Although the past few years have witnessed remarkable progress in the management of CVD, it continues to be a major global health burden with high disability and mortality rates(Reference Townsend, Wilson and Bhatnagar1). Current estimates indicate that the prevalence of CVD in the USA is increasing, with approximately 85·6 million Americans currently living with CVD(Reference Mozaffarian, Benjamin and Go2). Accordingly, further emphasis should be placed on preventing the occurrence and development of the disease, and a healthy lifestyle throughout life is widely thought to be the most important way to prevent CVD(Reference Arnett, Blumenthal and Albert3).
A healthy dietary habit may play the most important role in preventing premature death and disability of CVD compared with other habits, such as avoiding smoking and moderate physical activities. Ample evidence substantiates that dietary habits may influence the risk of CVD. A comprehensive review indicated that the Mediterranean diet is associated with better cardiovascular health outcomes, including decreased prevalence of coronary heart disease (CHD), ischaemic stroke and CVD in general(Reference Martínez-González, Gea and Ruiz-Canela4). Two randomised clinical studies revealed that vegetarian dietary patterns could reduce the risk of CVD mortality and morbidity(Reference Orlich, Singh and Sabaté5,Reference Crowe, Appleby and Travis6) . In addition, dietary patterns with proinflammatory potential were positively associated with CVD risk in a prospective observational study(Reference Li, Lee and Hu7). It is widely thought that cardiometabolic risk factors affected by diet include obesity, lipoprotein function and concentration, glucose-insulin homoeostasis, inflammation, oxidative stress, endothelial health, microbiome and others(Reference Mozaffarian8).
While studies have shown promising results regarding the benefits of healthy dietary patterns in preventing and managing CVD, no consensus has been reached on the effects of individual food items or categories on CVD. For instance, some intervention studies have shown that blood pressure reduction (diastolic/systolic blood pressure > 21·5/24·0 mm Hg) was significantly associated with an increased fruit and vegetables intake(Reference Appel, Moore and Obarzanek9–Reference John, Ziebland and Yudkin11), while other studies revealed little or no effect(Reference Berry, Mulla and Chowienczyk12,Reference Little, Kelly and Barnett13) . Similarly, research on the association between consumption of unprocessed red meat (i.e. beef, pork) and CHD has yielded heterogeneous findings. In this respect, three meta-analyses based on observational prospective studies revealed inconsistent results; one reported a significant 27 % increase in CHD incidence with red meat(Reference Abete, Romaguera and Vieira14), while the other two studies showed no association(Reference Micha, Wallace and Mozaffarian15,Reference Bechthold, Boeing and Schwedhelm16) . In addition, several other potential risk factors for CHD were identified through observational studies and randomised controlled trials, including salt intake(Reference Wang, Yeh and Shih17), less coffee(Reference Grosso, Micek and Godos18)and tea intake(Reference Chung, Zhao and Wang19), decreased cheese intake(Reference Zhang, Chen and Zhang20) and fish intake(Reference Bechthold, Boeing and Schwedhelm16). Conversely, studies also showed no association between CVD and these risk factors(Reference Aburto, Ziolkovska and Hooper21–Reference Zhang, Xiong and Cai25). These inconsistencies may be due to the presence of potential biases, such as confounding factors or reverse causality. Therefore, whether dietary habits play a causal role in the development of CVD remains undetermined.
Mendelian randomisation (MR) offers a novel approach to address these potential biases by utilising genetic variants as instrumental variables to assess the causal relationship between exposure to risk factors and disease outcomes(Reference Smith and Ebrahim26,Reference Smith and Ebrahim27) . Recent genome-wide association studies (GWAS) have shown that dietary habits are heritable traits(Reference Cole, Florez and Hirschhorn28,Reference Matoba, Akiyama and Ishigaki29) . Besides, a two-sample MR study confirmed a causal relationship between dietary influence and major depressive disorder(Reference Chen, Chen and Fang30). Herein, we conducted an MR analysis to investigate the causality of genetic liability for twenty dietary habits on the risk of developing CHD, hypertension (HP), heart failure (HF), myocardial infarction (MI), atrial fibrillation and ischaemic stroke.
