Quantarctica QGIS package. Thanks to Girish Prabhu (National Institute of Oceanography) for help with X-Ray Diffraction analysis and V. Purnachandra Rao (National Institute of Oceanography) for helping in the interpretation of X-ray Diffraction data. This is NCPOR contribution No. 56/2018.







