Most cancers result from a complex interaction of environmental factors, genetic susceptibility and lifestyle factors(Reference Guilbert1). Diet is an important component of lifestyle, and its role in the maintenance of good health and as a determinant of different types of chronic diseases, including cancer, has been extensively studied(Reference Guilbert1). Diets that emphasise the consumption of whole-grain foods, legumes, vegetables and fresh fruits and that limit animal fat have been associated with decreased cancer risk(Reference Guilbert1–Reference Key, Allen, Spencer and Travis4). Dietary patterns with high intake of fibres have also emerged as possible important preventive factors for cancer(Reference Guilbert1–Reference Key, Allen, Spencer and Travis4). On the other hand, diet can contribute to cancer risk through the consumption of food mutagens(Reference Jakszyn, Agudo, Ibanez, Garcia-Closas, Pera, Amiano and Gonzalez5), contained in contaminated foods or generated by frying, toasting and broiling of certain foods(Reference Bostrom, Gerde, Hanberg, Jernström, Johansson, Kyrklund, Rannug, Törnqvist, Victorin and Westerholm R6). In contrast to some extensively examined food mutagens, for example, aflatoxins, N-nitrosamines and heterocyclic amines, some other food contaminants, in particular polycyclic aromatic hydrocarbons (PAH) and other aromatic compounds, have received less attention. PAH may occur in fried and charcoal-grilled meat or in the food chain as a result of environmental pollution(Reference Jakszyn, Agudo, Ibanez, Garcia-Closas, Pera, Amiano and Gonzalez5–Reference Phillips7). PAH are an important class of carcinogens, capable of inducing the formation of DNA adducts leading to DNA damage after metabolic activation(8). Targets for PAH carcinogenicity are several organs, including lung and bladder(8). Some evidence has been also reported for an association between dietary PAH and colon cancer or adenomas(Reference Giovannucci, Rimm, Stampfer, Colditz, Ascherio and Willett9, Reference Probst-Hensch, Sinha, Longnecker, Witte, Ingles, Frankl, Lee and Haile10). In addition, increased levels of bulky DNA adducts have been detected in the colon mucosa of colon cancer patients and in early stages of colon carcinogenesis(Reference Alexandrov, Rojas, Kadlubar, Lang and Bartsch11, Reference Pfohl-Leszkowicz, Grosse, Carriere, Cugnenc, Berger, Carnot, Beaune and de Waziers12).
4-Aminobiphenyl (4-ABP) is a human bladder carcinogen formed during tobacco combustion, and detected both in main- and side-stream smoke(13, 14). Although tobacco smoke is the main source of 4-ABP human exposure, additional sources are known that may contribute to the total burden of human exposure, including diesel exhaust and heated cooking oils(Reference Ning and Xiaobai15–Reference Turesky, Freeman, Holland, Nestorick, Miller, Ratnasinghe and Kadlubar17). Upon metabolic activation, 4-ABP reactive species bind covalently to DNA to form adducts, that, if not repaired, may start the process of carcinogenesis. 4-ABP reactive species in the body also bind to other macromolecules, including Hb(Reference Hecht18).
Exploring the relationships between dietary habits and the levels of biomarkers related to exposure to aromatic compounds is highly relevant. Food can be the source of mutagenic aromatic compounds, but it can also exert a protective effect over the genotoxic potential of certain compounds. In particular, an inverse association between fruit and/or vegetable intake and carcinogen-DNA adducts has been recently reported, including observations in the European Prospective Investigation into Cancer and Nutrition (EPIC) study (limited to the Italian branch)(Reference Peluso, Airoldi, Magagnotti, Fiorini, Munnia, Hautefeuille, Malaveille and Vineis19–Reference Palli, Masala and Vineis21).
In the present study, we have investigated in the EPIC cohort the association between dietary items and bulky DNA adducts and 4-ABP-Hb adducts measured, respectively, in leucocytes and erythrocytes of non-smokers.
