Depression is a common mental disorder around the world. According to the WHO, about 280 million adults (5 % of the world population) suffer from depression symptoms(Reference Chen, Wang and Ji1). People with depression suffer from functional impairment and reduced quality of life(Reference Katschnig2). Depression is also associated with a higher risk of CHD(Reference Rudisch and Nemeroff3), stroke(Reference Krishnan4), and type 2 diabetes(Reference Carnethon, Biggs and Barzilay5) and thereby affects both individuals(Reference Lin, Yen and Chen6) and societies(Reference Ferrari, Charlson and Norman7).
Hence, it seems necessary to investigate various approaches to prevent depressive disorders among the general population or diminish depressive symptoms among people with existing depression. Currently, both pharmacological and non-pharmacological approaches are being used for treating depression. Even though there is improvement in developing antidepressant medications with lesser side effects, patients still experience residual symptoms(Reference Bokslag, van Weissenbruch and Mol8). Therefore, alternative non-pharmacological approaches for treating depressive symptoms may still be needed.
Evidence suggests that poor diet quality could be a risk factor for developing depression(Reference Bodnar and Wisner9). Of note, the optimum development of the central nervous system requires sufficient intake of n-3 PUFA such as EPA and DHA(Reference Hibbeln10). Evidence supports the protective effect of EPA and DHA in treating mental and mood disorders(Reference Hibbeln10,Reference Parker, Gibson and Brotchie11) . Over the past century, changes in the diet caused a noticeable decrease in the ratio of n-3 to n-6 fatty acids(Reference Parker, Gibson and Brotchie11). Epidemiologic studies have shown that patients with depression and mood disorders have a low dietary intake of long-chain n-3 fatty acids(Reference Hakkarainen, Partonen and Haukka12,Reference Conklin, Manuck and Yao13) .
Previous pairwise meta-analyses have reported conflicting results about the effects of supplementation with n-3 fatty acids on depressive symptoms(Reference Liao, Xie and Zhang14,Reference Deane, Jimoh and Biswas15) . A recent network meta-analysis indicated that high-dose n-3 fatty acids might be superior to low-dose supplements in reducing depressive disorders in patients with major depressive disorders(Reference Luo, Feng and Yang16). However, the optimum dose of n-3 fatty acids supplementation for reducing depressive symptoms has not been ascertained. Evaluating the potential dose-dependent effects of n-3 fatty acids on depressive symptoms can provide useful information for both patients and clinicians and, thus, may have important clinical implications. In addition, the potential efficacy of n-3 fatty acids on reversal of depression has not been well investigated. Therefore, we conducted a systematic review and dose–response meta-analysis of randomised controlled trials (RCT) to investigate the effectiveness of n-3 fatty acids for the prevention of depression, as well as for treating depressive symptoms in adults.
Methods
The review was planned and conducted in accordance with the Cochrane Handbook for Systematic Reviews of Interventions(Reference Higgins, Thomas and Chandler17) and the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework(Reference Schünemann, Oxman and Brozek18). The review protocol was registered with PROSPERO (CRD42022308241).
Data sources and searches
We searched PubMed, Scopus and Web of Science from inception to December 2022. Two investigators (RN and SZM) independently performed the literature search and screened the titles and abstracts and full texts. Disagreements were resolved by discussion with a third reviewer (SS-B). We also checked out the reference lists of published meta-analyses of RCT on the effect of n-3 fatty acids on depression and its symptoms. The systematic search was limited to articles published in English. The full search strategy is detailed in online Supplementary Table 2.
Study selection
Inclusion criteria for original controlled trials were as follows: (1) RCT (parallel or crossover design) with no limitation in intervention period, conducted in adults, regardless of medication use and health status, aged 18 years or older; (2) intervention with n-3 supplementation, including EPA and/or DHA or alpha-linolenic acid (ALA) in any type of advice, foodstuffs or oral supplements (oil, capsules or provided foodstuffs) against a control group; (3) considered one of these outcome including risk of depression as assessed by formal diagnosis or an appropriate scale, dichotomised to give risk of depression in participants without depression at baseline, or severity of depression as a continuous scale in participants with or without existing depression, and severity of depression or depression relapse in those with depression at baseline; and (4) provided the number of participants and events across study arms to estimate both relative and absolute effects for binary outcomes, or reported mean difference and its 95 % CI for continuous outcomes or reported required information to calculate these values.
Exclusion criteria
Trials that were conducted in adolescents (under 18 years of age), pregnant and lactating women were excluded from the analyses.
Outcomes
The primary outcomes of our systematic review were as follows (1) risk of developing depression among people without depression evaluated by formal diagnosis or a suitable scale (such as Geriatric Depression Scale (GDS), Becks’ Depression Inventory (BDI), etc.), dichotomised to provide depression risk among individuals without depression before intervention, (2) depression symptoms as a continuous scale in people with or without depression, and (3) depression remission as a dichotomous scale among patients with existing depression. Our secondary outcomes included quality of life(Reference Brenes19), medication reduction, and total and serious adverse events. Any scales that were used to measure depressive symptoms in the included trials were eligible for inclusion in the present meta-analysis.
Data extraction
Two authors (RN and SZM) independently and in duplicate conducted literature screening for eligibility. From studies that were considered eligible, the same two reviewers independently extracted the following data: author name, year of publication, population location, study design and duration, characteristics of the population (% female, mean age +/–sd, baseline BMI and health status), total sample size, intervention characteristics (dose of n-3 supplementation in the intervention group), weight status, drop-out, the scale used for evaluating depressive symptoms, baseline depression severity, comparison group, antidepressant usage (yes/no), physical activity (yes/no), behavioural support (yes/no), outcome measures and main results for the outcomes included.
Risk of bias assessment
To determine the risk of bias of the trials, we used the RoB 2.0 tools for individually randomised parallel-group and crossover trials(Reference Sterne, Savović and Page20). Two authors (RN and SZM) independently evaluated the study’s risk of bias. Disagreements were resolved by consulting a third investigator (SS-B).
Strategy for data synthesis
For reporting the results of the present systematic review, the effect size was considered as standardised mean difference (SMD) and its 95 % CI for continuous outcomes, and both relative (OR and its 95 % CI) and absolute (risk difference (RD) and its 95 % CI) effects for binary outcomes. Since included trials used different scale to measure depressive symptoms, we used SMD to standardise the effect estimates obtained from different scales.
For the analyses of continuous outcomes, we first extracted the mean and sd of changes from baseline to the end of the intervention in each study arm in each trial. If a trial did not report these changes, we used the reported means and sd of outcomes before and after the intervention using the Cochrane Handbook guidelines(Reference Chandler, Cumpston and Li21). For trials that reported standard errors instead of sd, we converted them to sd (Reference Higgins, Thomas, Chandler, Higgins, Thomas, Chandler, Cumpston, Li, Page and Welch22). If neither sds nor standard errors were reported in the trials, we used the average sd obtained from other trials for the analyses(Reference Furukawa, Barbui and Cipriani23). Second, we calculated SMD and its 95 % CI of change in continuous outcomes for each 1 g/d increment in n-3 fatty acids intake in each trial using the method introduced by Crippa and Orsini(Reference Crippa and Orsini24). Trial-specific changes in outcomes per each 1 g/d increment in n-3 fatty acids intake were pooled using the DerSimonian and Laird random-effects model(Reference DerSimonian and Laird25). For the analyses of binary outcomes (depression risk, depression remission and medication reduction), we calculated both relative and absolute effects using the number of participants and events in the intervention and control groups. With regard to trials that had multiple study arms, we included trials that implemented two or three study arms with different doses since dose–response meta-analysis allows to include these trials. With regard to trials that had two study arms, one with co-intervention and another without co-intervention, we selected those without co-intervention for inclusion. For trials that implemented several study arms as intervention that were eligible for inclusion, we combined their results using the methods described below(Reference Borenstein26). In order to rule out a possible placebo effect of n-3 fatty acids, we also showed the effects of the control groups (without n-3 fatty acids) for comparison. To report the results in the control group, we calculated the change in depressive symptoms in the control groups (final values minus baseline values) divided by baseline sd to compare the effect in the control groups with pooled SMD.
