In December 2019, an outbreak of pneumonia cases of unknown cause was reported by health facilities in Wuhan, Hubei province, China in which clusters of patients were associated with the seafood and wet animal wholesale market(Reference Zhu, Zhang and Wang1,Reference Chen, Zhou and Dong2) . On 7 January 2020, the novel coronavirus which caused the illness was identified in a throat swab sample(Reference Chen, Zhou and Dong2). On 11 February 2020, the WHO stated a new name for the malady: coronavirus disease-2019 (COVID-19)(Reference Lai, Shih and Ko3) and 1 month later declared it as a global pandemic(Reference Guo, Ye and Pan4). As stated by the WHO, until 6 September 2020, nearly 27 million cases of COVID-19 were reported of which 900 000 people died(5).
COVID-19 is a respiratory disease caused by the novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), that has reached pandemic status(Reference Puig-Domingo, Marazuela and Giustina6). Underlying diseases and other risk factors might contribute to the severity of COVID-19 patients. Chronic obstructive pulmonary disease, hypertension, diabetes, malnutrition, smoking habit, cerebrovascular disease, male sex, age over 65 years and obesity were reported to be associated with severe cases(Reference Lippi7–Reference Zabetakis, Lordan and Norton14). Although increased weight, and particularly obesity, has been associated with a more severe clinical course of COVID-19 and risk of fatality(Reference Simonnet, Chetboun and Poissy15,Reference Kalligeros, Shehadeh and Mylona16) , the course of the illness can lead to prolonged length of stay (LOS).
Changes in nutritional status and weight loss during hospitalisation are largely reported in some populations(Reference Borrego Utiel, Segura Torres and Pérez del Barrio17,Reference Valla, Baudin and Gaillard Le Roux18) , but still not explored in COVID-19 patients(Reference van Vliet, Gomes-Neto and de Jong19). In fact, few studies reported nutritional status of patients during hospitalisation for COVID-19(Reference Li, Zhang and Gong20). Symptoms and associated conditions might contribute to nutritional status deterioration, leading to cachexia(Reference Muscaritoli, Anker and Argilés21). Thus, the aim of this narrative review is to establish possible connections between COVID-19, prolonged hospitalisation and cachexia, and to propose nutritional recommendations for the prevention and treatment of such muscle wasting condition.
The literature review was conducted according to the SANRA Statement(Reference Baethge, Goldbeck-Wood and Mertens22) utilising PubMed, Lilacs, Google Scholar and Cochrane Library databases. First, to identify relevant publications about COVID-19 and cachexia, the combined search terms were used: (1) COVID-19 OR SARS-CoV-2, (2) cachexia OR muscle wasting and (3) diet OR nutrition. The inclusion criteria were studies published from January 2020 to 11 August 2020, published in English. Afterwards, to further discuss the relationship between COVID-19 infection, diet and loss of weight and muscle mass, relevant articles from the nutrition and cachexia area (including clinical characteristics and symptoms) were included.
How can COVID-19 induce weight loss?
The virus SARS-CoV-2 is commonly transmitted through respiratory droplets, contact and potentially via the faecal–oral route(23,Reference Jin, Yang and Ji24) . First, viral replication occurs in the upper respiratory tract and subsequently reaches the lower respiratory tract and other tissues and organs, including the gastrointestinal tract(Reference Jin, Yang and Ji24). In addition to respiratory symptoms, gastrointestinal symptoms caused by SARS-CoV-2 were also reported and they appear to exacerbate malnutrition in patients(Reference Li, Zhang and Gong20). Therefore, COVID-19 was reported to be associated with malnutrition in some studies(Reference Li, Zhang and Gong20,Reference Romano, Bilotta and Dauri25,Reference Stachowska, Folwarski and Jamioł-Milc26) . Diarrhoea, mild abdominal pain, nausea, vomiting, poor appetite and other symptoms were commonly reported and can cause reduction in food intake and/or absorption, and consequently weight loss(Reference Guan, Ni and Hu27).