Subjects and methods
Selection of subjects and collection of specimens
EPIC is a multicentre European study, coordinated by the International Agency for Research on Cancer, Lyon, in which more than 500 000 healthy volunteers were recruited in ten European countries (France, Denmark, Germany, Greece, Italy, The Netherlands, Norway, Spain, Sweden, UK) corresponding to twenty-three recruitment centres(Reference Bingham and Riboli22). The cohort includes subjects of both sexes, mostly in the age range 35–74 years at recruitment. Recruitment took place between 1993 and 1998. Detailed dietary and lifestyle histories collected mainly through self-administered questionnaires, plus a 24 h dietary recall through individual-to individual interview (in a 10 % sample), anthropometric measurements and a 30–40 ml blood sample are available. All questionnaire information is available in a computerised format. Signed informed consent forms were collected from all participants (except a sub-group of the Oxford cohort who gave consent on postal questionnaires).
GenAir is a case-control study nested within the EPIC cohort, aiming at studying the relationship between select cancer types and air pollution or environmental tobacco smoke. Cases are subjects with bladder, lung, oral, pharyngeal, or laryngeal cancers or leukaemia, all newly diagnosed after recruitment. Only non-smokers or ex-smokers since at least 10 years were eligible for the GenAir sub-study. Three controls per case were matched for exposure assessment and the analysis of questionnaire data, and two controls per case for laboratory analyses. Matching criteria were sex, age ( ± 5 years), smoking status (never or former smoker), country of recruitment, and follow-up time(Reference Peluso, Munnia and Hoek23).
GenAir has been approved by the Ethical Committee of the International Agency for Research on Cancer, and by the local Ethical Committees of the twenty-three centres.
The present study focuses on dietary determinants of DNA or Hb adducts in a healthy population, therefore the analysis is limited to the control population of GenAir.
Overall, 2977 controls met the protocol criteria. Of these subjects, 1564 had blood samples. Blood samples of controls for centres which have released an ethical approval have been sent to laboratories for investigation. The Malmö centre has decided not to allow the use of its blood samples, while Umeå participated only with erythrocytes. Therefore, DNA samples were available and successfully analysed among 1086 control subjects. Levels of 4-ABP-Hb adducts were analysed in 190 Hb samples.
Dietary variables
Dietary information on the frequency of consumption of more than 120 foods and drinks has been obtained by dietary questionnaires developed and validated in a pilot phase in each participating country. At enrolment, weight, height, and waist and hip circumferences have been measured for each participant. Detailed information has been collected on reproductive history, physical activity, smoking and alcohol drinking history, medical history, occupation, education level and other socio-economic variables.
Here we consider dietary variables that can be associated with levels of DNA adducts as suggested by previous investigations: intake of fruit, legumes, vegetables, alcohol, total energy, fibres, vitamins C and E, β-carotene (as estimated from food intake) and (only in four centres) folic acid (nine dietary items were analysed plus alcohol and BMI). Also BMI was considered as a potential predictor of DNA and Hb adducts. For details on the methods, see Peluso et al. and Airoldi et al. (Reference Peluso, Munnia and Hoek23, Reference Airoldi, Vineis and Colombi24). Also exposure levels for selected air pollutants (NO2, PM10 (particulate matter of 10 μm or less), SO2, ozone) have been estimated in GenAir; details on the estimation methods and results for lung cancer are given elsewhere(Reference Peluso, Munnia and Hoek23, Reference Vineis, Hoek and Krzyzanowski25).
[32P]DNA post-labelling technique
DNA was extracted and purified from the buffy coat using a method requiring enzymic digestion of RNA and proteins followed by phenol–chloroform extraction, with the exception of the Danish samples that were extracted and purified from lymphocytes using a technique based on a salting-out procedure(Reference Peluso, Munnia and Hoek23). Coded DNA was stored at − 80°C until laboratory analysis. Bulky DNA adducts were analysed blindly using the nuclease P1 modification of the [32P]post-labelling assay(Reference Peluso, Munnia and Hoek23) in the laboratory directed by M. P. Details of the technique have been previously reported(Reference Peluso, Munnia and Hoek23).
4-Aminobiphenyl-Hb extraction and quantitative analysis
Packed erythrocytes were selected at the International Agency for Research on Cancer and sent frozen to the Mario Negri Institute where they were stored at − 80°C until analysis. 4-ABP-Hb adducts were determined by parent arylamine after alkaline hydrolysis of the adducted Hb, and quantified by high-resolution GC-negative ion chemical ionisation-MS with selective ion monitoring as its pentafluoroacyl derivative, using the stable-isotope dilution technique. Details of the methodology used have been previously described(Reference Airoldi, Vineis and Colombi24).