We performed prespecified subgroup analyses based on baseline depression risk, defined as: (1) high risk, defined as people with clinically diagnosed depression, using any diagnostic criteria; (2) medium risk, defined as people with depression risk factors such as long-term conditions; and (3) low risk, defined as all other populations); duration of intervention (≤ 12, 12–24, ≥ 24 weeks for the severity of depression and ≤ 1 v. > 1 years for risk of depression); health status; and study risk of bias (low risk v. high risk/some concerns. Moreover, post hoc subgroup analyses were conducted based on supplement type (EPA, EPA + DHA, EPA + DHA + ALA), sex (men, women and both), weight status (normal weight, overweight/obese and not reported), and medication use (yes, no, mixed and not reported). According to eight criteria determined by the Instrument to assess the Credibility of Effect Modification Analyses (ICEMAN), we examined the credibility of subgroup differences(Reference Schandelmaier, Briel and Varadhan27). We used meta-regression analysis to compute the P-values for subgroup differences. We applied Egger’s(Reference Egger, Davey Smith and Schneider28) and Begg’s test(Reference Begg and Mazumdar29) for publication bias and evaluated the funnel plots for asymmetry. For assessing the heterogeneity across trials, we used the I2 statistic and performed a χ2 test (P heterogeneity > 0·10)(Reference Higgins, Sterne, Page, Higgins, Churchill, Chandler and Cumpston30). Finally, we conducted a dose–response meta-analysis to determine the dose-dependent effects of n-3 fatty acids (g/d) on depression risk and its symptoms(Reference Crippa and Orsini24). We used a ‘1-stage’ natural cubic spline regression model on the basis of a random-effects model(Reference DerSimonian and Laird31), assessing heterogeneity with the I2 statistic(Reference Higgins, Thompson and Deeks32). The 1-stage method, consisting of a weighted mixed effects model, was recently developed and(Reference Crippa, Discacciati and Bottai33) allowed us to make inferences about the average dose–response relationship between supplementation with n-3 fatty acids and depressive symptoms. Having no specific parametric assumptions about the shape of the association, we used restricted cubic splines of potassium with three knots at fixed percentiles (10%, 50% and 90 %)(Reference Orsini, Li and Wolk34). Estimates of the parameters were obtained using restricted maximum likelihood(Reference Orsini, Li and Wolk34). We used STATA software version 17.0 for our analyses. A two-tailed P-value less than 0·05 was considered statistically significant.
Grading the evidence
To evaluate the certainty of the evidence, we applied the GRADE approach(Reference Guyatt, Oxman and Kunz35). According to the GRADE, evidence acquired from RCT starts at high certainty that can be downregulated or upregulated based on predefined criteria. Detailed criteria used to apply the GRADE approach are explained in online Supplementary Table 14. In order to interpretation of the magnitude of effect sizes, the estimated SMD were interpreted as a trivial effect (0·0–0·2), a small effect (0·2–0·6), a moderate effect (0·6–1·2), a large effect (1·2–2·0), a very large effect (2·0–4·0) and an extremely large effect (≥ 4·0)(Reference Hopkins, Marshall and Batterham36,Reference Varangot-Reille, Suso-Martí and Romero-Palau37) .
Results
Systematic search
An outline of the search strategy is presented in online Supplementary Fig. 1. Our search in databases identified a total of 2611 records. After excluding 363 duplicates, and exclusion of twenty-one studies, we reviewed the rest of the records for eligibility, and of those, sixty-seven trials met the eligibility criteria(Reference Andrieu, Guyonnet and Coley38–Reference Zanarini and Frankenburg104) (online Supplementary Table 4). Reasons for exclusions are provided in online Supplementary Table 6.
Characteristics of original trials
Fifty-three trials reported information on depression severity, of those, seven trials reported information on depression remission (online Supplementary Table 7). Of the fifty-three studies, ten trials were carried out on healthy participants(Reference Dretsch, Johnston and Bradley53,Reference Gertsik, Poland and Bresee55,Reference Ginty, Muldoon and Kuan58,Reference Hashimoto, Kato and Tanabe62,Reference Jackson, Deary and Reay64,Reference Kiecolt-Glaser, Belury and Andridge67,Reference Sinn, Milte and Street94,Reference van de Rest, Geleijnse and Kok101–Reference Yurko-Mauro, McCarthy and Rom103) , twenty-five trials were carried out in depressed populations(Reference Antypa, Smelt and Strengholt39,Reference Bot, Pouwer and Assies41,Reference Carney, Freedland and Rubin43–Reference Carney, Freedland and Stein45,Reference da Silva, Munhoz and Alvarez49,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Jahangard, Sadeghi and Ahmadpanah65,Reference Jiang, Whellan and Adams66,Reference Lespérance, Frasure-Smith and St-André70,Reference Marangell, Martinez and Zboyan73,Reference Mazereeuw, Herrmann and Oh76,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79–Reference Park, Park and Kim81,Reference Ravi, Khalili and Abbasian87,Reference Rizzo, Corsetto and Montorfano88,Reference Rondanelli, Giacosa and Opizzi90,Reference Shinto, Marracci and Mohr92,Reference Silvers, Woolley and Hamilton93,Reference Su, Huang and Chiu96,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Tayama, Ogawa and Nakaya99,Reference Chang, Chen and Su105) , one study in Alzheimer’s disease patients(Reference Andrieu, Guyonnet and Coley38), two in participants with borderline personality disorder(Reference Bellino, Bozzatello and Rocca40,Reference Zanarini and Frankenburg104) , one in those with stress(Reference Bradbury, Myers and Meyer42), one in patients with myocardial infarction(Reference Giltay, Geleijnse and Kromhout57,Reference Haberka, Mizia-Stec and Mizia60) , one in patients with self-harm experience(Reference Hallahan, Hibbeln and Davis61), one in those with mild cognitive impairment(Reference Lee, Shahar and Chin69), one in those with psychological distress(Reference Lucas, Asselin and Mérette71), one in people at risk for psychotic disorders(Reference McGorry, Nelson and Markulev77), two in those with bipolar disorders(Reference McPhilemy, Byrne and Waldron78,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79) , one in those with schizophrenia(Reference Pawełczyk, Grancow-Grabka and Kotlicka-Antczak82), two in patients with Parkinson’s disease(Reference Pomponi, Loria and Salvati83,Reference Robinson, Gallego and John89) , one in patients with ischemic stroke(Reference Poppitt, Howe and Lithander84), one in women with premenstrual syndrome(Reference Sohrabi, Kashanian and Ghafoori95) and one those with cognitive decline(Reference Yurko-Mauro, McCarthy and Rom103). Twelve trials had a low risk of bias(Reference Antypa, Smelt and Strengholt39,Reference Bot, Pouwer and Assies41,Reference Bradbury, Myers and Meyer42,Reference Ginty, Muldoon and Kuan58,Reference Jiang, Whellan and Adams66,Reference Lespérance, Frasure-Smith and St-André70,Reference Lucas, Asselin and Mérette71,Reference Masoumi, Kazemi and Tavakolian74,Reference McGorry, Nelson and Markulev77,Reference Pomponi, Loria and Salvati83,Reference Robinson, Gallego and John89,Reference van de Rest, Geleijnse and Kok101) , sixteen trials were rated to have some concerns (Reference Chang, Chang and Yang46,Reference Dretsch, Johnston and