LEARNING OBJECTIVES
After reading this article you will be able to:
• describe the clinical characteristics of lithium intoxication
• outline risk factors for lithium intoxication
• understand the assessment and management of lithium intoxication.
Lithium has been widely recognised as the first-line treatment for bipolar disorder, with its anti-manic properties first identified in 1949 (Cade Reference Cade1949; McIntyre Reference McIntyre, Berk and Brietzke2020). Its primary use is as maintenance therapy in bipolar disorder, being an effective treatment for the prevention of illness recurrence, and in acute episodes of mania and depression (Goodwin Reference Goodwin, Haddad and Ferrier2016; Kessing Reference Kessing, Bauer and Nolen2018; Yatham Reference Yatham, Kennedy and Parikh2018). It has a long history of use in the treatment of depressive disorders, and can be used as augmentation therapy and as prophylaxis for unipolar depression (Cowen Reference Cowen and Anderson2015). Although an effective treatment, lithium's narrow therapeutic index requires caution with its use, to prevent toxicity. Target therapeutic serum levels for maintenance therapy in bipolar disorder are approximately 0.6–0.8 mmol/L, but in acute mania higher levels of 0.8–1.0 mmol/L, sometimes up to 1.2 mmol/L, are required (Stokes Reference Stokes, Kocsis and Arcuni1976; Goodwin Reference Goodwin, Haddad and Ferrier2016). As toxicity occurs at concentrations above 1.5 mmol/L, doses are gradually increased with careful monitoring of serum lithium concentrations, and kept at the minimum threshold concentration to achieve therapeutic effect. Once that is achieved, lithium levels are checked on a regular basis.
Lithium's mechanism of action in mood stabilisation remains unclear. It is postulated to modulate glutamate, inositol monophosphate and glycogen synthase kinase 3 pathways in the central nervous system (CNS) and to alter the release of noradrenaline, dopamine and serotonin. The role of lithium's pharmacodynamic properties in intoxication is uncertain (Meyer Reference Meyer and Brunton2011) and may be an area for future research. However, to understand the pathophysiology of lithium toxicity, the pharmacokinetic properties of lithium must be considered.
Pharmacokinetics
Absorption
When lithium is ingested orally, it is rapidly and completely absorbed by the gastrointestinal (GI) tract within 4–8 h, with serum concentrations of therapeutic doses peaking at 1–2 h after ingestion. Lithium is also available in extended-release formulations, which have limited water solubility and achieve peak serum levels after 5–6 h. Acute ingestion of large quantities of extended-release formulations can result in prolonged absorption, continuous rising of plasma concentrations and peak levels occurring after 48–72 h (Waring Reference Waring2006).
Distribution
Lithium is an alkali metal and one of the smallest elements in nature. It is therefore easily and rapidly distributed across the total body water (fluid) compartments, with a corresponding volume of distribution of 0.6–0.9 L/kg (Waring Reference Waring2006; Haussmann Reference Haussmann, Bauer and von Bonin2015). Lithium then begins a slow elimination by the kidneys, and a gradual equilibrium takes place between the intracellular and extracellular compartments.
Lithium distribution is complex and shows preferential uptake in certain compartments, such as the thyroid and kidney. Lithium concentrations in the brain are similar to those in the plasma. However, distribution of lithium to the brain is delayed by approximately 24 h and peak brain concentrations occur at least several hours later than the serum peak. This must be taken into consideration in cases of lithium intoxication, as toxic neurological symptoms may be seen several hours later than other features as tissue concentration increases (Finley Reference Finley, Warner and Peabody1995; Waring Reference Waring2006).
It takes 6 h to allow for the initial distribution of lithium – and accurate interpretation of serum concentration is not possible before this. It is most accurate at 12 h after lithium dosing.
Elimination
Lithium is a simple cation with negligible protein binding and is almost exclusively eliminated by the kidneys. It follows first-order kinetics; the elimination half-life is approximately 20–24 h after a single dose and up to 58 h in chronic use or in the elderly (Timmer Reference Timmer and Sands1999; Cates Reference Cates and Sims2005). Lithium is freely filtered by the glomerulus and approximately 80% is reabsorbed, predominantly along the proximal tubule and, to a lesser extent, along more distal tubular segments. Renal clearance in normal individuals is approximately 10–40 mL/min (Okusa Reference Okusa and Crystal1994). Total body clearance can be impeded by ongoing gastrointestinal absorption, particularly in overdoses of sustained-release preparations (Waring Reference Waring2006).
Patterns of toxicity
There are three patterns of lithium intoxication, each with their own pharmacokinetics, clinical features and outcomes. These are classified as acute, acute-on-chronic and chronic intoxication.