Statistical analysis
We have computed correlation coefficients, and regression coefficients in multivariate regression models. The models included age, sex, country, smoking (never/ex-smoker), educational level (in six categories) and BMI (continuous). In addition the intake of fibres, fruit, legumes, vegetables, and energy, and estimates of the intake of vitamins C and E, and β-carotene were considered (all continuous variables). We also considered estimated intake of folic acid (available in four centres), and four air pollutants. Descriptive statistics and frequency histograms indicated that adduct levels were not normally distributed, because of a large number of adduct values that were below the detection limit of the method. For levels of DNA adducts below the instrumental detection limit of 0·1 we have imputed the value of 0·1. Hb samples with undetectable 4-ABP adduct levels ( ≤ 20 pg/g Hb) were considered as having half the detection limit of the method (i.e. 10 pg/g Hb). Since only nine selected dietary items (based on previous hypotheses) plus alcohol and BMI have been selected we did not apply Bonferroni correction for multiple comparisons.
We used adducts as a continuous variable with and without log-transformation.
Results
Table 1 shows the mean levels and standard deviations of bulky DNA adducts and 4-ABP-Hb adducts, by relevant demographic variables. As it has already been stressed(Reference Peluso, Munnia and Hoek23), one limitation of the adduct technology is the large inter-individual variability (large standard deviations). Table 2 shows the mean levels of DNA and Hb adducts below and above the median levels of relevant dietary or anthropometric variables. For Hb adducts there were no appreciable differences for any dietary variable except for fibres, fruit and BMI. The distribution was skewed for both types of adducts, but particularly for Hb adducts, and the analyses were repeated after log-transformation, with only slight changes. Both types of adducts, but particularly Hb adducts, show lower mean levels for higher fibre intakes. Table 3 shows correlation coefficients between adduct levels and selected dietary variables. Statistically significant associations are evident for DNA adducts and the intake of fibres (correlation coefficient − 0·067; P = 0·02) and for Hb adducts and the intake of fibres (correlation coefficient − 0·15; P = 0·03) and fruit (correlation coefficient − 0·15; P = 0·04). No association was found between either adduct type and folate intake.
Table 1 DNA and 4-aminobiphenyl (4-ABP)-Hb adducts, by demographic variables and smoking
(Mean values and standard deviations)
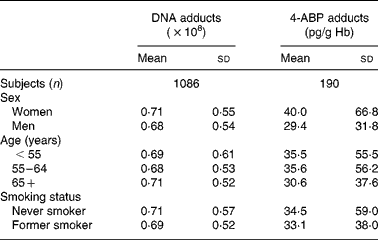
Table 2 Mean levels of bulky DNA adducts or 4-aminobiphenyl (4-ABP)-Hb adducts below and above the median level of fibre intake and other selected dietary variables and BMI (one or two missing values depending on item)
(Mean values with their standard errors)

* Based on t test.
Table 3 Correlation coefficients between selected dietary variables and adduct levels (Pearson correlation coefficients and P values)

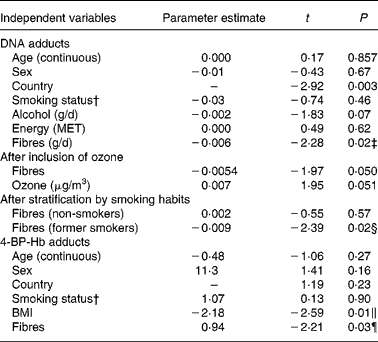
Table 4 shows multivariate regression models including age, sex, smoking status and country. Based on results shown in Table 3, alcohol and energy were added to the DNA adducts model, and BMI to the 4-ABP-Hb adducts model. A statistically significant association is shown between fibres and DNA adducts. The association with fibre intake became slightly weaker when ozone, an air pollutant, was included into the model. Other air pollutants (PM10, SO2, NO2) had no clear effect on adduct levels; details are given elsewhere(Reference Vineis, Hoek and Krzyzanowski25). Also, the association with fibres was apparently present only in former smokers. An association between fibre intake and 4-ABP-Hb adducts is suggestive but falls short of significance. An association between BMI and Hb adducts is also suggested but disappeared after log-transformation of the adduct variable (Table 4).