Bradley53,Reference Gertsik, Poland and Bresee55,Reference Jahangard, Sadeghi and Ahmadpanah65,Reference Mazereeuw, Herrmann and Oh76,Reference McPhilemy, Byrne and Waldron78,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Park, Park and Kim81,Reference Pawełczyk, Grancow-Grabka and Kotlicka-Antczak82,Reference Poppitt, Howe and Lithander84,Reference Rondanelli, Giacosa and Opizzi90,Reference Shinto, Marracci and Mohr92–Reference Sinn, Milte and Street94,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Watanabe, Matsuoka and Kumachi102,Reference Yurko-Mauro, McCarthy and Rom103) and the other twenty-five trials considered to have a high risk of bias(Reference Andrieu, Guyonnet and Coley38,Reference Bellino, Bozzatello and Rocca40,Reference Carney, Freedland and Rubin43–Reference Carney, Freedland and Stein45,Reference Cohen, Joffe and Guthrie48,Reference da Silva, Munhoz and Alvarez49,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Giltay, Geleijnse and Kromhout57,Reference Haberka, Mizia-Stec and Mizia60–Reference Hashimoto, Kato and Tanabe62,Reference Jackson, Deary and Reay64,Reference Kiecolt-Glaser, Belury and Andridge67,Reference Lee, Shahar and Chin69,Reference Marangell, Martinez and Zboyan73,Reference Nemets, Stahl and Belmaker80,Reference Ravi, Khalili and Abbasian87,Reference Rizzo, Corsetto and Montorfano88,Reference Sohrabi, Kashanian and Ghafoori95,Reference Su, Huang and Chiu96,Reference Tayama, Ogawa and Nakaya99,Reference Zanarini and Frankenburg104) . In total, twenty trials were carried out in populations with overweight or obesity (BMI ≥ 25 kg/m2)(Reference Andrieu, Guyonnet and Coley38,Reference Bot, Pouwer and Assies41–Reference Carney, Freedland and Rubin43,Reference Carney, Freedland and Stein45,Reference Cohen, Joffe and Guthrie48,Reference Giltay, Geleijnse and Kromhout57,Reference Ginty, Muldoon and Kuan58,Reference Jahangard, Sadeghi and Ahmadpanah65–Reference Kiecolt-Glaser, Belury and Andridge67,Reference Lee, Shahar and Chin69,Reference Lucas, Asselin and Mérette71,Reference Poppitt, Howe and Lithander84,Reference Sinn, Milte and Street94,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Tayama, Ogawa and Nakaya99,Reference van de Rest, Geleijnse and Kok101) , eight trials were conducted in participants with normal weight (18 < BMI < 25 kg/m2)(Reference Antypa, Smelt and Strengholt39,Reference Hallahan, Hibbeln and Davis61,Reference Hashimoto, Kato and Tanabe62,Reference Jackson, Deary and Reay64,Reference Park, Park and Kim81,Reference Robinson, Gallego and John89,Reference Rondanelli, Giacosa and Opizzi90,Reference Sohrabi, Kashanian and Ghafoori95) and the other twenty-seven trials did not report the weight status of the participants(Reference Bellino, Bozzatello and Rocca40,Reference da Silva, Munhoz and Alvarez49,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Dretsch, Johnston and Bradley53,Reference Gertsik, Poland and Bresee55,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Haberka, Mizia-Stec and Mizia60,Reference Kiecolt-Glaser, Belury and Andridge67,Reference Lespérance, Frasure-Smith and St-André70,Reference Marangell, Martinez and Zboyan73,Reference Masoumi, Kazemi and Tavakolian74,Reference Mazereeuw, Herrmann and Oh76–Reference Nemets, Stahl and Belmaker80,Reference Pawełczyk, Grancow-Grabka and Kotlicka-Antczak82,Reference Pomponi, Loria and Salvati83,Reference Ravi, Khalili and Abbasian87,Reference Rizzo, Corsetto and Montorfano88,Reference Shinto, Marracci and Mohr92,Reference Silvers, Woolley and Hamilton93,Reference Su, Huang and Chiu96,Reference Watanabe, Matsuoka and Kumachi102–Reference Chang, Chen and Su105) . The intervention duration was 12 weeks or shorter in thirty-one trials(Reference Antypa, Smelt and Strengholt39–Reference Carney, Freedland and Rubin44,Reference Cohen, Joffe and Guthrie48,Reference da Silva, Munhoz and Alvarez49,Reference Haberka, Mizia-Stec and Mizia60,Reference Hallahan, Hibbeln and Davis61,Reference Jackson, Deary and Reay64–Reference Kiecolt-Glaser, Belury and Andridge67,Reference Lespérance, Frasure-Smith and St-André70,Reference Lucas, Asselin and Mérette71,Reference Marangell, Martinez and Zboyan73,Reference Masoumi, Kazemi and Tavakolian74,Reference Mazereeuw, Herrmann and Oh76,Reference Nemets, Stahl and Belmaker80,Reference Park, Park and Kim81,Reference Poppitt, Howe and Lithander84,Reference Ravi, Khalili and Abbasian87,Reference Rizzo, Corsetto and Montorfano88,Reference Rondanelli, Giacosa and Opizzi90,Reference Silvers, Woolley and Hamilton93,Reference Sohrabi, Kashanian and Ghafoori95,Reference Su, Huang and Chiu96,Reference Tayama, Ogawa and Nakaya99,Reference Zanarini and Frankenburg104,Reference Chang, Chen and Su105) , between 12 and 24 weeks in nine trials(Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Ginty, Muldoon and Kuan58,Reference Pomponi, Loria and Salvati83,Reference Robinson, Gallego and John89,Reference Shinto, Marracci and Mohr92,Reference Sinn, Milte and Street94,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Yurko-Mauro, McCarthy and Rom103) , and longer than 24 weeks in thirteen trials(Reference Andrieu, Guyonnet and Coley38,Reference Carney, Freedland and Rubin43,Reference Dretsch, Johnston and Bradley53,Reference Gertsik, Poland and Bresee55,Reference Giltay, Geleijnse and Kromhout57,Reference Hashimoto, Kato and Tanabe62,Reference Lee, Shahar and Chin69,Reference McGorry, Nelson and Markulev77–Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Pawełczyk, Grancow-Grabka and Kotlicka-Antczak82,Reference van de Rest, Geleijnse and Kok101,Reference Watanabe, Matsuoka and Kumachi102) . Four clinical trials used DHA for supplementation(Reference Marangell, Martinez and Zboyan73,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Silvers, Woolley and Hamilton93,Reference Yurko-Mauro, McCarthy and Rom103) , four trials used EPA(Reference Bot, Pouwer and Assies41,Reference Carney, Freedland and Rubin44,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Nemets, Stahl and Belmaker80,Reference Zanarini and Frankenburg104) , thirty-seven trials used a combination of DHA and EPA(Reference Andrieu, Guyonnet and Coley38–Reference Bellino, Bozzatello and Rocca40,Reference Bradbury, Myers and Meyer42,Reference Carney, Freedland and Rubin43,Reference da Silva, Munhoz and Alvarez49,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Dretsch, Johnston and Bradley53,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Ginty, Muldoon and Kuan58,Reference Haberka, Mizia-Stec and Mizia60–Reference Hashimoto, Kato and Tanabe62,Reference Jiang, Whellan and Adams66,Reference Kiecolt-Glaser, Belury and Andridge67,Reference Lee, Shahar and Chin69–Reference Lucas, Asselin and Mérette71,Reference McGorry, Nelson and Markulev77–Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Park, Park and Kim81–Reference Poppitt, Howe and Lithander84,Reference Ravi, Khalili and Abbasian87–Reference Robinson, Gallego and John89,Reference Shinto, Marracci and Mohr92,Reference Sinn, Milte and Street94,Reference Su, Huang and Chiu96,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Tayama, Ogawa and Nakaya99,Reference van de Rest, Geleijnse and Kok101,Reference Watanabe, Matsuoka and Kumachi102,Reference Chang, Chen and Su105) and eight trials used EPA + DHA + ALA for supplementation(Reference Carney, Freedland and Stein45,Reference Cohen, Joffe and Guthrie48,Reference Gertsik, Poland and Bresee55,Reference Giltay, Geleijnse and Kromhout57,Reference Jahangard, Sadeghi and Ahmadpanah65,Reference Masoumi, Kazemi and Tavakolian74,Reference Mazereeuw, Herrmann and Oh76,Reference Sohrabi, Kashanian and Ghafoori95) .