Acute
Acute intoxication occurs outside of the setting of regular lithium treatment: for example by intentionally overdosing on somebody else's tablets. Acute cases are generally mild, owing to rapid distribution of lithium across body compartments and subsequent falling of serum levels (Jaeger Reference Jaeger, Sauder and Kopferschmitt1993). For this reason, a higher level of lithium is required to cause toxicity in acute cases (Bailey Reference Bailey and McGuigan2000). In comparison with chronic poisoning, the risk of neurotoxicity is low, as lithium concentrations have not yet built up in the brain in acute poisoning (Chen Reference Chen, Shen and Lu2004).
Acute-on-chronic
Acute-on-chronic intoxication refers to acute overdose and poisoning in the setting of regular lithium treatment. In contrast to chronic intoxication, acute and acute-on-chronic cases do not tend to show consistent correlation between serum concentrations and clinical features (Bailey Reference Bailey and McGuigan2000).
Chronic
Chronic intoxication, the most common pattern, refers to toxicity over the course of long-term treatment. In chronic intoxication the rate of lithium ingested is greater than the elimination rate, usually over a period of weeks. This may occur due to inappropriately high target concentrations, insufficient monitoring or comorbid illnesses such as chronic kidney disease (Waring Reference Waring2006).
Acute-on-chronic and chronic cases of intoxication are more likely to display clinical toxicity, as the lithium half-life is longer in these cases (50 h v. 13 h in acute toxicity) (Bailey Reference Bailey and McGuigan2000). This is probably multifactorial and relates to the intracellular lithium accumulation and inhibited lithium efflux with chronic use (Jaeger Reference Jaeger, Sauder and Kopferschmitt1993; Okusa Reference Okusa and Crystal1994). Patients are therefore exposed to high lithium concentrations for a prolonged period, and longer exposure to elevated lithium concentrations is more toxic than exposure to high concentrations for shorter periods (Chen Reference Chen, Shen and Lu2004; Waring Reference Waring, Laing and Good2007). It requires time for lithium to be distributed to intracellular compartments such as the CNS and brain, which occurs with chronic use (Hanak Reference Hanak, Chevillard and El Balkhi2015). Further, the rate of lithium elimination exceeds the rate at which it is distributed out of intracellular sites such as the brain – a phenomenon that is more evident with chronic treatment. Chronic toxicity has increased risk for neurotoxicity and a worse prognosis in comparison with acute intoxication (Chen Reference Chen, Shen and Lu2004). It is unclear whether higher lithium concentration affects renal clearance of lithium, or whether reduced renal clearance causes higher concentration (Bailey Reference Bailey and McGuigan2000).
Epidemiology
There are few population-based studies reporting on rates of lithium toxicity. A case–control study identified a prevalence rate of 2% for lithium toxicity over a 5-year study period (Heath Reference Heath, Billups and Gaughan2018), whereas a large Swedish population-based study identified a rate of 7% over a 17-year period (Ott Reference Ott, Stegmayr and Renberg2016). Lithium intoxication is a common occurrence over several years of treatment but annual incidence remains low, with an incidence rate of 1/100 lithium-treated patients developing intoxication per year of lithium treatment in the Swedish observational study (Ott Reference Ott, Stegmayr and Renberg2016).
Across various studies, intoxication occurs nearly twice as frequently in women. Chronic intoxication is the most common pattern (Offerman Reference Offerman, Alsop and Lee2010; Ott Reference Ott, Stegmayr and Renberg2016). In general, the incidence of acute-on-chronic intoxication may be underestimated, with one survey identifying this category as the most frequent (Bailey Reference Bailey and McGuigan2000). Although acute intoxication accounts for the least common pattern of intoxication (Mowry Reference Mowry, Spyker and Cantilena2014), a prospective evaluation of referrals to a national poisons centre in Scotland found this category to be the most frequently reported (Waring Reference Waring, Laing and Good2007). Table 1 shows the prevalence of the various patterns of intoxication among individuals requiring hospital admission in a Canadian study over a 5-year period (Offerman Reference Offerman, Alsop and Lee2010) compared with Swedish observational data (Ott Reference Ott, Stegmayr and Renberg2016).The mortality rate is 0.8% (Offerman Reference Offerman, Alsop and Lee2010), a significant improvement in comparison with the mortality rate in the 1970s, which was reported at 15% (Hansen Reference Hansen and Amdisen1978).