Table 4 Multivariate regression models*
MET, metabolic equivalent values; ABP, aminobiphenyl.
* The dependent variable is DNA adducts or 4-ABP-Hb adducts. All independent variables except sex, country and smoking (never or ex-smoker) are continuous. Ozone was measured in 1990–98.
† Former v. never smokers.
‡ After log-transformation of DNA adducts, t = − 2·02 (P = 0·04).
§ After log-transformation of DNA adducts, t = − 2·14 (P = 0·03).
∥ After log-transformation of 4-ABP-Hb adducts, t = − 1·46 (P = 0·15).
¶ After log-transformation of 4-ABP-Hb adducts, t = − 1·95 (P = 0·05).
Discussion
In the EPIC investigation we have recently examined prospectively the ability of bulky DNA adducts to predict cancer and we have found that leucocyte DNA adducts may predict lung cancer risk among never and former smokers(Reference Airoldi, Vineis and Colombi24). In addition we have provided evidence that, particularly among women, elevated 4-ABP-Hb adducts may help identify subjects at higher risk of environmental tobacco smoke-related cancers(Reference Airoldi, Vineis and Colombi24).
In the present study, we have investigated the association between dietary items and bulky DNA adducts and 4-ABP-Hb adducts measured, respectively, in leucocytes and erythrocytes of non-smokers. The rationale came from the presence of mutagenic PAH and aromatic compounds in food, and from previous observations relating high intakes of fruit and vegetables to decreased levels of adducts. No dietary item was associated with the levels of aromatic DNA or Hb adducts, except the intake of fibres, independently of levels of vitamins or folate intake.
When exposure to an air pollutant, ozone, was included into the regression model, the role of fibres was slightly weakened. Also, the association with fibres was present only in former smokers, an observation that suggests complex interactions between exposure to carcinogens and to protective compounds. In a study in healthy volunteers we have found that diet can have an important effect on the induction of DNA repair genes(Reference Guarrera, Sacerdote and Fiorini26). One can speculate the expression of DNA repair genes is increased by fibre intake or correlates of fibre intake (such as polyphenols), particularly in subjects who have had extensive DNA damage in the past such as ex-smokers.
Whereas the effect of fruit and vegetable intake on the formation of adducts of different carcinogens has been widely investigated, studies on the effects of dietary fibres are scanty. The relationship of fruit and vegetable consumption to bulky DNA-adduct formation was already examined by us in a case-control study on bladder cancer(Reference Peluso, Airoldi, Magagnotti, Fiorini, Munnia, Hautefeuille, Malaveille and Vineis19). The level of leucocyte DNA adducts was shown to decrease with increasing levels of vegetable consumption in controls. In addition, the association between case or control status, and the level of adducts (below or above the median value) was stronger in subjects who consumed less than one portion of vegetables per d (OR 7·80; 95 % CI 3·0, 20·3) than in heavy consumers (OR 4·98 for consumers of two portions per d; OR 1·97 for consumers of three or more portions per d). In another study among healthy subjects, inverse associations emerged between levels of bulky DNA adducts and plasma retinol (P = 0·02), α-tocopherol (P = 0·04) and γ-tocopherol (P = 0·03), but not carotenoids (except a borderline inverse association with β-carotene; P = 0·08)(Reference Mooney, Bell and Santella27).
Concerning 4-ABP adducts, in a previous study among bladder cancer patients we found that 4-ABP-DNA adducts in bladder biopsies were inversely related to fruit and vegetable intake(Reference Airoldi, Orsi, Magagnotti, Coda, Randone, Casetta, Peluso, Hautefeuille, Malaveille and Vineis20). In a more recent study we reported an inverse correlation also between 4-ABP-Hb adduct levels and fruit and vegetable consumption in non-smokers(Reference Airoldi, Vineis and Colombi24). Moreover, 4-ABP-Hb adducts were reportedly modulated by the intake of carotenoids in the control population of a large case-control study on smoking-related bladder cancer, this effect being confined to current smokers(Reference Castelao, Yuan and Gago-Dominguez28).