Thirteen trials (fourteen effect sizes) reported information about the effects of n-3 supplementation on depression risk (online Supplementary Table 8). These trials were published between 2008 and 2019. Four trials implemented behavioural support(Reference Derosa, Cicero and D’Angelo52,Reference Kromhout, Giltay and Geleijnse68,Reference Maltais, de Souto Barreto and Pothier72,Reference Geleijnse, Giltay and Kromhout106) , while the other ten trials did not(Reference Chew, Clemons and Agrón47,Reference Dangour, Allen and Elbourne50,Reference Ferreira, Rosser and Craufurd54,59,63,Reference Mazaherioun, Saedisomeolia and Javanbakht75,Reference Pratt, Reiffel and Ellenbogen85,Reference Rauch, Schiele and Schneider86,Reference Sanyal, Abdelmalek and Suzuki91,Reference Torkildsen, Wergeland and Bakke100) . Nine trials had a low risk of bias(Reference Dangour, Allen and Elbourne50,Reference Derosa, Cicero and D’Angelo52,Reference Ferreira, Rosser and Craufurd54,59,Reference Kromhout, Giltay and Geleijnse68,Reference Mazaherioun, Saedisomeolia and Javanbakht75,Reference Rauch, Schiele and Schneider86,Reference Sanyal, Abdelmalek and Suzuki91,Reference Torkildsen, Wergeland and Bakke100) , and four trials were considered to have a high risk of bias(Reference Chew, Clemons and Agrón47,63,Reference Maltais, de Souto Barreto and Pothier72,Reference Pratt, Reiffel and Ellenbogen85) (online Supplementary Table 9). The primary studies used different scales to recognise participants with depression or at risk of depression. For instance, GDS(Reference Andrieu, Guyonnet and Coley38,Reference Giltay, Geleijnse and Kromhout57,Reference Lee, Shahar and Chin69,Reference Maltais, de Souto Barreto and Pothier72,Reference Rizzo, Corsetto and Montorfano88,Reference Rondanelli, Giacosa and Opizzi90,Reference Sinn, Milte and Street94,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference van de Rest, Geleijnse and Kok101,Reference Yurko-Mauro, McCarthy and Rom103) , BDI(Reference Antypa, Smelt and Strengholt39,Reference Carney, Freedland and Rubin43–Reference Chang, Chang and Yang46,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Gharekhani, Khatami and Dashti-Khavidaki56,Reference Ginty, Muldoon and Kuan58,Reference Haberka, Mizia-Stec and Mizia60,Reference Hallahan, Hibbeln and Davis61,Reference Jahangard, Sadeghi and Ahmadpanah65,Reference Jiang, Whellan and Adams66,Reference Mazaherioun, Saedisomeolia and Javanbakht75,Reference Mazereeuw, Herrmann and Oh76,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Ravi, Khalili and Abbasian87,Reference Silvers, Woolley and Hamilton93,Reference Tayama, Ogawa and Nakaya99) , Hamilton Depression Rating Scale (HAMD)(Reference Bellino, Bozzatello and Rocca40,Reference Carney, Freedland and Rubin43,Reference Carney, Freedland and Rubin44,Reference Chang, Chang and Yang46,Reference Jiang, Whellan and Adams66,Reference Marangell, Martinez and Zboyan73,Reference Mazereeuw, Herrmann and Oh76,Reference Pomponi, Loria and Salvati83,Reference Su, Lai and Yang97) , Montgomery–Asberg Depression Rating Scale (MADRS)(Reference da Silva, Munhoz and Alvarez49,Reference Jahangard, Sadeghi and Ahmadpanah65,Reference Lespérance, Frasure-Smith and St-André70,Reference Shinto, Marracci and Mohr92,Reference van de Rest, Geleijnse and Kok101,Reference Zanarini and Frankenburg104) , Clinical Global Impression (CGI)(Reference da Silva, Munhoz and Alvarez49), Hospital Anxiety and Depression Scale (HDRS)(Reference McPhilemy, Byrne and Waldron78–Reference Nemets, Stahl and Belmaker80,Reference Silvers, Woolley and Hamilton93) , Calgary Depression Scale (CDS)(Reference Pawełczyk, Grancow-Grabka and Kotlicka-Antczak82), Brief Psychiatric Rating Scale (BPRS)(Reference Robinson, Gallego and John89), Center for Epidemiologic Studies Depression Scale (CES-D)(Reference Kiecolt-Glaser, Belury and Andridge67,Reference Park, Park and Kim81,Reference Tayama, Ogawa and Nakaya99,Reference van de Rest, Geleijnse and Kok101) , The Depression, Anxiety and Stress Scale (DASS)(Reference Bradbury, Myers and Meyer42,Reference Jackson, Deary and Reay64) , Patient Health Questionnaire (PHQ)(Reference Cohen, Joffe and Guthrie48,Reference Dretsch, Johnston and Bradley53,Reference Ravi, Khalili and Abbasian87,Reference Watanabe, Matsuoka and Kumachi102) , The General Health Questionnaire(Reference Poppitt, Howe and Lithander84), The Diagnostic and Statistical Manual of Mental Disorders (DSM)(Reference Marangell, Martinez and Zboyan73,Reference Nemets, Stahl and Belmaker80,Reference Shinto, Marracci and Mohr92) , Zagazig Depression Scale (ZDS)(Reference Dretsch, Johnston and Bradley53), Self-Rating Depression Scale (SDS)(Reference Hashimoto, Kato and Tanabe62), and Young Mania Rating Scale (YMRS)(Reference McPhilemy, Byrne and Waldron78) in the form of continuous or dichotomised scales were commonly used to assess the outcomes.
The definition of depression remission also varied considerably across trials. For example, one trial defined depression remission as the GDS score less than 11(Reference Rondanelli, Giacosa and Opizzi90), two trials defined it as the BDI-II score ≤ 8(Reference Carney, Freedland and Rubin43,Reference Carney, Freedland and Rubin44) and the other four trials defined it as the Hamilton Rating Scale for Depression score ≤ 7(Reference Gertsik, Poland and Bresee55,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Pomponi, Loria and Salvati83,Reference Hallahan, Ryan and Hibbeln107) .
Primary outcomes
Fourteen trials with 16 412 participants in the intervention group and 16 343 in the control group reported data about the effect of n-3 fatty acids on the risk of depression(Reference Chew, Clemons and Agrón47,Reference Dangour, Allen and Elbourne50,Reference Derosa, Cicero and D’Angelo52,Reference Ferreira, Rosser and Craufurd54,59,63,Reference Kromhout, Giltay and Geleijnse68,Reference Maltais, de Souto Barreto and Pothier72,Reference Mazaherioun, Saedisomeolia and Javanbakht75,Reference Pratt, Reiffel and Ellenbogen85,Reference Rauch, Schiele and Schneider86,Reference Sanyal, Abdelmalek and Suzuki91,Reference Torkildsen, Wergeland and Bakke100,Reference Geleijnse, Giltay and Kromhout106) . Supplementation with n-3 fatty acid did not significantly reduce the risk of depression (OR: 0·95, 95 % CI 0·79, 1·15; GRADE = moderate) (Fig. 1, online Supplementary Fig. 2 and Table 1).
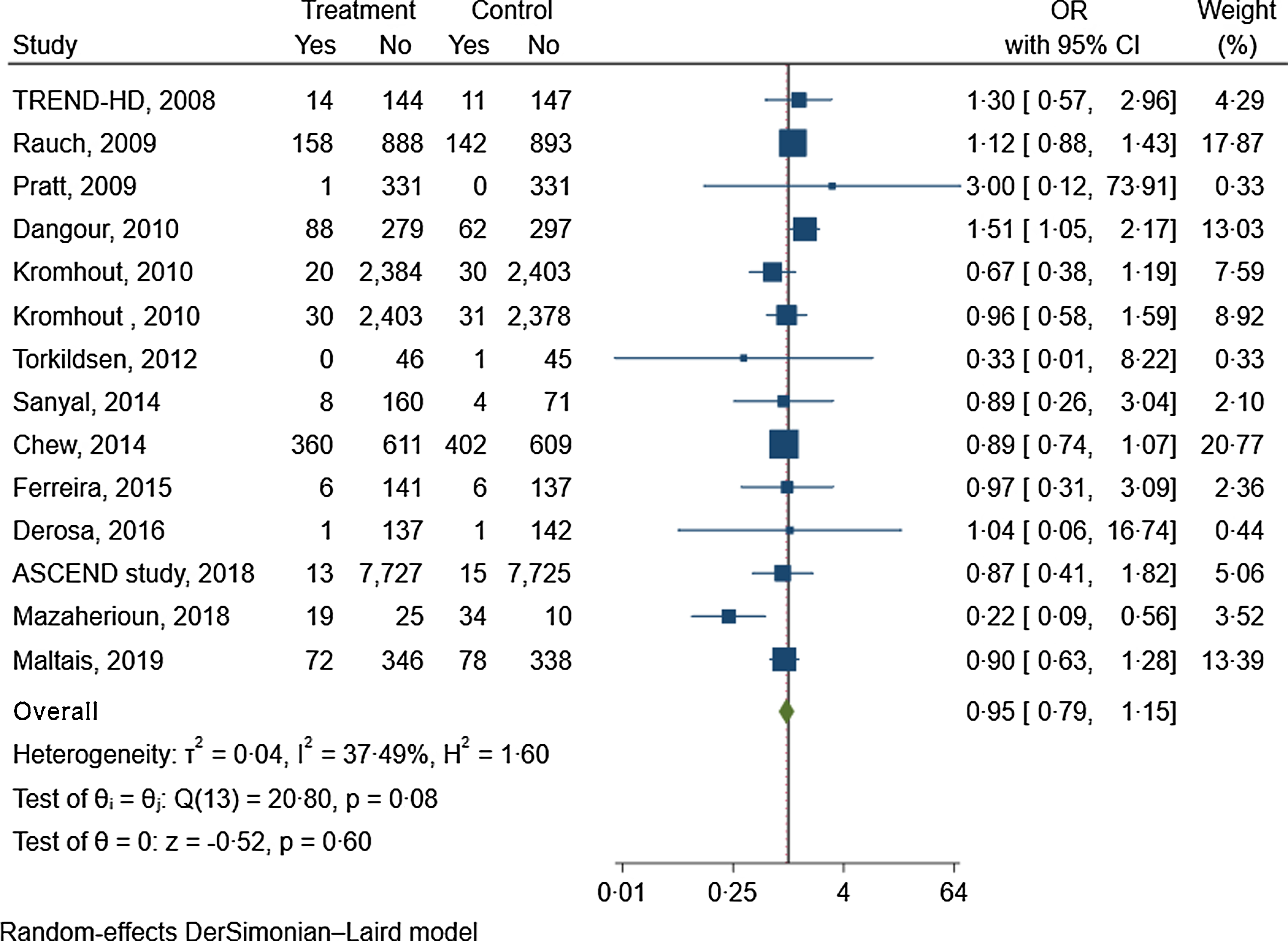
Fig. 1. The effects of n-3 fatty acids supplementation on depression risk.
Table 1. The effect of n-3 fatty acids supplementation on primary and secondary outcomes

Online Supplementary Table 10 shows the subgroup analyses of the effects of n-3 fatty acids supplementation on the risk of depression based on risk of bias, intervention duration, physical activity, behavioural support and degree of adherence to the intervention. The results remained non-significant in all subgroups (online Supplementary Table 10). The ICEMAN tool revealed no credible difference between the subgroups (online Supplementary Table 11)(Reference Schandelmaier, Briel and Varadhan27). Figure 2 showed the dose-dependent effects of n-3 fatty acids on the risk of depression. The analysis showed that the risk of depression did not change materially with the increase of the dosage of intervention (P nonlinearity = 0·71, P dose-response = 0·49; n 14, Table 2).