TABLE 1 Patterns of lithium intoxication among individuals requiring hospital admission
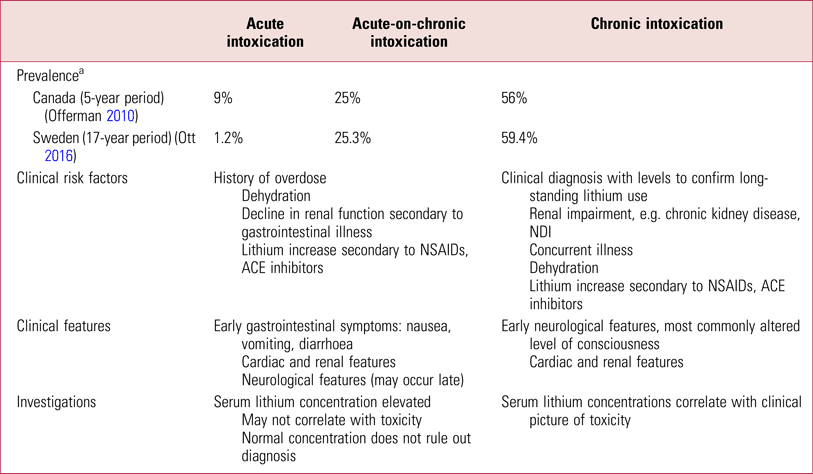
NSAID, non-steroidal anti-inflammatory drug; ACE, angiotensin-converting enzyme; NDI, nephrogenic diabetes insipidus.
a. Prevalence where the pattern of intoxication was unknown was 10% in the Canadian study and 14.3% in the Swedish study.
Sources: Bailey & McGuigan, Reference Bailey and McGuigan2000; Waring et al, Reference Waring, Laing and Good2007; Offerman et al, Reference Offerman, Alsop and Lee2010; Ott et al, Reference Ott, Stegmayr and Renberg2016.
Aetiology
Lithium intoxication may be the result of several different risk factors (Table 2). Medications such as thiazide diuretics and non-steroidal anti-inflammatory drugs (NSAIDs) interact with lithium and increase its serum concentration by reducing its renal clearance. As a monovalent cation, lithium resembles sodium in size and charge. In states of sodium depletion or sodium avidity (e.g. extracellular volume depletion due to vomiting or diarrhoea), the proximal tubular absorption of lithium increases, therefore increasing the risk of lithium toxicity. Both thiazide diuretics and NSAIDs alter renal lithium clearance by triggering increased tubular reabsorption of sodium (and lithium). In the case of thiazide diuretics, this occurs in response to the natriuresis caused by the drug. In the case of NSAIDs, it is postulated to be due to the inhibition of prostaglandin production, which decreases renal blood flow, promoting sodium avidity (Finley Reference Finley, Warner and Peabody1995, Reference Finley2016).
TABLE 2 Risk factors in lithium intoxication
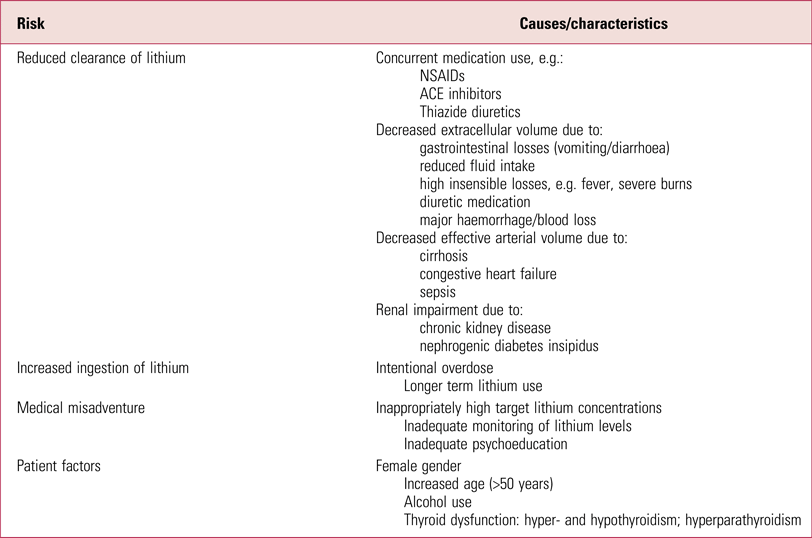
Sources: Okusa & Crystal, Reference Okusa and Crystal1994; Oakley et al, Reference Oakley, Whyte and Carter2001; Webb et al, Reference Webb, Solomon and Ryan2001; Bedford et al, Reference Bedford, Weggery and Ellis2008; Haussmann et al, Reference Haussmann, Bauer and von Bonin2015; Baird-Gunning et al, Reference Baird-Gunning, Lea-Henry and Hoegberg2017.