Another possible important implication to weight loss is the acute inflammatory chain in response to the SARS-CoV-2 infection. The virus has spike (S) proteins, a glycoprotein that has high affinity with the angiotensin-converting enzyme 2 receptor, which is the mediator of virus entry(Reference Jin, Lian and Hu28,Reference Zhang and Liu29) . Angiotensin-converting enzyme 2 in the gastrointestinal tract has been identified in several studies, but it is widely expressed in various organs considered target for SARS-CoV-2 in humans, such as the nasal mucosa, bronchus, lung, heart, oesophagus, kidney and bladder(Reference Jin, Yang and Ji24,Reference Jia30) .
Once the virus entry occurs, the rapid viral replication and a series of reactions begin such as cellular damage, the cytokine storm and antibody-dependent enhancement(Reference Jin, Yang and Ji24,Reference Jia30,Reference Briguglio, Pregliasco and Lombardi31) . The process referred above as ‘cytokine storm’ is characterised as the presentation of the viral antigens to the natural killer and CD8-positive cytotoxic T cells in the context of major tissue histocompatibility(Reference Briguglio, Pregliasco and Lombardi31).
After this event, it is possible that massive epithelial and endothelial cell death and vascular leakage happen, triggering the production of several pro-inflammatory cytokines and chemokines, which are responsible for the aggressive inflammation caused by SARS-CoV-2(Reference Jin, Yang and Ji24,Reference Jia30) . In this regard, acute phase proteins such as C-reactive protein, ferritin, TNFα, IL family factors, NF-κB, interferon-γ, fibroblast growth factor and others are synthesised(Reference Li, Zhang and Gong20). Antibody-dependent enhancement can promote interactions between virus-anti-S protein-neutralising antibodies and target cell receptors that can increase inflammatory response as well(Reference Jin, Yang and Ji24).
All these processes are directly related to the increase of muscle proteolysis, albumin consumption and impaired metabolism of macronutrients which can contribute to the onset of malnutrition and cachexia(Reference Jia30). A cross-sectional study evaluating malnutrition in 182 COVID-19 hospitalised elderly patients (mean age 68·5 (sd 8·8) years) in China found that subjects classified as malnourished showed significantly lower albumin levels and calf circumference(Reference Li, Zhang and Gong20). However, changes in fat deposits and age-related loss of skin elasticity may contribute to errors in estimating muscle mass in the elderly(Reference Mendes, Pinho and Santana32).
Considering that patients with COVID-19 show an increased inflammatory response upon hospital admission(Reference Zabetakis, Lordan and Norton14), other signs and symptoms which can lead to weight and muscle loss should be monitored. Table 1 shows some studies in which symptoms may contribute to weight loss in COVID-19 patients. Notably, there is lack of information regarding some symptoms in these reports. It is also important to emphasise the lack of information regarding nutritional status, including BMI and weight loss in these studies. BMI was reported in a few studies in COVID-19 patients, although this marker of nutritional status has a significant association with the illness severity(Reference Simonnet, Chetboun and Poissy15,Reference Cai, Chen and Wang39–Reference Hajifathalian, Kumar and Newberry41) . Older age and the presence of co-morbid conditions are almost invariably associated with impaired nutritional status and sarcopenia, independently of BMI(Reference Laviano, Koverech and Zanetti42).