Materials and methods
Study design
We applied a two-sample MR approach to investigate the causal relationship between dietary habits and CVD, including CHD, HP, MI, HF, atrial fibrillation and ischaemic stroke. The study framework is shown in Fig. 1. To ensure the validity of our MR analysis, we considered three fundamental assumptions: (1) The genetic variants used as instrumental variables are strongly associated with the exposure of interest (twenty dietary habits in this study); (2) the instrumental variables have an impact on the outcome (CAD in this study) only through the exposure and (3) the instrumental variables are independent of other factors that influence the outcome(Reference Boef, Dekkers and le Cessie31).

Fig. 1. The central framework of Mendelian randomisation analyses of the causal relationship of twenty dietary habits on the risk of CVD. Assumption 1: IV are strongly associated with the exposure; Assumption 2: IV have an impact on outcome through the exposure only; Assumption 3: IV are independent of other factors that influence outcome. SNP, single nucleotide polymorphism; CHD, coronary heart disease; HP, hypertension; HF, heart failure; MI, myocardial infarction; AF, atrial fibrillation; IS, ischaemic stroke.
Data sources for dietary habits
The genome-wide associations for twenty dietary habits used in our study were derived from UK Biobank (UKBB) GWAS summary statistics provided by Benjamin Neale’s lab (http://www.nealelab.is/uk-biobank/) (Reference Sudlow, Gallacher and Allen32). UKBB is a large prospective study with over 500 000 participants aged 40–69 years recruited between 2006 and 2010. To prevent selective reporting bias, we evaluated all dietary habit variables (n 20) in the UKBB GWAS results obtained from Neil’s laboratory to explore potential causal associations with CVD. At the same time, we conducted an extensive review of relevant studies, as mentioned in the introduction. We observed that the impact of dietary variables on heart disease remains controversial in observational studies. It is worth noting that certain dietary factors have not been extensively studied in relation to heart disease (such as hot drink temperature). In the UKBB, information on the twenty dietary habits was collected retrospectively by a shortened food frequency touchscreen questionnaire at baseline. Data on the twenty single food intakes consist of quantitative continuous variables (the number of heaped tablespoons of cooked vegetables per day), categorical variables (the main type of food consumed) and ordinal non-quantitative variables (frequency of consumption of oily fish). The questions on dietary habits and their corresponding answers are summarised in online Supplementary Table S1, based on publicly available information on the UKBB website (http://biobank.ctsu.ox.ac.UK/crystal/label.cgi?id=100 052). The submission would not be accepted if the answer were unrealistic.
Selection of genetic instruments
After obtaining GWAS data for twenty dietary habits from the UKBB, we performed various quality control procedures to select appropriate genetic instruments. First, we extracted single nucleotide polymorphisms (SNP) that were highly associated with each dietary habit (P < 5 × 10−8). Second, we conducted Linkage disequilibrium-based clumping with r2 < 0·01 and set a window size of 10 000 kb to evaluate whether there is a linkage disequilibrium between SNPs of one trait located on the same chromosome. In this case, SNP with the largest P values were discarded to ensure the independence between IVs. Third, we assessed the strength of the genetic instruments and mitigated potential weak instrument bias by calculating F-statistics. The F-statistics were computed using the formula: F = [R2 × (N − k − 1) ]/[k (1 − R2)](Reference Pierce, Ahsan and Vanderweele33). We calculated the R2 using the following equation: R2 = [2 × EAF×(1 − EAF) × beta2]/[2 × EAF × (1 − EAF) × beta2 + 2 × EAF × (1 − EAF) × n × se2](2). A threshold of an F-statistic higher than 10 indicated a strong genetic variant. Additionally, we excluded SNP associated with the outcome traits (at P < 5 × 10–8) and not present in the outcome data. We employed the MRPRESSO method to identify and remove SNP and outliers to address potential horizontal pleiotropy. Furthermore, we used Phenoscanner (www.phenoscanner.medschl.cam.ac.UK) to exclude SNP associated with confounding factors to account for potential confounding.
