CVD is a leading cause of death worldwide, with deaths expected to reach 23 million by 2030(Reference Laslett, Alagona and Clark1). According to the American Heart Association, costs associated with CVD are expected to grow to nearly $1·1 trillion by 2035(Reference Dunbar, Khavjou and Bakas2). Considering the increasing prevalence and healthcare burden of CVD, advancements in interventions and treatment approaches are in urgent need.
Although the manifestation of CVD is multifaceted and complex, major contributing factors are considered to be increased levels of reactive oxygen species (ROS) and inflammation in the vasculature(Reference Steven, Frenis and Oelze3). Elevated levels of ROS specifically have been reported to scavenge endothelial cell-derived nitric oxide (NO), a potent vasodilator that is essential for intact endothelial function(Reference Schiffrin4,Reference Park, Rossman and Gifford5) . Reduced NO bioavailability due to elevated ROS in the vasculature ultimately attenuates endothelium-dependent vasodilatory function(Reference Mudau, Genis and Lochner6). Excessive ROS production may also induce vascular damage that could lead to diseases, including atherosclerosis, hypertension and diabetes(Reference Guzik, Mussa and Gastaldi7,Reference Rodrigo, Gonzalez and Paoletto8) . Additionally, ROS-associated attenuated vascular function and disease conditions impair blood flow and oxygen delivery to skeletal muscle, which may also impair tissue saturation and oxygen utility capacity(Reference Goto, Higashi and Kimura9). It has been reported that a high ingestion of fruits and vegetables, in general, is associated with a lower risk of CVD mortality(Reference McCullough, Peterson and Patel10), with epidemiological data suggesting that the intake of flavonoids, which are potent plant-based antioxidants, is associated with a lower risk of CVD development and mortality(Reference McCullough, Peterson and Patel10,Reference Kim and Je11) . Therefore, antioxidant supplements containing flavonoids may be an ideal therapy to protect against ROS-induced vascular dysfunction that may lead to CVD development.
Anthocyanins are considered the largest class of flavonoids in fruits and vegetables(Reference Khurana, Venkataraman and Hollingsworth12). Anthocyanin-rich foods have been shown to mediate endothelium-dependent vasodilation by increasing endothelial NO synthase (eNOS) activity and NO production in the vasculature(Reference Anselm, Socorro and Dal-Ros13,Reference Brixius, Willms and Napp14) . It has also been demonstrated that anthocyanins can scavenge ROS, protect against inflammation, reduce blood pressure (BP), improve skeletal muscle oxygen utility capacity and enhance skeletal muscle recovery following exercise(Reference Wu, Gao and Guo15–Reference Chai, Davis and Zhang21). In addition to anthocyanins, the anti-inflammatory compound called bromelain, which is a mixture of proteases extracted from immature fruits or stems of pineapples(Reference Shing, Chong and Driller22), has also been shown to improve muscle function and reduce inflammation by reducing prostaglandin production and neutrophil migration(Reference Miller, Bailey and Barnes17,Reference Fitzhugh, Shan and Dewhirst19,Reference Buford, Cooke and Redd23) . Additionally, bromelain has been shown to have other cardiovascular benefits such as protection against endothelial damage as well as possessing antithrombotic properties(Reference Felton24,Reference Bahde, Palmes and Minin25) . Therefore, anthocyanins and bromelain have several benefits for scavenging ROS and reducing inflammation, which may help improve vascular function, BP and skeletal muscle oxygen utility capacity. A combination of anthocyanins and bromelain may potentially be an ideal therapeutic intervention for maintaining and/or improving cardiovascular health. However, to our knowledge, no prior studies have examined the acute impacts of these powerful antioxidants in combination in humans. The purpose of this study was to examine the impacts of a supplement rich in anthocyanins and bromelain (combined hawthorn berry extract, tart cherry extract and bromelain supplement (hereafter BE)) on total antioxidant capacity (TAC), endothelial function, resting heart rate (RHR), BP, skeletal muscle oxygen utility capacity and muscular fatigability in healthy young adults. It was hypothesised that an acute intake of BE would improve endothelial function by improving total TAC, which would then reduce resting BP while improving oxygen utility capacity during exercise and muscular fatigue index in this population.
Methods
Participants
Healthy adults (n 18, age 19–35 years, nine males and nine females) who were recreationally active volunteered to participate in this study. Exclusion criteria included (1) any cardiovascular, neurological, metabolic, respiratory or renal diseases, (2) any musculoskeletal conditions or injuries, (3) presence or history of stomach ulcers, (4) prescribed medications or over-the-counter medications, (5) pregnant, trying to become pregnant or breastfeeding women, (6) any history of smoking/current smoker and (7) any allergies to fruits or vegetables. The procedures used in this study were approved by the Institutional Review Board at the University of Nebraska Medical Center, and carried out in accordance with the Declaration of Helsinki. All participants gave written informed consent prior to study enrolment, during which the experimental procedures, probable risks and potential benefits were explained. This study was registered with https://clinicaltrials.gov/ (NCT04312022).