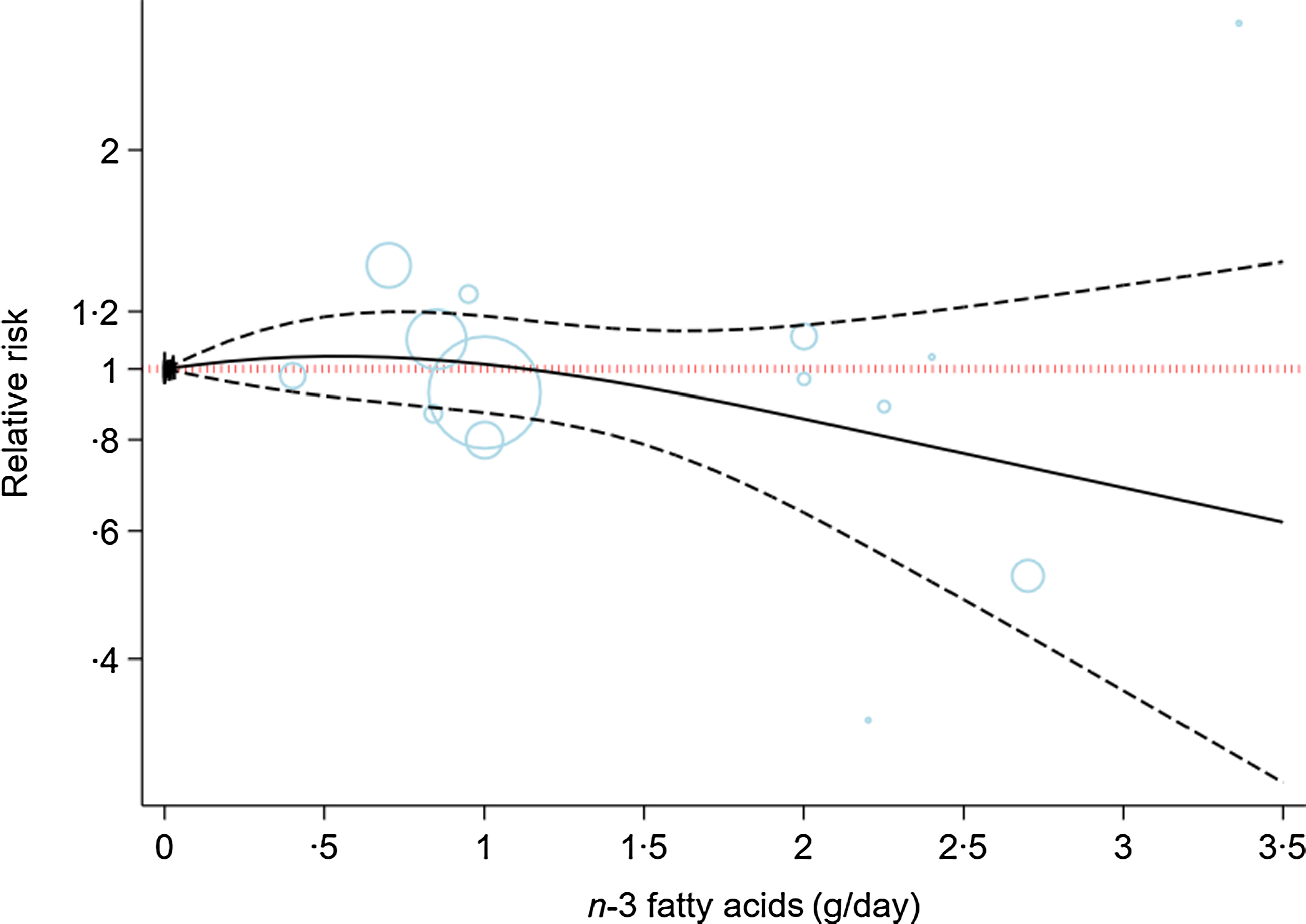
Fig. 2. Dose-dependent effect of n-3 fatty acids on risk of depression. Solid lines represent standardised mean difference and dashed lines represent 95 % CI.
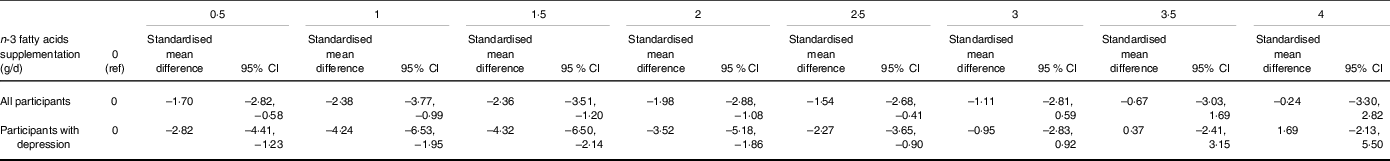
Table 2. The effects of n-3 fatty acids supplementation on severity of depression in the non-linear dose–response meta-analysis (standardised mean difference and 95 % CI)
Fifty-three trials with 5110 participants in the intervention group and 5057 in the control group reported data about the effect of n-3 fatty acids (each 1 g/d) on the severity of depression(Reference Andrieu, Guyonnet and Coley38–Reference Chang, Chang and Yang46,Reference Cohen, Joffe and Guthrie48,Reference da Silva, Munhoz and Alvarez49,Reference Dashti-Khavidaki, Gharekhani and Khatami51,Reference Dretsch, Johnston and Bradley53,Reference Gertsik, Poland and Bresee55–Reference Ginty, Muldoon and Kuan58,Reference Haberka, Mizia-Stec and Mizia60–Reference Hashimoto, Kato and Tanabe62,Reference Jackson, Deary and Reay64–Reference Jiang, Whellan and Adams66,Reference Lee, Shahar and Chin69–Reference Lucas, Asselin and Mérette71,Reference Marangell, Martinez and Zboyan73,Reference Masoumi, Kazemi and Tavakolian74,Reference Mazereeuw, Herrmann and Oh76–Reference Poppitt, Howe and Lithander84,Reference Ravi, Khalili and Abbasian87–Reference Rondanelli, Giacosa and Opizzi90,Reference Shinto, Marracci and Mohr92–Reference Su, Huang and Chiu96,Reference Tajalizadekhoob, Sharifi and Fakhrzadeh98,Reference Tayama, Ogawa and Nakaya99,Reference van de Rest, Geleijnse and Kok101–Reference Zanarini and Frankenburg104) . Each 1 g/d n-3 fatty acid supplementation resulted in a large improvement in the severity of depression (SMD: −1·38, 95 % CI −1·69, −1·07; I2 = 97 %, GRADE = moderate) (online Supplementary Fig. 3 and Table 1).
Online Supplementary Table 12 shows the subgroup analyses of the effects of n-3 fatty acids (each 1 g/d) supplementation on the severity of depression. Of note, supplementation with n-3 fatty acids resulted in a larger reduction in depressive symptoms among those with existing depression (SMD: -3·03, 95 % CI –4·27, –1·79; n 25 trials with 1830 participants). There was no significant subgroup difference based on risk of bias, length of intervention, baseline depression risk and type of supplement (EPA v. DHA v. combined). There was no credible differences across subgroups (online Supplementary Table 13)(Reference Schandelmaier, Briel and Varadhan27). The funnel plot and Egger’s test (P = 0·01) and Begg’s test (P = 0·001) showed some evidence of publication bias (online Supplementary Fig. 4).
Figure 3 indicates the dose-dependent effects of n-3 fatty acids on the severity of depression. The analysis showed that supplementation of n-3 fatty acids up to 2 g/d resulted in a large reduction in the severity of depression (SMD2 g/d: −1·98; 95 % CI −2·88, −1·08), followed by a trivial decrease in the severity of depression at higher doses (P dose-response < 0·001, P nonlinearity = 0·021; n 53, Table 2).