Data sources for CVD
Summary genetic statistics for CHD and MI were extracted from a large-scale GWAS meta-analysis from the CARDIoGRAMplusC4D consortium, which included up to 184 305 subjects across forty-eight studies (77 % of whom were of European descent, 60 801 CHD cases and 123 504 controls, 43 676 MI cases and 128 199 controls)(Reference Nikpay, Goel and Won34). Summary-level data for stroke were obtained from the MEGASTROKE consortium, compromising 446 696 participants of European descent (40 585 cases and 406 111 controls). Summary statistics for atrial fibrillation were derived from by a meta-analysis of GWAS, comprising 55 114 cases and 482 295 control samples of European ancestry(Reference Roselli, Chaffin and Weng35). Summary data for HF were obtained from the HERMES consortium, comprising 977 323 participants of European descent (47 309 cases and 930 014 controls) from twenty-six studies. HP GWAS data were extracted from the FinnGen Study, compromising thirteen cohorts and biobanks. The data were adjusted for age, sex, genotyping batch and ten principal components. In total, 15 870 cases were defined as having essential or primary hypertension, and 74 345 controls were without essential hypertension or any other hypertensive diseases. The detailed definition of essential hypertension and outcome data resources are provided in Table S3 and S4. We retrieved these summary data and extracted the SNP associated with the twenty dietary habits.
Mendelian randomisation analyses
After harmonising the SNP between exposures and outcomes and excluding palindromic SNP with an allele frequency > 0·3, we conducted three different approaches of MR, including random-effect inverse-variance weighted (IVW), MR Egger and weighted median to assess the effect of variant heterogeneity and pleiotropy. We used the IVW method as the main MR analysis, which estimates the causal effect by performing a weighted regression of the SNP-outcome effect on the SNP exposure effect. In this method, the intercept is constrained to zero, assuming there are no other pathways through which the genetic variants affect the outcome besides the exposure of interest. We conducted additional MR analyses, including MR-Egger and weighted median, to enhance the robustness of our findings, although these methods may yield wider confidence intervals due to lower efficiency(Reference Bowden, Davey Smith and Burgess36). In cases where the results of these three methods were inconsistent, a stricter instrument p-value threshold was implemented to reperform another MR analysis. We further examined the horizontal pleiotropy using the MR-Egger intercept test and leave-one-out analyses.
Sensitivity analysis is crucial to detect the pleiotropy and heterogeneity for MR estimates. Cochran’s Q test (P < 0·05) from the IVW method is used to represent heterogeneity. We further examined the intercept obtained from the MR-Egger regression and leave-one-out analyses for horizontal pleiotropy (P < 0·05)(Reference Burgess and Thompson37). A funnel plot was also conducted to examine the potential directional pleiotropy. SNP and outliers identified by MRPRESSO were removed to correct horizontal pleiotropy.
We performed power analyses for significant and nominally significant MR via an online tool (https://shiny.cnsgenomics.com/mRnd/) to assess the likelihood of a non-zero causal relationship between the dietary habits and their corresponding CVD (online Supplementary Table S5).
Statistical analysis
Statistical analysis was performed using the two-sample MR package (version 0.5.6) and MRPRESSO (version 1.0) in R (version 4.2.2). We checked for potential mediators, including obesity, BMI, serum lipids, type 2 diabetes and smoking in PhenoScanner (www.phenoscanner.medschl.cam.ac.uk) to assess their influence on the SNP. A threshold of P < 0·000417 (Bonferroni correction P = 0·05/120) was considered significant for establishing a causal relationship. The risk of CVD was quantified using OR with a 95 % CI per unit in our MR results.
Results
The F statistics of all extracted SNP ranged from 29 to 811, indicating strong instruments. Besides, all power values were greater than 0·8, indicating a high confidence level in the results’ significance.