Chronic renal impairment and nephrogenic diabetes insipidus (NDI) are important risk factors to consider, as they both occur commonly in the setting of long-term lithium treatment (Gupta Reference Gupta, Kripalani and Khastgir2013). Impaired urinary concentrating ability is seen in up to 40% of patients on lithium, and approximately 12% develop NDI (Stone Reference Stone1999; Gupta Reference Gupta, Kripalani and Khastgir2013). The cause is multifactorial, involving lithium's interference with the effects of anti-diuretic hormone (ADH), aquaporin-2 synthesis and prostaglandin production. This is usually reversible during the first 6 years of treatment but may become permanent with long-term treatment (≥15 years) and may result in NDI (Grandjean Reference Grandjean and Aubry2009; Gupta Reference Gupta, Kripalani and Khastgir2013). Whether lithium intoxication itself has long-term effects on renal function remains uncertain and represents a potential avenue for further research (Ott Reference Ott, Stegmayr and Renberg2016).
Clinical features
Lithium intoxication has a heterogeneous clinical presentation with varying clinical features, from asymptomatic to clinical signs of severe toxicity. Any lithium-treated patient who develops altered level of consciousness, vomiting, gross tremor or cerebellar signs should be considered to have lithium intoxication and managed as such until the situation is clarified.
The CNS is the most significantly affected system in lithium toxicity, and altered level of consciousness is the most commonly reported feature (Offerman Reference Offerman, Alsop and Lee2010). CNS features include drowsiness, coarse tremor, slurred speech, hyperreflexia, agitation, muscle weakness and ataxia. With increasing severity, increased confusion, disorientation and seizures may occur, progressing to more impaired levels of consciousness and eventually coma (Jaeger Reference Jaeger, Sauder and Kopferschmitt1993; Bailey Reference Bailey and McGuigan2000; Ott Reference Ott, Stegmayr and Renberg2016). Gastrointestinal features include nausea, vomiting and diarrhoea. Renal symptoms of polyuria and polydipsia occur, and impaired urinary concentrating ability is seen in acute intoxication (Erden Reference Erden, Karagöz and Başak2013). Acute renal failure occurs rarely (Gupta Reference Gupta, Kripalani and Khastgir2013). Cardiac features also develop, including bradycardia, prolonged QTc intervals, arrhythmias and, in severe cases, cardiovascular collapse (Bailey Reference Bailey and McGuigan2000).
Other neurological manifestations occasionally seen include extrapyramidal features, generalised polyneuropathies and neuromuscular features such as fasciculations and myoclonus (Grandjean Reference Grandjean and Aubry2009). Most symptoms and signs of lithium intoxication resolve as serum lithium levels normalise, but there is a risk of long-term neurological sequelae such as the syndrome of irreversible lithium-effectuated neurotoxicity (SILENT), which predominantly manifests as cerebellar dysfunction, with features of ataxia, dysarthria and dysmetria (Adityanjee 1987).
There may be differences in the clinical presentation depending on which pattern of lithium toxicity has occurred. Individuals with acute toxicity are more likely to be asymptomatic, despite high lithium concentrations, and gastrointestinal symptoms are thought to occur earlier and more frequently. By contrast, chronic toxicity is predominantly associated with gradually developing neurological signs and may present with overt signs and symptoms despite relatively low lithium levels (Timmer Reference Timmer and Sands1999; Grandjean Reference Grandjean and Aubry2009).
Differential diagnosis
The differential diagnosis (Box 1) includes a range of presentations associated with neurological features and alterations in mental status or levels of consciousness. It is essential to rule out neuroleptic malignant syndrome (NMS) and serotonin syndrome. Both may occur in the setting of lithium treatment and may have fatal outcomes. Lithium toxicity, NMS and serotonin syndrome can all present with altered consciousness and neurological features. NMS will be distinguishable by fever >38°C, generalised (lead-pipe) rigidity, bradykinesia and autonomic instability, along with raised creatinine kinase. Distinguishing features of serotonin syndrome include agitation, shivering, diarrhoea, hyperreflexia of the lower limbs, and myoclonus (including ocular clonus) (Gupta Reference Gupta, Kripalani and Khastgir2013; Wadoo Reference Wadoo, Ouanes and Firdosi2021).
BOX 1 Differential diagnosis
Iatrogenic effect
Psychotropic medication-related adverse event:
• serotonin syndrome
• seuroleptic malignant syndrome
Intoxication
Drug toxicity, e.g. due to:
• anticholinergics
• cocaine
• phencyclidine (PCP)
Withdrawal syndrome, e.g. due to:
• alcohol
• benzodiazepines
Endocrine dysfunction
Thyroid dysfunction:
• thyrotoxicosis
• hypothyroidism
Glucose disturbance:
• hypoglycaemia
Neurological effect
Trauma, e.g. head trauma
Infection, e.g. central nervous system infection
Vascular, e.g. cerebrovascular accident
Assessment and management
Recognition
The first step in the management of lithium intoxication is prompt recognition and early referral for medical intervention. Clinicians must be vigilant and screen for toxicity symptoms as well as for risk factors for intoxication. Both patient and clinician may miss early features of intoxication, and therefore continuous psychoeducation is essential. An awareness of potentially fatal outcomes of lithium intoxication is necessary, with increased risk in cases of chronic and acute-on-chronic intoxication.