Table 1. COVID-19 symptoms potentially related to weight loss and cachexia
(Mean values and standard deviations; medians and interquartile ranges (IQR))
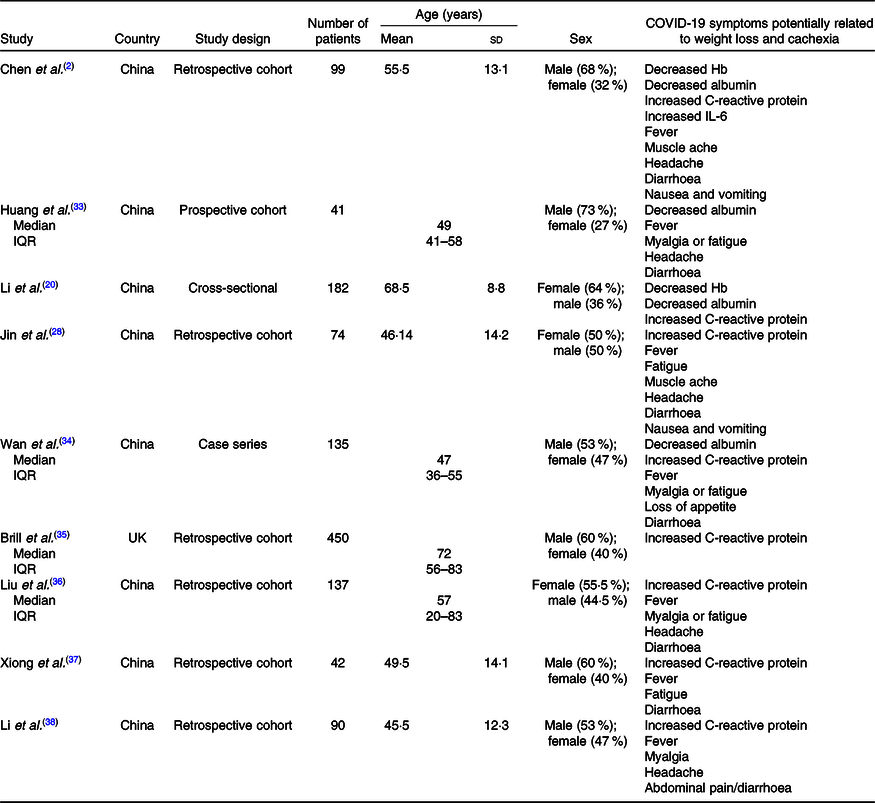
Based on available clinical observations, it is evident that although people of all ages can become infected and present weight loss, elderly people with low immunity and patients with chronic diseases have a worse prognosis and have a higher risk of cachexia. So, it is important to highlight the importance of weight loss monitoring and nutritional status vigilance for these patients. Nutritional therapy should be regarded as first-line treatment and implemented into standard of practice(Reference Romano, Bilotta and Dauri25), representing primary guarantee for promoting disease recovery.
Interrelations between prolonged hospitalisation, weight loss and risk of cachexia
SARS-CoV-2 manifestation and COVID-19 disease might be asymptomatic or present moderate to severe symptoms. In moderate and more severe cases, hospitalisation is necessary and complications can include acute respiratory distress syndrome, acute cardiac complications, multiple organ dysfunction syndrome, septic shock and death(Reference Iddir, Brito and Dingeo43). These complications have been described as the cytokine storm, in which viral replication triggers an abnormally strong release of cytokines and other immune-related stimuli, resulting in inflammation(Reference Zabetakis, Lordan and Norton14) and weight loss(Reference Li, Zhang and Gong20).
Identification of risk and presence of malnutrition should be an early step in the general assessment of all patients, with regard to more at-risk categories including older adults and individuals suffering from chronic and acute disease conditions, as COVID-19. Malnutrition can be defined as any nutritional imbalance which can happen both in underweight and overweight individuals(Reference Barker, Gout and Crowe44). This imbalance can be originated from different conditions such as insufficient nutrient intake, higher energy requirements, impaired absorption and/or changes in nutrient utilisation and transport(Reference White, Guenter and Jensen45). In addition, malnourished patients have higher risks of poorer outcomes such as longer length of hospital stay, muscle wasting, postoperative complications, depression of the immune system and mortality(Reference Barker, Gout and Crowe44).
It is well known that a healthy nutritional status is important for immune system support and to prevent severe infections(Reference Zabetakis, Lordan and Norton14). Nevertheless, the nutritional status of people with viral infections was not described to be a risk factor in the emergence of viral diseases which could be due to a lack of data, making it even more important to be reported(Reference Zhang and Liu29). In COVID-19 patients, these challenges appear to result from the direct effects of the SARS-CoV-2 virus on the gastrointestinal tract and are compounded by the elevated sedation required for this patient(Reference Arkin, Krishnan and Chang46). Also, the elevated doses of sedatives and opioids required to facilitate mechanical ventilation in patients with COVID-19 can contribute to intestinal dysmotility and weight loss(Reference Romano, Bilotta and Dauri25) which may lead to cachexia. Fig. 1 illustrates, in a didactic way, how the frequent manifestations presented by patients with COVID-19 can induce weight loss and, consequently, cachexia.