In our comprehensive MR analysis investigating the genetic predisposition towards dietary habits and their influence on CVD, we identified several significant or nominally significant associations (Table 1). We found evidence of a protective causal effect of genetic predisposition towards cheese intake on MI (IVW OR = 0·67; 95 % CI = 0·544, 0·826; P = 1·784 × 10−4; 60 SNP) and HF (IVW OR = 0·646; 95 % CI = 0·513, 0·814; P = 2·135 × 10−4; 53 SNP). Poultry intake was found to be a detrimental factor for HP (IVW OR = 4·306; 95 % CI = 2·158, 8·589; P = 3·416 × 10−5; 6 SNP), while dried fruit intake was protective against HP (IVW OR = 0·473; 95 % CI = 0·348, 0·642; P = 1·683 × 10−6; 28 SNP). Except for the effect of cheese on HF, the Cochran-Q test derived P values were > 0·05. Since we used the random effect IVW as the primary outcome, the observed heterogeneity was acceptable(Reference Burgess, Davey Smith and Davies38). The MR-Egger intercept (P > 0·05) confirmed the absence of directional pleiotropy was identified (Fig. 2). Furthermore, the leave-one-out plot indicated no individual SNP influenced the pooled IVW estimates (Fig. 3).
Table 1. Significant and nominal significant Mendelian randomisation estimates from dietary habits on genetically predicted CVD
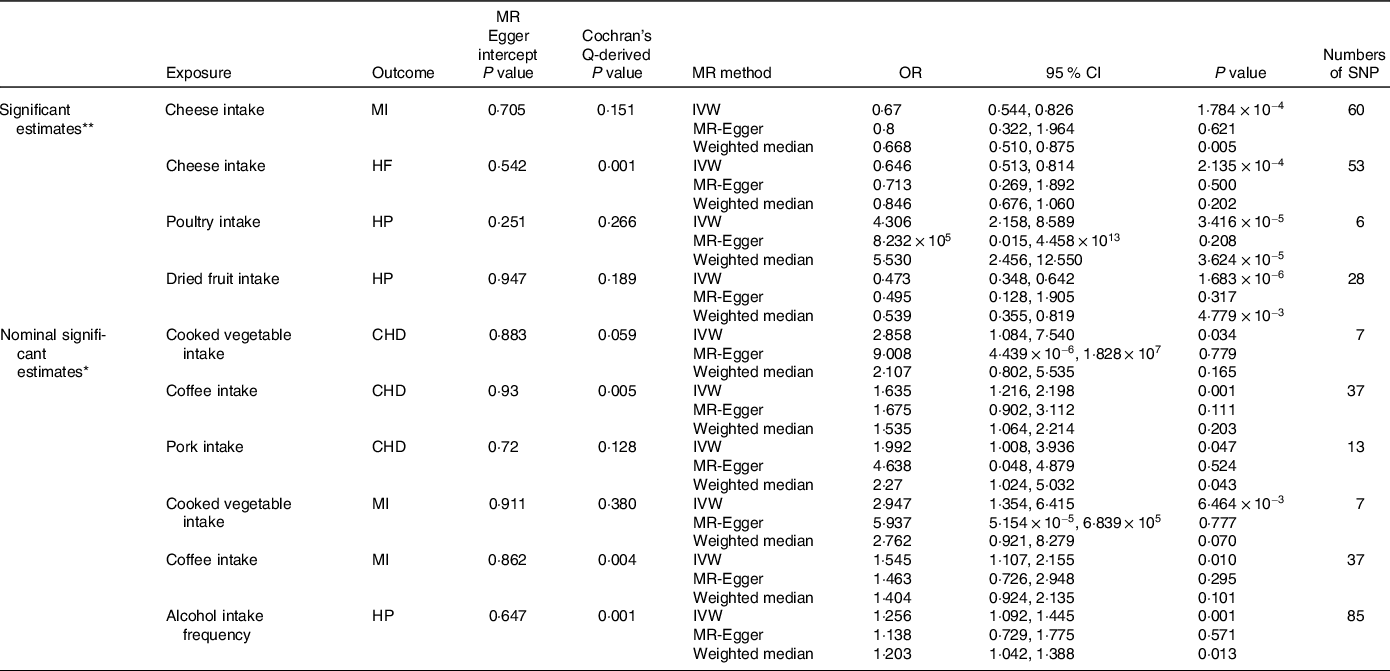
MR, Mendelian randomisation; IVW, inverse-variance weighted; MI: myocardial infarction; HF, heart failure; HP, hypertension; CHD, coronary heart disease.
Cochran’s Q-derived P value and MR-Egger intercept-derived P value < 0·05 is significant.
* Nominal significant estimate is defined as IVW-derived P < 0·05.