Referral
When lithium toxicity is suspected, blood tests including serum lithium concentration and renal function should be performed. Generally, a referral to hospital may be indicated to allow for the timely completion of tests (Haussmann Reference Haussmann, Bauer and von Bonin2015).
Box 2 shows key clinical factors to assess in each case of lithium intoxication, which will influence management and monitoring requirements. In cases of overdose, additional drug ingestion must be clarified.
BOX 2 Key clinical factors to assess in lithium intoxication
• Acute ingestion or not
• Time course: acute, acute-on-chronic, chronic
• Signs and symptoms and time since onset
• Time since acute ingestion
• Time of last lithium intake
• Regular lithium dose and duration of use
• Lithium dose changes
• Formulation: extended-release versus immediate-release
• Prior lithium concentration measures (if available)
• Medical conditions (Table 2): e.g. chronic kidney disease, nephrogenic diabetes insipidus
Investigation
Serum lithium concentration should be measured on presentation. Clinical findings of toxicity commonly occur at serum concentrations >1.5 mmol/L (Ott Reference Ott, Stegmayr and Renberg2016). However, serum lithium concentrations should only be viewed as a guide to potential toxicity and must be interpreted in relation to the clinical findings and renal function.
Serum lithium concentration measurements should be repeated every 2–4 h during initial management. Once they are decreasing they can be checked 6–12 hourly (Haussmann Reference Haussmann, Bauer and von Bonin2015). Table 3 provides a list of recommended investigations.
TABLE 3 Investigations in suspected lithium toxicity
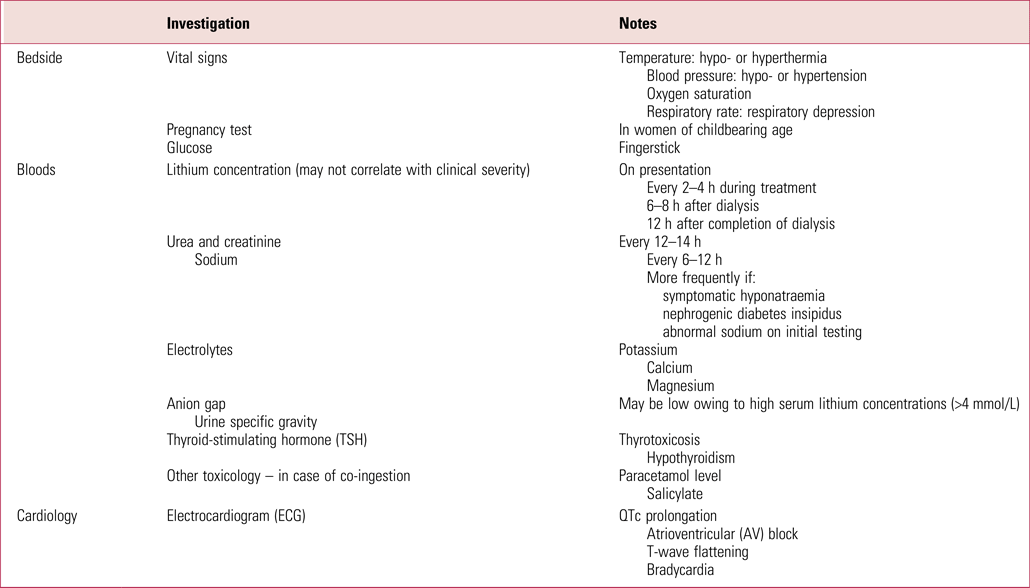
Sources: Decker et al, Reference Decker, Goldfarb and Dargan2015; Haussman et al, Reference Haussmann, Bauer and von Bonin2015; Gitlin et al, Reference Gitlin2016; Baird-Gunning et al, Reference Baird-Gunning, Lea-Henry and Hoegberg2017.
Serial lithium measurements are necessary owing to lithium's delayed distribution to the CNS and brain. Serial concentrations are also useful for determining the type of intoxication that has taken place: a level that peaks and quickly falls suggests acute intoxication, whereas levels that persist or rebound indicate chronic saturation of the tissues (Haussmann Reference Haussmann, Bauer and von Bonin2015). Serial testing is also required in cases of overdose of sustained-release lithium to establish the extent of toxicity that has taken place (Grandjean Reference Grandjean and Aubry2009).
Renal function is important as this may be disturbed in lithium toxicity, which can prolong the clearance of lithium and exposure to toxic levels (Grandjean Reference Grandjean and Aubry2009). Persistent electrolyte disturbances may require an intensive care unit (ICU) admission.