Possible mechanisms for the inverse association between fibre intake and adduct formation include:
(1) Bacterial fermentation of dietary fibre produces SCFA, including butyrate, that can protect the colonic mucosa barrier(Reference Toden, Bird, Topping and Conlon29), increase apoptotic response to genotoxic carcinogens(Reference Le Leu, Brown, Hu and Young30) and enhance glutathione transferase π expression(Reference Treptow-van Lishaut, Rechkemmer, Rowland, Dolara and Pool-Zobel31);
(2) Dietary fibre can decrease the levels of DNA damage by enhancing the action of antioxidant components contained in phytochemicals; the protective effects may be mainly due to some components, including phenolic polysaccharides and polyphenols, which are present in the cell walls of various plants and that can be released by bacterial enzymes in the colon. Indeed, flavonoids, polyphenols and other plant compounds have been shown to be capable of inhibiting DNA adduct formation(Reference Rho and Kim32, Reference Malaveille, Hautefeuille, Pignatelli, Talaska, Vineis and Bartsch33), possibly by their antioxidant activity or by interfering with the metabolic pathways of activation/detoxification of food mutagens(Reference Malaveille, Hautefeuille, Pignatelli, Talaska, Vineis and Bartsch33);
(3) The protective effects of an increased intake of fibre may be related to increased faecal bulk or reduced transit time(Reference Cummings, Bingham, Heaton and Eastwood34), thereby diluting potential toxins and carcinogens and reducing their contact time with the colonic epithelium.
It is unlikely that the present results can be explained by bias or confounding. The study had a prospective nature, and dietary ascertainment took place several years before adduct measurement, which was blind as to dietary variables. Alcohol and several of the potential confounding factors, such as age, sex, smoking status, BMI, energy intake, intake of fruit, legumes, vegetables, meat, vitamins and folate have also been evaluated in the present study.
In summary, after finding a clear protective effect of fibres in colon carcinogenesis(Reference Bingham, Day and Luben35), the present study from the same population (the EPIC prospective study) suggests that fibres can modify the levels of bulky DNA adducts and 4-ABP-Hb adducts.
Acknowledgements
We are grateful to Julie Britton for comments on the manuscript.
This paper was made possible by a grant of the European Community (5th Framework Programme) to P. V. (grant QLK4–CT–1999–00 927) and a grant of the Compagnia di San Paolo to the ISI Foundation. All authors are independent from funders. Mortality data for the Netherlands were obtained from Statistics Netherlands.
Also, the work described in the paper was carried out with the financial support of: Europe Against Cancer Program of the European Commission (SANCO); ISCIII, Red de Centros RCESP, C03/09; Deutsche Krebshilfe; Deutsches Krebsforschungszentrum; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund (FIS) of the Spanish Ministry of Health; Spanish Regional Governments of Andalucia, Asturias, Basque Country, Murcia and Navarra; Cancer Research UK; Medical Research Council, UK; Stroke Association, UK; British Heart Foundation; Department of Health, UK; Food Standards Agency, UK; Wellcome Trust, UK; Greek Ministry of Health; Greek Ministry of Education; Italian Association for Research on Cancer (AIRC); Italian National Research Council; Dutch Ministry of Public Health, Welfare and Sports; World Cancer Research Fund; Swedish Cancer Society; Swedish Scientific Council; Regional Government of Skåne, Sweden; Norwegian Cancer Society; Research Council of Norway.
There are no conflicts of interests to declare.
Individual authors' contributions were: M. P. and A. M. performed the analysis of DNA adducts; L. A. and A. C. analysed Hb adducts; F. V. performed the statistical analyses; K. O., O. R.-N., F. C.-C., J. L., H. B., A. T., D. P., V. K., R. T., S. P., B. H. B.-D.-M., P. H. P., M. K., A. A., C. Martinez, M. D., A. B., M. J. T., J. R. Q., G. B., L. J., B. J., N. E. D., T. J. K., R. S., R. K. and S. B. collected the data in the local EPIC centres; H. A., A. D., S. G., E. G., C. Malaveille and G. M. were part of the working group that performed laboratory analyses in GenAir and contributed to the interpretation of the results; E. R. is the EPIC coordinator; P. V. coordinated the GenAir investigation and wrote the manuscript with M. P. and L. A.