** Significant estimate is defined as IVW-derived P < 4·17 × 10−4.

Fig. 2. Scatter plots of significant estimates from genetically predicted dietary habits on CVD. (a) cheese intake on MI; (b) cheese intake on HF; (c) poultry intake on HP; (d) dried fruit intake on HP.

Fig. 3. Leave-one-out plots of significant estimates from genetically predicted dietary habits on CVD. (a) cheese intake on MI; (b) cheese intake on HF; (c) poultry intake on HP; (d) dried fruit intake on HP.
Additionally, we identified several nominally significant variables in our analysis. Cooked vegetables, coffee and pork consumption was associated with an increased risk of CHD. Coffee, cooked vegetables and alcohol intake frequency was associated with increased risk of MI and HP, respectively (Table 1). To assess the presence of horizontal pleiotropy, we conducted Cochran-Q tests, leave-one-out plots, MR-Egger intercept tests and funnel plots (online Supplementary Fig. S1–S3). The MR-Egger intercept tests yielded P-values greater than 0·05, indicating no evidence of horizontal pleiotropy.
To assess potential confounding effects, we performed a sensitivity analysis by removing specific SNP (rs10938397, rs13107325, rs1051730, rs1421085 and rs782221) that were associated with known confounders such as obesity, body traits, lipid levels, type 2 diabetes and smoking, or directly affected MI, HF or HP. After removing these SNP, we re-evaluated the MR results and found that the causal associations remained consistent with the previous findings.
Discussion
This study represents the first and largest MR study to explore the causal relationship between twnety dietary habits and CVD. Our results suggest that different dietary intakes may have protective or deleterious effects on CVD, consistent with the literature. We found that certain dietary intakes may have protective effects on CVD, such as cheese intake on myocardial infarction and heart failure and dried fruit intake on hypertension. Conversely, we observed the deleterious effects of poultry intake on HP. Furthermore, our study revealed a higher risk of CHD associated with increased intake of cooked vegetables, coffee and pork. Besides, coffee, cooked vegetable and alcohol intake frequency was associated with increased risk of MI and HP.
Dairy fat, characterised by a high content of saturated fatty acids, is widely thought to increase CHD mortality(Reference Burgess, Davey Smith and Davies38). Hence, only low-fat dairy products are recommended for a healthy diet. Surprisingly, dairy fat intake was associated with ischaemic heart disease when cheese was excluded from the analysis(Reference Zemel and Sun39), while several related studies have found no or even an inverse association between cheese and CVD(Reference Díaz and Decker40,Reference Nongonierma and FitzGerald41) . The inconsistency in the results of previous studies may be explained by the heterogeneity in study design and methods, including different FFQ, population differences and other residual confounding factors. A two-sample MR study, utilising single nucleotide polymorphisms as instrumental variables provides a robust approach to assess the causal relationship between cheese intake and CVD while minimising the impact of residual confounding factors. In the present study, we found that cheese intake has a beneficial effect on IM and HF, consistent with the results of previous studies(Reference Hernández-Ledesma, Miralles and Amigo42). Several potential mechanisms may explain the protective effects of cheese on CVD. First, cheese is rich in calcium, which has been reported to reduce total and LDL cholesterol concentrations(Reference O"Keeffe and Fitzgerald43) by binding to intestinal fatty acids to form insoluble soaps that may inhibit fatty acid absorption. Moreover, Ca is reportedly negatively correlated with stroke(Reference Phelan, Aherne-Bruce and O"Sullivan44) and hypertension(Reference Yamaguchi, Kawaguchi and Yamamoto45). Second, cheese contains a variety of bioactive proteins that protect vascular endothelial function through a variety of putative mechanisms, which include reducing NADPH oxidase(Reference Zemel and Sun39), inhibiting lipid peroxidation(Reference Díaz and Decker40), scavenging free radicals(Reference Nongonierma and FitzGerald41,Reference Hernández-Ledesma, Miralles and Amigo42) and enhancing antioxidant enzyme capacity(Reference O"Keeffe and Fitzgerald43,Reference Phelan, Aherne-Bruce and O"Sullivan44) . These studies suggest that dairy proteins may protect endothelial function by limiting the production of reactive oxygen species. In addition, microarray analysis of the aorta from a spontaneously hypertensive rat model showed that targeted changes in the expression of genes related to vascular function were alterations in pro-/ anti-inflammatory transcription factors and increases in endothelial NO synthase and gap junction protein 40, including peroxisome proliferator-activated receptor γ and NF-κB, respectively(Reference Yamaguchi, Kawaguchi and Yamamoto45). Third, cheese is a probiotic food, containing many live microorganisms. A clinical trial(Reference Khan, Kwon and Shivappa46) demonstrated that two probiotics in cheese, Lactobacillus and Bifidobacterium, yielded beneficial effects on immunity, inflammation and cardiovascular risk factors.