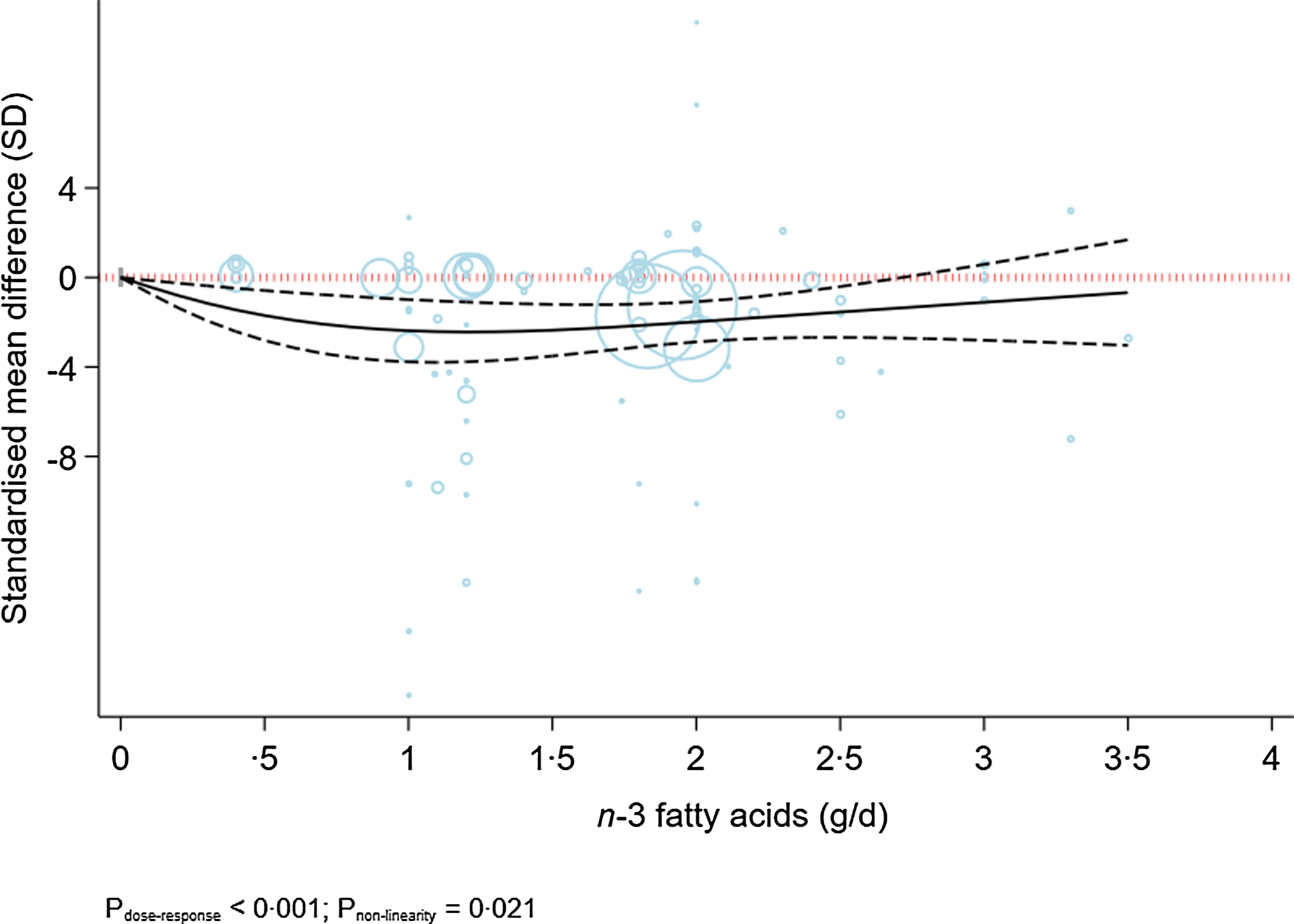
Fig. 3. Dose-dependent effect of n-3 fatty acids on severity of depression. Solid lines represent standardised mean difference and dashed lines represent 95 % CI.
Figure 4 indicates a sensitivity analysis of the dose-dependent effects of n-3 fatty acids on the severity of depression in patients with existing depression. The analysis indicated a modest U-shaped effect, with the highest decline in the severity of depression at a dose of 1·5 g/d (MD1·5 g/d: −4·32; 95 % CI −6·50, −2·14) (P dose-response < 0·001, P nonlinearity = 0·002; n 33, Table 2). A sensitivity analysis of participants without depression indicated a linear reduction in depressive symptoms along with the increase in dose of intervention ((P dose-response < 0·001, P nonlinearity = 0·08; n 20, Fig. 5).
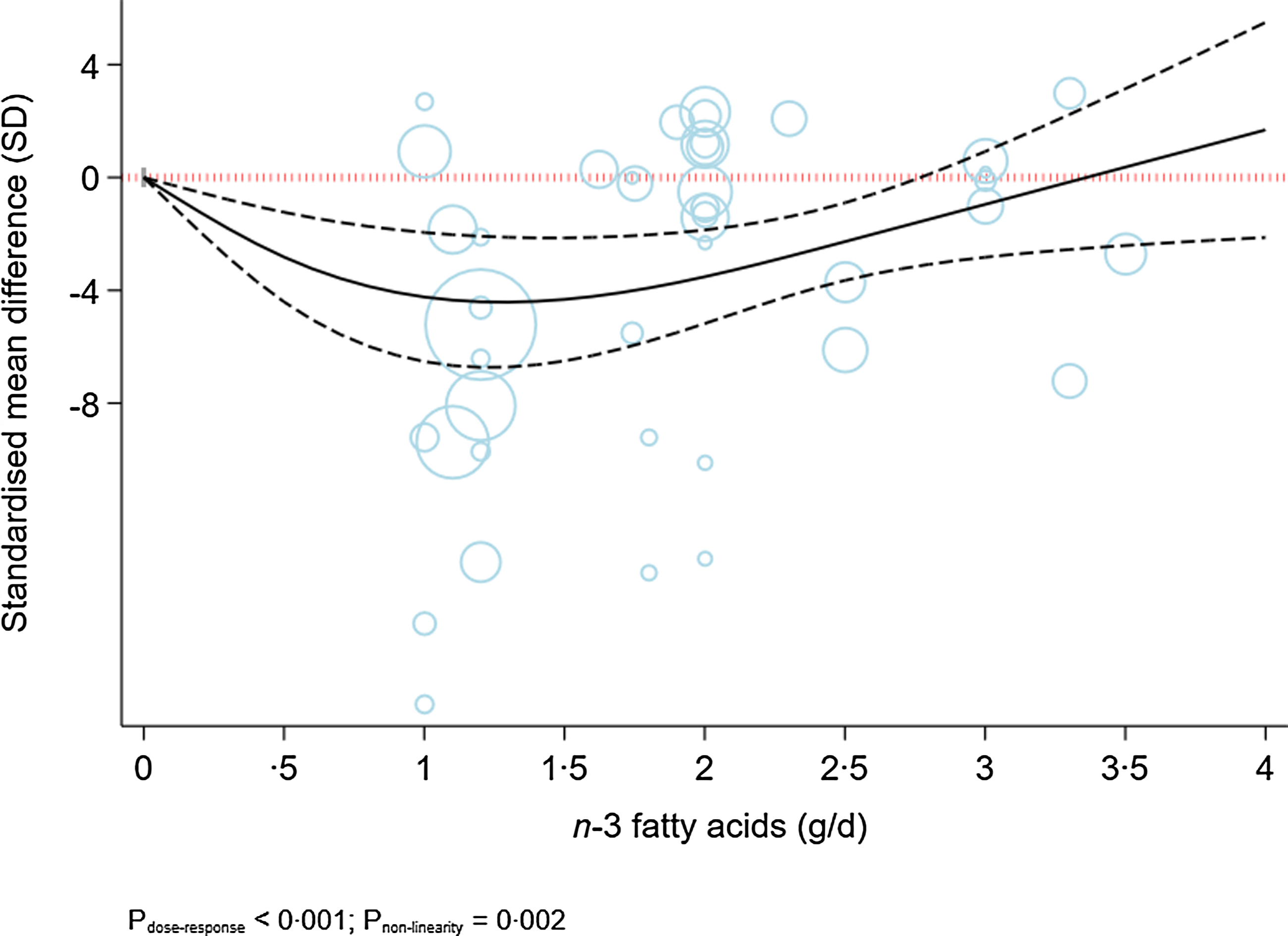
Fig. 4. Dose-dependent effect of n-3 fatty acids on severity of depression in depressed individuals. Solid lines represent standardised mean difference and dashed lines represent 95 % CI.