Fig. 1. Symptoms in COVID-19 inpatients related to the onset of cachexia. CRP, C-reactive protein.
Cachexia is a complex metabolic syndrome associated with underlying illness and characterised by loss of muscle with or without loss of fat mass(Reference Baracos, Martin and Korc47). Cachexia can also be defined as ‘weight loss in the presence of illness, combined with three or more of the following five criteria: decreased handgrip strength, fatigue, anorexia, low fatty free mass index (FFMI) or abnormal biochemistry (high C-reactive protein, low hemoglobin or low albumin)’(Reference Evans, Morley and Argilés48). Many of these factors that must be associated with weight loss for the diagnosis of cachexia are frequent symptoms in COVID-19 patients. Table 2 shows the interrelations between the signs and symptoms present in cachexia syndrome and COVID-19 patients.
Table 2. Interrelations between the signs and symptoms present in cachexia and COVID-19 patients

* The signs and symptoms usually observed in COVID-19 patients.
To date, data regarding decreased muscle strength and low fat-free mass index were not reported in recent studies in patients with COVID-19. However, they could be further explored using a hand dynamometer for assessing muscle strength and computed tomography (CT) acquired as part of standard COVID-19 to the determination of body composition and diagnosis of muscle mass reduction in these patients. The use of imaging diagnostics for body composition makes maximal use of existing information and could help to recognise and treat patients at increased risk of wasting with targeted pathways. In fact, some studies have already used CT images to describe the body composition of patients with COVID-19(Reference Gualtieri, Falcone and Romano49–Reference Petersen, Bressem and Albrecht51), showing that an inadequate body composition increases the risk of complications from the disease. In general, cross-sectional analysis of single CT images, typically landmarked at the 3rd lumbar vertebra (L3), is conducted(Reference Baracos, Mazurak and Bhullar52,Reference Mourtzakis, Prado and Lieffers53) . It is possible that some CT images of patients with COVID-19 do not include the L3 region, but it is still possible to estimate body composition in other regions of the abdomen, as the thoracic area(Reference Derstine, Holcombe and Goulson54). The information obtained through CT analysis, quantifying the patient’s muscle mass, can improve the care of the critically ill patient, establishing early conducts for the cachexia management.
Fatigue is a debilitating symptom commonly reported by COVID-19 patients(Reference Li, Huang and Wang55). Its aetiology and pathophysiology are not well understood yet, still it is suggested that both central and peripheral mechanisms are involved in the physical expression of fatigue(Reference Jason, Evans and Brown56). Up to date, the exact duration of this symptom is not reported by the studies as well, it is still unclear for how long patients can experience COVID-19-related fatigue. This could be due to the ‘cytokine storm’ leading to inflammation and anorexia, both responsible for muscle loss, weakness and fatigue. Also, infections contribute to higher basal energy expenditure during the immune system activation in which fever is a common symptom(Reference Childs, Calder and Miles57). Still, studies regarding the aetiology of fatigue, its duration and associated diseases in COVID-19 patients have to be better explored.
Anorexia has also been reported as a common symptom in recent meta-analyses(Reference Shang, Xu and Jiang58–Reference Zhu, Ji and Pang60). It is a complex and multifactorial symptom which can occur in the presence of acute inflammatory disease adverse reactions to treatment, depression, altered liver function and hypoxia(Reference Tian, Rong and Nian61,Reference Saper, Romanovsky and Scammell62) . Diarrhoea, nausea and vomiting were less frequent gastrointestinal symptoms than anorexia(Reference Zhu, Ji and Pang60). It is important to mention the presence of dysgeusia and anosmia of COVID-19 as possible contributors of anorexia.