Treatment
Admission
Hospital admission should be considered for individuals with clinical signs and symptoms of toxicity (this should be instigated regardless of lithium concentration, as serum levels may not correlate with degree of neurological symptoms) and for those with a serum level >2.0 mmol/L on a background of chronic lithium treatment. Approximately 80% of individuals with lithium intoxication require admission (Offerman Reference Offerman, Alsop and Lee2010), with an emphasis on stabilising the patient and minimising the time of exposure to toxic lithium levels (Decker Reference Decker, Goldfarb and Dargan2015).
Admission to an ICU is required if there are severe and life-threatening symptoms such as cardiovascular collapse, respiratory depression, severe neurological symptoms, hyperthermia requiring immediate cooling, or renal failure, or if there are very high lithium concentrations (>4.0 mmol/L). Intubation is required in approximately 5% of patients with lithium intoxication (Offerman Reference Offerman, Alsop and Lee2010).
Stabilisation
In all cases, initial management is as with any case of poisoning, using an ABCDE (airway, breathing, circulation, disability, exposure) approach to achieve airway management, ensuring adequate breathing and oxygen saturation, and circulatory support.
Medication review
In cases of mild toxicity, discontinuation of lithium may be all that is required.
It is essential to remove any medications that may be exacerbating lithium intoxication by pharmacokinetic interactions limiting renal excretion, such as NSAIDS, angiotensin-converting enzyme (ACE) inhibitors and diuretics, and medications with pharmacodynamic interactions with increased risk of neurotoxicity or cardiovascular adverse effects. Any medications potentially contributing to symptoms of toxicity should be reviewed and discontinued if safe to do so. This includes any medication that may also cause cardiac arrhythmias or QTc prolongation, neurotoxicity (e.g. antiepileptics) or electrolyte imbalance.
Fluid resuscitation
In chronic cases of toxicity, dehydration and volume depletion is a frequent cause. Fluid infusion with normal saline is required to correct and/or prevent volume depletion and restore electrolyte and fluid balance (Grandjean Reference Grandjean and Aubry2009). This should occur alongside regular monitoring of serum sodium concentration to monitor for hypernatraemia. Hypernatraemia may occur when there is an inadequate level of intravenous hydration and a lack of access to free water intake in someone with NDI or impaired urinary concentrating ability secondary to lithium. If this occurs, replacement of the calculated total body water deficit is required, using normal or hypotonic saline (Stone Reference Stone1999).
Acute intoxication
Absorption preventive measures are used in acute intoxication only, and include gastric lavage and whole-bowel irrigation.
Gastric lavage
There is limited evidence to support the use of gastric lavage, but it may be helpful if administered within 1 h of ingestion (Teece Reference Teece and Crawford2005; Waring Reference Waring2006).
Whole-bowel irrigation
Whole-bowel irrigation with polyethylene glycol solution is considered when acute poisoning by extended-release formulations occurs, with recommendations for use when at least 80 mg/kg of lithium are ingested. It should be initiated within 12 h of ingestion (Wilting Reference Wilting, Egberts and Heerdink2009) in alert patients with no confusion allowing the patient to drink the solution or for nasogastric tube insertion to allow for the rapid ingestion of the fluid (1–2 L/h) until there is clear rectal effluent. However, this intervention is also based on limited evidence and caution is required as it may cause hypokalaemia (Reumkens Reference Reumkens, Masclee and Winkens2017). Bowel obstruction or haemodynamic instability would be contraindications to the use of whole-bowel irrigation.
Forced diuresis
Forced diuresis is the administration of intravenous fluid, sometimes along with diuretics, to enhance renal clearance of medication. Although potentially helpful in patients with volume depletion, it is no longer used and is in fact contraindicated owing to a lack of evidence for its efficacy and as it may exacerbate water and electrolyte imbalance (Waring Reference Waring2006).
Activated charcoal
Activated charcoal has no effect on lithium toxicity, but may be helpful in cases of polysubstance intoxication (Okusa Reference Okusa and Crystal1994).
Dialysis
In severe cases, treatment should be commenced with extracorporeal treatments (ECTRs). ECTRs used in toxin removal include intermittent haemodialysis, continuous renal replacement therapies (CRRTs) and, to a lesser extent, peritoneal dialysis. Solute removal from the intravascular compartment can occur by diffusion or convection (or both) (Decker Reference Decker, Goldfarb and Dargan2015). In a large study of lithium intoxication cases, 14% of patients received haemodialysis, 71% of whom had chronic intoxication (Offerman Reference Offerman, Alsop and Lee2010).