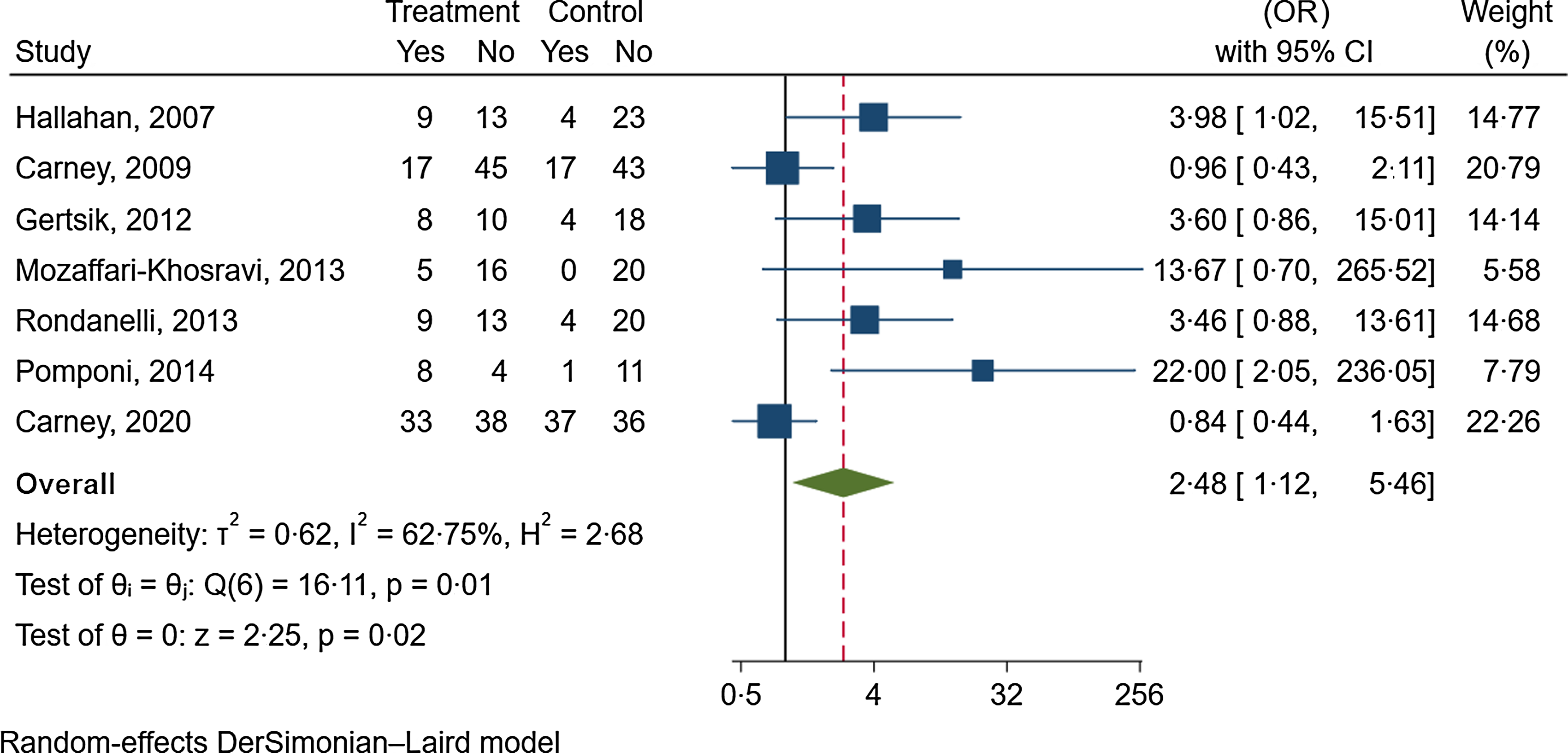
Fig. 5. The effects of n-3 fatty acids supplementation on depression remission.
Seven trials with 113 participants in the intervention group and 128 participants in the control group reported data about the effect of n-3 fatty acids on depression remission(Reference Carney, Freedland and Rubin43,Reference Carney, Freedland and Rubin44,Reference Gertsik, Poland and Bresee55,Reference Hallahan, Hibbeln and Davis61,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Pomponi, Loria and Salvati83,Reference Rondanelli, Giacosa and Opizzi90) . The follow-up duration was between 8 to 52 weeks (median follow-up duration: 12 weeks). n-3 fatty acid supplementation significantly increased the odds of depression remission by 148 % (OR: 2·48, 95 % CI 1·12, 5·46; I2 = 63 %, GRADE = low) (online Supplementary Fig. 5 and Table 1).
Secondary outcomes
The effects of n-3 fatty acids on secondary outcomes are shown in online Supplementary Fig. 6–22 and Table 1. Supplementation with n-3 fatty acids did not increase adverse events but improved overall quality of life and some aspects of quality of life such as role emotion and vitality (Table 1).
Grading of the evidence
The certainty of evidence was rated moderate for the effects of supplementation with n-3 fatty acids on the risk of depression and severity of depression. The certainty of evidence was rated very low to low for other outcomes (online Supplementary Table 14).
Discussion
Herein, we investigated the RCT of the effect of n-3 fatty acids supplementation on the risk of depression among the general population, as well as the effects of n-3 supplementation on depression symptoms. The analyses indicated that supplementation with n-3 fatty acids did not reduce the risk of developing depression among those without depression but resulted in a large improvement in depressive symptoms and increased remission rate among patients with existing depression.
Comparison with previous reviews
Evidence regarding the effects of n-3 fatty acids supplementation on depressive symptoms is conflicting. A meta-analysis of twenty-eight trials suggested that supplementation with n-3 fatty acids improved depressive symptoms in adults(Reference Martins108). A meta-analysis of twenty-six trials with 2160 participants indicated that supplementation with n-3 fatty acids did not significantly affect depressive symptoms in adults; however, they found some evidence of a significant improvement in trials that implemented EPA supplementation(Reference Liao, Xie and Zhang14). Another meta-analysis of thirty-one randomised trials with 41 470 adults with or without depression indicated that supplementation with n-3 fatty acids did not reduce the risk of depression severity when assessed as a binary outcome(Reference Deane, Jimoh and Biswas15). In comparison with previous reviews, we included a larger number of trials and evaluated the dose-dependent effects.
Subgroup analyses
In the subgroup analyses of depression severity, there was a significant subgroup difference by health status, where trials that were conducted among patients with existing depression indicated a larger improvement than those conducted in other populations, especially healthy populations. Previous reviews demonstrated that supplementation with EPA had stronger effects on improving depression symptoms than DHA(Reference Liao, Xie and Zhang14,Reference Hallahan, Ryan and Hibbeln107,Reference Mocking, Harmsen and Assies109) . Although our subgroup analysis by type of intervention indicated a larger effects in trials that implemented EPA supplementation, there was no significant difference across subgroups by type of intervention (EPA v. DHA v. combined). In any case, because comparisons between EPA + DHA v. pure EPA or DHA in clinical investigations are limited, comparative effects of EPA and DHA in depression therapy needs to be more investigated in future research.
Risk of depression
Although our findings indicated that supplementation with n-3 can significantly improve depressive symptoms, we could not find any significant relation between supplementation with n-3 fatty acids and the risk of depression among the general population. Similarly, a recent systematic review and meta-analysis of thirteen trials suggested that increasing n-3 fatty acids intake probably has little or no effect on the risk of developing depression among those without depression at baseline(Reference Deane, Jimoh and Biswas15). Of thirteen trials that were included in the previous meta-analysis, most of the meta-analysis weight (over 90 %) came from three trials that assessed depression symptoms dichotomously(Reference Chew, Clemons and Agrón47,Reference Dangour, Allen and Elbourne50,Reference Rauch, Schiele and Schneider86) . The other ten trials reported depression events based on a 15-score GDS, mainly in the form of an adverse event. However, unlike the clinical trials, a pooled analysis of thirty-one observational studies in 255 076 participants with 20 000 cases of depression indicated that highest v. lowest category of fish intake was associated with a 22 % lower risk of depression among the general population(Reference Grosso, Micek and Marventano110). With regard to no effects in healthy population, we think that although supplementation with n-3 fatty acids did not reduce the risk of developing depression, this finding does not imply on no effects of n-3 fatty acids on depressive symptoms. Indeed, meta-analyses of prospective cohort studies indicated that higher intake of n-3 fatty acids and fish, their main dietary sources, was associated with a lower risk of depression(Reference Grosso, Micek and Marventano110,Reference Yang, Kim and Je111) . Null effect of supplementation with n-3 fatty acids may be due the fact that individuals in the included trials did not have n-3 fatty acids deficiency or had sufficient serum n-3 fatty acids concentrations. Due to inadequate information, we could performed subgroup analyses by baseline n-3 fatty acids intake or their serum concentrations.
Depression remission
Besides improvement in depressive symptoms, our findings indicated that supplementation with n-3 could result in depression remission among patients with existing depression. A recent meta-analysis of trials with an intervention duration longer than 6 months did not find an evidence of the effect of supplementation with n-3 on depression remission(Reference Deane, Jimoh and Biswas15). However, they included only one trial for depression remission. It is suggested that the assessment of depression remission needs participants with depression at baseline and at least 6 months of intervention with n-3 fatty acids to equilibrate fatty acids throughout our bodies(Reference Browning, Walker and Mander112). However, the average intervention duration of the trials in the present meta-analysis was 12 weeks, the certainty of evidence was rated low, and the definition on depression remission varied considerably across trials. One trial defined depression remission as the GDS score less than 11(Reference Rondanelli, Giacosa and Opizzi90), two trials defined it as the BDI-II score ≤ 8(Reference Carney, Freedland and Rubin43,Reference Carney, Freedland and Rubin44) , and the other four trials defined it as the Hamilton Rating Scale for Depression score ≤ 7(Reference Gertsik, Poland and Bresee55,Reference Mozaffari-Khosravi, Yassini-Ardakani and Karamati79,Reference Pomponi, Loria and Salvati83,Reference Hallahan, Ryan and Hibbeln107) . Therefore, our findings on depression remission are not conclusive and need to be examined in more long-term RCT.