There is expressive evidence of low albumin, other inflammation biomarkers, anorexia and fatigue as common symptoms in patients with COVID-19. Observational studies show low albumin in severe cases(Reference Wan, Xiang and Fang34,Reference Aziz, Fatima and Lee-Smith63) . A meta-analysis has reported that severe cases of COVID-19 were associated with hypoalbuminaemia, but the relation between the disease and the biomarker is still unclear(Reference Aziz, Fatima and Lee-Smith63). Albumin circulating levels should not be considered as a nutritional marker in patients with inflammatory response(Reference Muscaritoli, Anker and Argilés21,Reference Laviano, Koverech and Zanetti42) , but this biochemical marker has good relation with muscle mass and high-sensitive C-reactive protein.
High high-sensitive C-reactive protein usually observed in COVID-19 patients, confirming its viral aetiology as well as a biomarker of cachexia, is commonly altered in patients as a result of the ‘cytokine storm’ which increases the severity of COVID-19(Reference Wan, Xiang and Fang34,Reference Li, Huang and Wang55,Reference Luo, Liu and Qiu64,Reference Liu, Li and Xu65) . The rapid recruitment of neutrophils and macrophages causes an exacerbated reaction to infections producing pro-inflammatory cytokines and modifying the fragile balance between the host-damaging reaction and a controlled immune response(Reference Picchianti Diamanti, Rosado and Pioli66). The acute phase protein is a sensitive biomarker in which the up-regulated synthesis occurs in tissue damage, malignant neoplasia, infections and inflammation(Reference Pepys and Hirschfield67).
Low Hb is a biomarker present in the cachexia syndrome, and it was reported in COVID-19 patients in a few studies(Reference Chen, Zhou and Dong2,Reference Li, Zhang and Gong20) . However, it may be worth evaluating the role of Hb in the pathophysiology of COVID-19 and investigating its relation to the illness and adverse outcomes. Sotoudeh et al.(Reference Sotoudeh and Sotoudeh68) reported that the mortality rate of COVID-19 patients is lower in countries with a higher prevalence of haemoglobinopathies. This finding does not prove the direct association, and the observed finding can be due to other confounding variables or poor patient detection in tropical countries. A systematic review shows that six studies described low Hb in patients with COVID-19, while four studies found that all patients had Hb within the reference interval(Reference Alnor, Sandberg and Gils69). However, a limitation for this alteration is that most studies reported laboratory analysis from the patients at admission.
Few studies reported how the severity of COVID-19 leads to longer LOS compared with milder cases(Reference Liu, Zhou and Zhou70,Reference Suleyman, Fadel and Malette71) . Bhatraju et al. showed that the median LOS among survivors of critically ill COVID-19 patients was 17 d (interquartile range, 16–23), and the median length of intensive care unit (ICU) stay among survivors was 14 d (interquartile range, 4–17). Prolonged length of hospital stay may be accompanied by loss in weight and muscle mass(Reference Lovesley, Parasuraman and Ramamurthy72), leading to cachexia(Reference Arthur, Noone and Van Doren73,Reference Fukuta, Saito and Murata74) . LOS is usually lacking information in COVID-19 reports, and it should be given more attention considering it is an adverse outcome. Thus, nutrition therapy in patients with cachexia can be effective, and it was shown to significantly reduce LOS(Reference De Waele, Mattens and Honoré75).
Prevention and treatment of COVID-19 cachectic patients through diet
Although most critically ill patients with COVID-19 are overweight or obese(Reference Simonnet, Chetboun and Poissy15), they are often sick at home for days to weeks prior to being admitted to the hospital, thus increasing their likelihood of being malnourished upon presentation(Reference Arkin, Krishnan and Chang46).
Malnutrition differs from cachexia due to its complex nature which combines an underlying disease, metabolic alterations and sometimes a reduced nutrient availability which might play an important role on the onset of the syndrome(Reference Muscaritoli, Anker and Argilés21), while malnutrition is not described as a syndrome but an altered state of nutrition due to different causes. Therefore, cachexia must be distinguished from other causes of muscle loss. The clinical consequences of cachexia depend as much on the weight loss as on the systemic inflammation, which accompany the development of cachexia(76). Muscle, bone and fat tissue loss are also reported in cachexia(Reference Christensen, Kistorp and Schou77,Reference Okoshi, Capalbo and Romeiro78) . Prolonged length of hospital stay is another situation that may be accompanied by loss in weight and muscle mass(Reference Lovesley, Parasuraman and Ramamurthy72). Although nutrition therapy is essential to promote better outcomes for patients, it is still often overlooked in clinical practice(Reference Barker, Gout and Crowe44).