Owing to its physical and pharmacokinetic properties, lithium is very effectively and rapidly dialysed (Okusa Reference Okusa and Crystal1994). Clinical guideline recommendations for the initiation and cessation of dialysis are inconsistent, but because of the risk of permanent neurological sequelae with lithium toxicity, the decision to initiate haemodialysis should be made in the first 8–12 h of admission (Timmer Reference Timmer and Sands1999). There is a lack of randomised trial data on which to base treatment decisions, and further contributing to the inconsistency in clinical guidelines is the heterogeneity of clinical presentations, differing categories of intoxication and the lack of correlation between serum concentrations and clinical toxicity, particularly in acute scenarios.
Recommended indications for haemodialysis are displayed in Table 4. These recommendations are based on guidelines developed by the Extracorporeal Treatments in Poisoning (EXTRIP) workgroup via expert consensus (Decker Reference Decker, Goldfarb and Dargan2015) and take more of a clinical approach than previous guidelines, which suggested haemodialysis for serum lithium levels >4 mmol/L in acute toxicity and >2.5 mmol/L in chronic toxicity (Baird-Gunning Reference Baird-Gunning, Lea-Henry and Hoegberg2017). Patients falling outside of the categories listed in Table 4 need careful consideration on a case-by-case basis, which will be guided by the lithium absorption, distribution and elimination factors in each case (Grandjean Reference Grandjean and Aubry2009). This will be informed by the type of toxicity that has taken place, the formulation of lithium involved and clinical features such as comorbid illnesses preventing extensive intravenous hydration (Haussmann Reference Haussmann, Bauer and von Bonin2015). Haemodialysis is also recommended for those with serum levels that continue to rise owing to continued gastrointestinal absorption. A suggested guide to determine efficacy has been described as attainment of serum levels <1.0 mmol/L 6–8 h after dialysis (Waring Reference Waring2006; Decker Reference Decker, Goldfarb and Dargan2015).
TABLE 4 Indications for dialysis

Sources: Decker et al, Reference Decker, Goldfarb and Dargan2015; Baird-Gunning et al, Reference Baird-Gunning, Lea-Henry and Hoegberg2017.
Rebound of serum lithium levels is seen 6–12 h after haemodialysis, as lithium is redistributed from deeper tissue compartments to the plasma (Okusa Reference Okusa and Crystal1994; Decker Reference Decker, Goldfarb and Dargan2015). Therefore, accurate assessment and appropriate treatment should take place within this period (Dawson Reference Dawson and Whyte1999). It is recommended that the lithium concentration should be measured 6 h after haemodialysis to check that levels are decreasing. An increase in serum lithium concentration of >1.0 mmol/L within 8 h of dialysis is a recommended indication for repeat dialysis (Baird-Gunning Reference Baird-Gunning, Lea-Henry and Hoegberg2017), and dialysis should be repeated until serum lithium concentrations are <1 mmol/L for at least 6 h after treatment and there is also clinical improvement (Okusa Reference Okusa and Crystal1994; Decker Reference Decker, Goldfarb and Dargan2015). It is often the case that clinical improvement is slower than reductions in lithium concentrations. Studies have indicated that at least two haemodialysis treatment courses may be required to treat lithium intoxication (Okusa Reference Okusa and Crystal1994).
Dialysis modality
Intermittent haemodialysis
Intermittent haemodialysis is the most rapid and effective way of clearing lithium from the plasma. It utilises ultrafiltration for fluid removal and uses predominantly diffusion for solute removal. Depending on blood flow, haemodialysis can achieve a lithium clearance of up to 180 mL/min (Bouchard Reference Bouchard, Roberts and Roy2014). Although intermittent haemodialysis is well tolerated in most patients, it can cause haemodynamic compromise in individuals with cardiovascular instability such as cardiac arrhythmia or hypovolaemic or septic shock. Patients require close haemodynamic monitoring and may require inotropic or vasopressor support during dialysis.
Continuous renal replacement therapy
Continuous renal replacement therapies such as continuous arteriovenous haemodiafiltration (CAVHD) and continuous venovenous haemodiafiltration (CVVHD) may also have a role as second-line extracorporeal treatments; they clear lithium at a rate of 20–40 mL/min (Decker Reference Decker, Goldfarb and Dargan2015; Waring Reference Waring2006). In comparison with intermittent haemodialysis, these methods of ECTR are slower and occur over a longer period. CRRT utilises diffusion, convection or both for solute removal (Jha Reference Jha and Padmaprakash2018). CRRT is helpful for patients who are haemodynamically compromised or for whom there may be a delay in initiating haemodialysis (Waring Reference Waring2006).
Peritoneal dialysis
Peritoneal dialysis may be considered if haemodialysis or CRRT are contraindicated or delayed (Waring Reference Waring2006). This method is significantly less effective, with lithium clearance rates only marginally higher than that of renal clearance, at 9–14 mL/min (Brown Reference Brown and Pawlikowski1981).