Dose–response analyses
In the present dose–response meta-analysis, we observed that supplementation with n-3 fatty acids could significantly decrease the severity of depression, with the greatest reduction at a dose of 1 g/d (MD1 g/d: −2·38) in the main analysis. A sensitivity analysis among patients with existing depression showed a modest U-shaped effect, with the highest decline in the severity of depression at a dose of 1·5 g/d of n-3 fatty acid (MD1·5 g/d: −4·32). Taken together, our findings suggest that the beneficial effect of n-3 fatty acids supplementation was more evident between the doses of 1 and 1·5 g/d and, as a result, higher doses could not confer additional benefits.
In case of safety of high-dose n-3 fatty acids in short terms, it has been reported that doses up to 4 g of n-3 PUFA daily are not associated with an increased risk of major bleeding(Reference Mori113). Moreover, it has been illustrated that even when patients receive antiplatelet and anticoagulants, there is no risk of excessive bleeding from n-3 fatty acids(Reference Guu, Mischoulon and Sarris114). Of course, there are minor side effects such as fishy smell, hiccups and nausea, rather than any serious ones(Reference Chang, Tseng and Chen115). Higher dosages of supplementation with n-3 fatty acids (3–6 g/d) in several trials did not result in any serious adverse effect (Reference Peoples, McLennan and Howe116–Reference Buckley, Shewring and Turner121).
In case of safety of high-dose n-3 fatty acids in long terms, a RCT with 52 weeks of intervention with 4 g/d n-3 fatty acids revealed that this dosage was safe and tolerated by hepatitis C virus patients(Reference Su, Lai and Yang97). Evidence from a RCT on patients at high cardiovascular risk, after 5 years of intervention with 4 g/d n-3 fatty acids v. corn oil, the adverse events were more commonly observed in the n-3 fatty acids group than the comparator group(Reference Nicholls, Lincoff and Garcia122). However, to make conclusion with certainty, more studies with long-term follow-ups are needed.
To precisely designate the effect of n-3 fatty acids supplementation, we need to access data that present the effects of n-3 fatty acids supplementation solely and yet several trials in our meta-analysis used antidepressant alongside the n-3 fatty acids supplements. In comparison with n-3 monotherapy, taking n-3 supplements with antidepressants may be more effective(Reference Mocking, Harmsen and Assies109), suggesting that combined supplementation with n-3 fatty acids and antidepressant medications may potentiate the efficacy of drugs. PUFA have the ability to modulate neuronal membrane–antidepressant interactions and inflammatory pathways(Reference Rapaport, Nierenberg and Schettler123). On the other hand, n-3 fatty acids have the potential to disrupt the functioning of serotonin neurotransmitters(Reference Kodas, Galineau and Bodard124). Deeper analysis into the interaction between n-3 PUFA and antidepressants is needed. We performed subgroup analysis of the effects of n-3 fatty acids supplementation on the severity of depression on medication use (online Supplementary Table 12) The subgroup analysis showed that the severity of depression did not change significantly across different categories of medication use (medication use, no medication use, mixed and not reported) and results were significant in all subgroups. What is to be done? If studies involve both groups of participants – those who use antidepressant medications and those who do not –, they should include a large sample data so that analysis can be conducted separately. It should be clear what type of antidepressants participants used.
Placebo effect
In general, in trials evaluating the effects of a specific intervention on depressive symptoms, in comparison with a placebo or sham intervention, the effects of placebo on depressive symptoms should be considered(Reference Fountoulakis, McIntyre and Carvalho125). The placebo effect is defined as the therapeutic effect caused by a placebo that is not due to any inherent properties of the placebo. This phenomenon is a challenge for researchers and may result in an overestimated effect estimate when evaluating the effects of a specific intervention, in comparison with the placebo, on depressive symptoms. Previous meta-analyses of randomised trials on depression suggested that the magnitude of the effect due to the placebo was about 35–40 %, suggesting a large effect estimate(Reference Jones, Razza and Weissman126,Reference Furukawa, Cipriani and Atkinson127) . Therefore, the magnitude of the effect estimates found in the present meta-analyses may have been overestimates and, thus, should be interpreted with caution.
When comparing intervention and placebo group, making sure that blinding is correctly taking place is a must. Are participants and research crew actually blind? In a study by Rabkin et al., depressed patients who were given imipramine, phenelzine or a placebo were asked to identify which group they had been placed in. Most patients and doctors were able to tell whether the patients had received an active medication or a placebo(Reference Rabkin, Markowitz and Stewart128). It is a fact that patients are being told that there are possible side effect at the beginning of the study, and they (and by extension research crew) could identify the assigned group. Patients respond better to medications when they are aware they are receiving them than when they suspect they could be receiving a placebo(Reference Papakostas and Fava129). Moreover, when patients are aware that they could be receiving a placebo, the placebo reaction is less pronounced than when they are made to believe they are receiving the real medication(Reference Rutherford, Wall and Brown130). This could be one possible reason for the marginally different results between the medication and placebo.
Mechanisms
One possible mechanism for n-3 fatty acids is its vital role in fluidity and preserving the function of cell membrane(Reference Bourre, Dumont and Piciotti131) through displacing cholesterol from the membrane(Reference Yehuda, Rabinovitz and Mostofsky132), which is crucial for neurotransmitter binding and the signalling within the cell(Reference Heron, Shinitzky and Hershkowitz133).
Another role for n-3 fatty acids is in the production of pro-inflammatory immune chemicals such as IL-1β, −2 and −6, interferon-γ (IFN-γ) and TNF-α. Such cytokines can lower neurotransmitter precursor availability, activate the hypothalamic–pituitary axis, and alter the metabolism of neurotransmitters and neurotransmitter mRNA(Reference Maes and Smith134). Overexpression of monocyte-associated pro-inflammatory cytokines and chemokines has been seen in depressed patients(Reference Suarez, Krishnan and Lewis135). An elevation in such inflammatory cytokines by psychological stress could be inhibited by n-3 fatty acids as antidepressant(Reference Maes and Smith134). Moreover, n-3 fatty acids could be beneficial in reducing the severity of depression by modulating brain-derived neurotrophic factor, which supports the survival and growth of neurons through development and adulthood(Reference Shimizu, Hashimoto and Okamura136).
Is there a sole solution? To fairly respond to this question, we should be asking, is there a sole cause for depression. There are two main category relating depression: biology and psychology, that each one contains multiple domains (e.g. neuroticism, cognitive fusion, emotional clarity, rumination, and so on). Different combinations of reasons are linked to various types of depression, requiring different types of intervention for patients. It is important for clinicians to go through the diagnosing process cautiously and prescribe psychosocial therapies, when needed, alongside the biochemical ones.
Strengths and limitations
Our systematic review and meta-analysis was the first study to evaluate the dose-dependent effects of n-3 supplementation on the severity of depression and depression risk. Our broad search included participants at different baselines of depression severity. In comparison with previous reviews, we included a high number of RCT with considerable participants regardless of their health status, estimated both relative and absolute effects for binary outcomes, used the GRADE approach for evaluating the certainty of the evidence, and used the minimal clinically important difference (MCID) thresholds (0·0–≥ 4·0) for evaluating whether the results were clinically important.
As for limitations of our study, the geographical and ethnic variables affecting depression were not examined in the present meta-analysis. The variety of methods that were used for the assessment of depression symptoms and also high levels of heterogeneity, which persisted even after subgroup analysis, may also limit clinical interpretation. The large heterogeneity in the data may be due to the variation in participant’s characteristics, intervention duration and type of outcome assessment. The number of trials that used EPA or DHA supplementation as monotherapy was limited (four trials for each), which made it difficult to interpret the efficacy of EPA and DHA singularly. In addition, for depression remission, we had limited data obtained from short-term trials, and thus, our findings about the effects of n-3 fatty acids on depression remission should be interpreted with caution.
Conclusions
Based on moderate-certainty evidence, our study showed that supplementation with n-3 fatty acids could lead to a large and clinically important improvement in the severity of depression among patients with existing depression. The greatest reduction was seen at 1–1·5 g/d, with no additional benefits for higher dosages of n-3 fatty acids supplementation. Supplementation with n-3 fatty acids had no effects on the risk of developing depression among participants without baseline depression. Based on low-certainty evidence obtained from short-term trials, supplementation with n-3 fatty acids may increase remission rate in patients with existing depression, finding that needed to be confirmed in future research.
Acknowledgement
We would like to acknowledge all participants who made this research possible. This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
S. S-B. designed the research; R. N. and S. Z-M. conducted research; A. J. analysed data; S. Z-M. and A. J. revised the tables and images; R. N. and S. Z-M. wrote the first draft of the manuscript; S. S-B. and A. J. provided important revisions for the final content. All authors reviewed and approved the study content.
There are no conflicts of interest.
The data sets generated or analysed during the current study are not publicly available but are available from the corresponding author at reasonable request.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114523002052