Established interventions to treat cachexia are available for specific underlying diseases, for example, progestogens(Reference Argilés, Anguera and Stemmler79), corticosteroids and other different approaches including, for example, myostatin antagonists, ghrelin agonists, selective androgen receptor antagonists were described(Reference Ebner, Steinbeck and Doehner80). To impair muscle mass loss, exercises should be included in the treatment even in the individual with advanced cachexia to reduce muscle mass and physical function loss(Reference Argilés, Busquets and López-Soriano81).
The European Society for Clinical Nutrition and Metabolism and other authors proposed general nutritional recommendations for all stages of COVID-19(Reference Romano, Bilotta and Dauri25,Reference Stachowska, Folwarski and Jamioł-Milc26,Reference Barazzoni, Bischoff and Breda82) . However, there is no data in the literature regarding nutritional recommendations for the prevention and treatment of cachexia in COVID-19 patients. The European Society for Clinical Nutrition and Metabolism document aims to provide concise statements from experts and practical guidelines for the nutritional management of patients with COVID-19, whether for adults with polymorbidity or those in the ICU setting, which are independent factors associated with malnutrition and negative outcomes(Reference Barazzoni, Bischoff and Breda82). In summary, this reference guides an energy supply between 27 and 30 kcal/kg per d (113 and 126 kJ/kg per d) (according to the nutritional status), and a protein supply of 1 g/kg.
A recent review summarised the clinical Chinese observations and compared them with the references brought by European Society for Clinical Nutrition and Metabolism’s guidelines(Reference Stachowska, Folwarski and Jamioł-Milc26). Zhang & Liu(Reference Zhang and Liu29) proposed several treatment options (including nutritional interventions) for the novel coronavirus, based on a review of the current literature(Reference Hajifathalian, Kumar and Newberry41). There are macronutrient recommendations for patients with COVID-19 pointed out in those studies. For lipid and carbohydrate needs, it is possible to consider an energy ratio from fat and carbohydrates from total estimated energy in a percentual distribution of 30:70 for subjects with no respiratory deficiency to 50:50 in ventilated patients(Reference Barazzoni, Bischoff and Breda82). It can also be considered 2 g/kg per d, not exceeding 150 g/d for carbohydrate and 1·5 g/kg per d for fat in critically ill patients(Reference Romano, Bilotta and Dauri25).
In COVID-19 intubated and ventilated ICU patients, enteral nutrition should be started. European Society for Clinical Nutrition and Metabolism suggests that in the early phase of the acute illness, a hypoenergetic nutrition of 20 kcal/kg per d (84 kJ/kg per d) should be administered (not exceeding 70 % of estimated energy) with increments up to 80–100 % after the third day. During critical illness, 1·3 g/kg protein equivalents per d can be delivered progressively. The use of enteral n-3 fatty acids may improve oxygenation despite strong evidence still not being available(Reference Barazzoni, Bischoff and Breda82,Reference Singer, Blaser and Berger83) . Up to date, the studies described in literature discuss nutritional management in ICU patients with cachexia due to other causes and report the importance of using specific nutrients, especially amino acids, to minimise excessive muscle mass loss(Reference Singer, Blaser and Berger83).
Leucine has been shown to be a potent stimulator of protein synthesis via the mammalian target of rapamycin complex pathway. It has recently been shown to be effective in elderly subjects with sarcopenia(Reference Morley, Argiles and Evans84). It may be extrapolated for COVID-19 patients with cachexia, but this use needs more studies. Arginine and glutamine are nonessential amino acids that are widely discussed in the literature for critically ill patients, but their role in muscular recovery is still unclear(Reference Singer, Blaser and Berger83). A recent report on nutritional support in patients with COVID-19 reaffirmed the use of arginine for immunity and healing, as well as the role of glutamine in preserving intestinal function, but not in muscle recovery in either nutrient(Reference Romano, Bilotta and Dauri25).