Interestingly, we substantiated the harmful effect of poultry intake on HP, but consumption of beef, pork, processed meat and lamb/mutton did not significantly influence HP. There is an increasing consensus suggesting that unprocessed red meat increases the risk of hypertension(Reference Han and Levings47,Reference Tam and Redman48) , although there are conflicting data on the relationship between blood pressure and poultry. Poultry consumption has been associated with an increased risk of HP in some studies(Reference Miglio, Chiavaro and Visconti49), while others reported contrasting results(Reference Vlassara, Cai and Tripp50). Three prospective cohorts from America(Reference Vlassara, Cai and Tripp50) consistently demonstrated a significant association between animal flesh consumption and an increased risk of HP, independent of other dietary factors. Besides, higher consumption of poultry was associated with a higher risk for HP in two of the three cohorts. However, our results differ from previous studies in terms of the positive association observed between processed and red meat consumption and hypertension. The disparity in findings could be attributed to the inherent limitations and biases present in observational studies. It is highly conceivable that previous studies might not have consistently classified meat types, which could have contributed to the inconsistent outcomes. The increased risk of hypertension in poultry may be attributed to the formation of Milrad reaction products in cooked meat(Reference Cepas, Collino and Mayo51), such as heterocyclic amines and advanced glycation end products (AGE). The Milrad reaction products reportedly increase oxidative stress and inflammation, both of which are potential factors in the development of HP(Reference Szypowska, Regulska-Ilow and Zatońska52,Reference Canto-Osorio, Denova-Gutierrez and Sánchez-Romero53) . Prospective studies conducted in Poland have shown a positive association between a proinflammatory diet and high-fat poultry(Reference Szypowska, Regulska-Ilow and Zatońska52). A proinflammatory diet is associated with higher dietary energy expenditure(Reference Khan, Kwon and Shivappa46,Reference Canto-Osorio, Denova-Gutierrez and Sánchez-Romero53) , as fat cells store excess energy leading to adipose tissue hyperplasia and hypertrophy. This process triggers the release of high levels of proinflammatory molecules, including tumor necrosis factor receptors, interleukin-1 receptors, NF-kB transcription factors and Toll-like receptors from macrophages(Reference Han and Levings47). More importantly, the excess lipid is redistributed to other organs (e.g. blood vessels), causing the expression of proinflammatory mediators, differentiation of monocytes into macrophages and recruitment of macrophages. This can lead to a vicious cycle of impaired endothelial function and vascular inflammation(Reference Tam and Redman48), contributing to the development and progression of hypertension. In addition to poultry consumption, we found a negative correlation between dried fruit intake and the risk of HP. One clinical study supported the role of raisin intake in BP reduction(Reference Puglisi, Vaishnav and Shrestha54). Dried fruit is a nutritionally concentrated form of fresh fruit withlow water content. The health benefits of frequent dried fruit consumption include improvements in CVD and cardiometabolic syndrome (e.g. endothelial function, inflammation, lipid levels and blood pressure) and insulin homoeostasis.