Discharge criteria
Once the patient has been treated and stabilised, and lithium effectively eliminated, discharge from hospital can be considered. Our recommendation is that this can be safely done once clinical improvement occurs and serum lithium levels have been <1.5 mmol/L for 1–2 days. However, in cases of large-volume ingestion of sustained-release preparations of lithium, it may be necessary to continue to observe for 3–4 days (Timmer Reference Timmer and Sands1999). Considerations can then be made as to whether to restart lithium.
Restarting lithium
Individuals who have previously responded well to lithium with no contraindications to restarting treatment should have their lithium reintroduced when it is safe to do so. This should be a multidisciplinary team decision, with the patient's involvement, taking the risks and benefits of continued lithium treatment into account. Risks to consider include the risk of acute overdose, the risks with medical comorbidities such as chronic kidney disease and NDI, and the likelihood of future lithium intoxication (Baird-Gunning Reference Baird-Gunning, Lea-Henry and Hoegberg2017). These must be weighed against the benefit of maintenance lithium therapy in bipolar disorder.
To date, there are no guidelines on the reintroduction of lithium after lithium intoxication and evidence remains lacking in this area. Our recommendation is to restart treatment once serum lithium levels have been within, or below, the therapeutic range for at least 24 h.
Psychoeducation is crucial in those recommencing treatment, with a view to preventing recurrence of intoxication. Careful dose titration and monitoring of serum levels is required. There may be a need to reassess target lithium concentrations given the patient's longitudinal history and comorbidities. Medications that interact with lithium to increase toxicity risk should be discontinued. Patients must also be counselled on the long-term risk of irreversible neurological sequelae (Adityanjee 1987).
Conclusions
Lithium intoxication, although potentially life-threatening, can be safely managed in most cases and the mortality rate is low. There are few studies reporting on the prevalence and incidence of lithium intoxication, but its relatively low incidence suggests that current lithium monitoring procedures are effective in minimising risk of lithium toxicity. In cases of suspected lithium intoxication, clinical features of lithium toxicity are more important than serum lithium levels, and any lithium-treated patient who develops vomiting, confusion or cerebellar signs should be assumed to have lithium toxicity until the situation is clarified. Clinicians should have a low threshold to check lithium levels if clinical features of concern occur.
There is no specific antidote for lithium intoxication. The pattern of lithium exposure and subsequent intoxication category have important implications for management and prognosis.
Many treatment approaches are available, but randomised trial data regarding their efficacy are lacking. The mainstay of treatment is stabilisation of the patient and minimisation of the time of toxic exposure to lithium. More trial data are required to better establish specific criteria for treatment interventions, to clarify when to initiate specific interventions such as dialysis, and to better determine differential treatment outcomes. Clear guidelines do not yet exist regarding when to restart lithium and when to discharge from hospital following intoxication. Prevention of lithium intoxication is crucial, and education of clinicians and patients is key to ensuring that risk factors for intoxication are minimised and clinical features are recognised early.
Data availability
Data availability is not applicable to this article as no new data were created or analysed in this study.
Author contributions
All authors contributed to the writing of the manuscript. J.L. conceived the idea of the article and oversaw the project. N.M. carried out a literature review and did the primary writing of the article. L.R. contributed to the sections related to renal medicine and extracorporeal treatments.
Funding
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Declaration of interest
None.
MCQs
Select the single best option for each question stem
1 The following features are risk factors for lithium toxicity except:
a dehydration
b NSAIDs
c chronic kidney disease
d epilepsy
e female gender.
2 Gastrointestinal symptoms are most commonly seen:
a in acute lithium toxicity
b in acute-on-chronic lithium toxicity
c in chronic lithium toxicity
d in all forms of lithium toxicity
e as a normal side-effect of therapeutic lithium.
3 The following are used in the management of lithium toxicity except:
a correction of dehydration and sodium depletion
b activated charcoal
c discontinuation of lithium
d gastric lavage
e haemodialysis.
4 The most effective way of eliminating lithium from the serum is:
a continuous renal replacement therapy
b whole-bowel irrigation
c haemodialysis
d peritoneal dialysis
e fluid resuscitation.
5 Recommended indications for haemodialysis in lithium intoxication include all except:
a serum lithium levels >5 mmol/L
b serum lithium levels >4 mmol/L with renal impairment
c if the expected time to achieve serum lithium level <1 mmol is >36 h
d severe clinical features regardless of toxicity
e ingestion of extended-release lithium.
MCQ answers
1 d 2 a 3 b 4 c 5 e








eLetters
No eLetters have been published for this article.