n-3 PUFA, β-hydroxy-β-methylbutyrate (HMB) and l-carnitine(76,Reference Esfahani, Sahafi and Derakhshandeh85,Reference Holeček86) are some other target nutrient suggestions for the recovery process. n-3 PUFA have shown to optimise tissue recovery(76). A systematic review with HMB concluded that this amino acid metabolite attenuates exercise-induced muscle damage and enhances muscle hypertrophy and strength(Reference Holeček86). l-Carnitine supplementation has protective effects on several mechanisms of muscle loss, improving protein synthesis(Reference Esfahani, Sahafi and Derakhshandeh85).
It is also important to monitor and assess micronutrient levels and supplement accordingly. Overall, low levels or intakes of micronutrients such as vitamins A, E, D, B6 and B12, Zn and Se have been associated with adverse clinical outcomes during viral infections(Reference Romano, Bilotta and Dauri25,Reference Barazzoni, Bischoff and Breda82) . For the assessment of micronutrients in COVID-19 patients, vitamins A and D, vitamin B, vitamin C, n-3 PUFA, as well as Se, Zn and Fe should also be considered(Reference Mendes, Pinho and Santana32,Reference Hajifathalian, Kumar and Newberry41).
For cachectic patients, no guidelines exist for its prevention or treatment. Appetite stimulants, such as megestrol acetate and glucocorticoids, have been shown to increase appetite and weight(Reference De Waele, Mattens and Honoré75). However, in the acute phase of the COVID-19 disease and in the presence of intubation, these drugs are considered futile. Nowadays, clinicians should consider personalised nutritional treatment as target to prevent and treat cachexia for each patient. These interventions would include, as possible according to the general state of a patient, nutritional counselling, assessing and treating symptoms that have an impact on energetic intake, and a rational combination of pharmacological approaches directed at underlying pathophysiology(Reference Fearon, Strasser and Anker87). Laboratory measurements of nutritional status, such as albumin levels, may be useful in certain cases. Table 3 summarises the purpose of nutritional recommendations for COVID-19 patients with cachexia during critical illness and/or admitted in a critical care unit.
Table 3. Prevention and treatment of malnutrition and cachexia in COVID-19 patients

ESPEN, European Society for Clinical Nutrition and Metabolism; HMB, β-hydroxy-β-methylbutyrate.
The risk of refeeding syndrome in critically ill patients with COVID-19 should be monitored, as cachexia increases nutritional deficits(Reference Arkin, Krishnan and Chang46). In addition, electrolyte disturbances increase the risk of refeeding syndrome and contribute to arrhythmias and hemodynamic instability. So far, we are not aware of specific recommendations for patients after extubation and in the state of rehabilitation, but it is important to pay attention to the risk of dysphagia and swallowing assessment procedures should be applied to assess the possibility of implementing texture-adapted food in this condition(Reference Barazzoni, Bischoff and Breda82).
Conclusions
Governments of countries around the globe should be dealing with the possibility of prolonged hospitalisation of COVID-19 patients and its enormous strains on the healthcare system. The deterioration of nutritional status, and consequently cachexia, increases the risk of mortality and needs to be treated with attention as other complications. Ensuring adequate nutrition in patients with COVID-19 who presented cachexia or associated symptoms has proven to be challenging due to intestinal alterations and inflammatory profile which complicate nutritional management. There is, however, little hard evidence of nutritional health approaches in assisting COVID-19 treatment or its management including cachexia. Nevertheless, COVID-19 is still a recently discovered disease, measures regarding maintenance of nutritional status and prevention of cachexia in hospitalised patients should be better explored for effective nutritional interventions in the future.
Acknowledgements
There are no sources of support.
This research received no external funding.
The authors' contributions were as follows: I. P. A. V. and N. M. S. contributed to data curation, analysing the data, interpretation of the findings, writing the original draft, review and editing. S. C. V. C. L. contributed to the review and editing and writing the original draft. A. P. T. F. contributed in the conceptualisation, data curation, methodology, project administration, writing the original draft, and review and editing.
The authors declare no conflict of interest.