Although some estimates did not pass Bonferroni correction, their IVW derived P < 0·05 should also be treated with caution(Reference Chen, Kong and Pan55). Interestingly, we found that cooked vegetable intake can be a risk factor for CHD and MI, attributed to the effects of cooking, which can dramatically alter the chemical composition of vegetables and affect the concentration and bioavailability of bioactive compounds, such as heat-sensitive, antioxidant and water-soluble nutrients(Reference Miglio, Chiavaro and Visconti49). It has been established that protein glycosylation occurs in food preparation processes such as baking, cooking and frying. High temperature and long cooking time are also conducive to the glycosylation reaction(Reference Vlassara, Cai and Tripp50) and formation of AGE. By interacting with their corresponding receptor, AGE can exacerbate oxidative stress by generating reactive oxygen species via NADPH oxidase in mitochondria. This, in turn, adversely impacts mitochondrial function and subsequently alters cellular metabolism, particularly in pathological circumstances(Reference Cepas, Collino and Mayo51). Therefore, AGE are considered a risk factor for the onset of diet-related diseases such as CVD.
Our two-sample MR Study, which assessed the association between genetic predisposition to twenty dietary habits and CVD, has several strengths. First, implementing MR analysis allows for the mitigation of residual confounding and the minimisation of issues related to reverse causality, frequently encountered in traditional observational studies. Second, a meticulous screening process was employed to carefully select robust genetic instruments, eliminate SNP associated with outcomes and mitigate potential confounding variables. Third, the study employed three complementary MR methods to assess and address any potential violations of the MR assumption.
This study offers a novel perspective to explore the causal relationship between dietary habits and the risk of CVD. Future studies should elucidate the mechanism underlying the association between diet and CVD or explore dietary treatment modalities to prevent or treat different types of CVD.
Limitation
The limitations inherent in MR studies affected the robustness of the present study findings to a certain extent. First, the instrumental variable approach using genetic variation may introduce pleiotropy and residual confounding, where the genetic variants affect outcomes through pathways other than the exposure of interest. This could potentially lead to biased estimates. However, our study observed robust effect estimates across different MR models, and sensitivity analyses based on various assumptions did not indicate any horizontal pleiotropy. Additionally, the genetic variants used as instruments in our study may not completely capture the biological pathways through which dietary habits affect cardiovascular health. Second, the participants included in our study were predominantly of European descent, limiting the generalisability of the causal relationship between dietary habits and CVD to other populations. Third, although the effect of diet on CVD may differ by sex and age group, we only analysed the overall effect adjusted for sex and age due to limited GWAS summary data. Furthermore, certain dietary patterns, such as the Mediterranean diet, could not be examined due to insufficient data. Finally, some dietary habits were treated as ordered variables, and the assumption of a linear effect of SNP on diet may introduce bias towards zero in GWAS results, potentially resulting in false-negative findings regarding the effect of dietary habits on CVD. We were also unable to confirm the presence of a nonlinear causal relationship between dietary habits and CVD. Linear regression-based GWAS is reportedly unsuitable for examining the nonlinear association between exposure and outcome. Despite these limitations, our MR results still serve as valid tests of the null hypothesis of causality, although some negative causal relationships in our study may have been underestimated. Accordingly, we recommend the prospective collection of detailed dietary data across different populations for future MR analyses or implementing randomised controlled trials with adequate sample sizes.
Conclusion
This is the first comprehensive MR analysis to reveal an association between dietary habits and CVD. Multiple dietary habits exhibit protective or harmful effects on CVD. Accordingly, adopting healthy dietary habits may prevent and reduce the risk of CVD. However, further validation and investigation of the underlying mechanisms are warranted.
Acknowledgement
We want to acknowledge all participants and investigators from UK biobank, the FinnGen study, MEGASTROKE consortium, CARDIoGRAMplusC4D consortium, HERMES consortium and Roselli et al. for the atrial fibrillation GWAS meta-analysis.
This study was supported by the Natural Science Foundation of Guangdong Province, China (Grant No. 2023A1515010587).
J. P., M. M. Y. and X. G. designed the study, drafted the manuscript and verified the data; M. M. Y., X. G. conducted statistical analyses; L. Z. X. drafting the work and revising it critically for important intellectual content; Z. Z. L., X. S. Y. and J. Y. O. provided substantial contribution to interpretation of data; J.P. was accountable for all aspects of the work. All authors checked the paper critically and approved the final manuscript.
This study only used publicly summary data which has been approved to conduct human experimentation by an ethical standards committee. Hence, additional ethical approval was not required in current study.
The authors declare no conflict of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452300140X