Study design
A randomised, double-blind, placebo-controlled, crossover study design with a 2-week washout period was used. After study enrolment, participants were randomly assigned to either the BE supplement group or the placebo group (Fig. 1). All data collections were performed at the same time of day (±1 h) after an overnight fast, and participants were asked to abstain from caffeine, alcohol, high intake of antioxidant-rich foods or supplements and excessive exercise for at least 48 h prior to their visits. Participants were also informed to not change their dietary habits during the study period. Descriptive measurements of height, weight, BMI, body composition and hand grip strength were taken at both BE and placebo visits. Baseline measurements of RHR, BP, blood sampling for TAC, endothelial function, skeletal muscle oxygen utility capacity during exercise and muscular fatigue index were assessed. Participants then consumed either the BE or placebo, and all baseline measures were repeated 1 h after BE or placebo intake. A 1-h digestion period was chosen because anthocyanins have been shown to reach peak levels in the blood within 1 h post-consumption(Reference Matsumoto, Takenami and Iwasaki-Kurashige26,Reference Stoner, Sardo and Apseloff27) . After 2 weeks, each participant returned for a second visit and the same protocol was repeated with the intervention (BE or placebo) they did not receive during the first visit. All women (n 9) were tested during the early-to-mid follicular (days 1–10) and late-luteal phases (over day 19) of the menstrual cycle in order to avoid confounding effects of endogenous oestrogen on autonomic function(Reference Saeki, Atogami and Takahashi28).
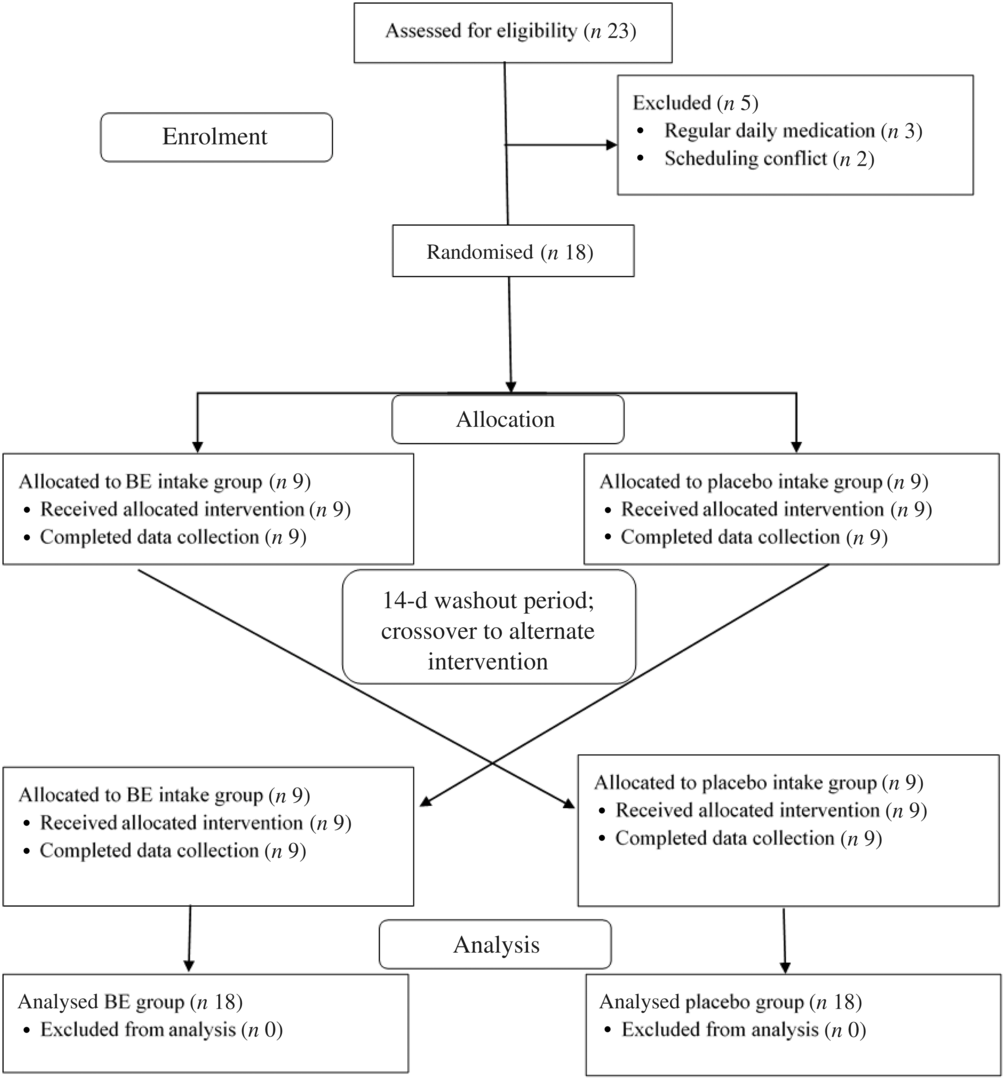
Fig. 1. Double-blinded, randomised, crossover study design, study participant allocation and analysis. BE, combined hawthorn berry extract, tart cherry extract and bromelain supplement.
Supplementation
The supplement used in this study contained three plant-based ingredients, including (1) hawthorn berry extract, derived from the hawthorn berry (Crataegus spp.), (2) tart cherry powder (Prunus cerasus), a rich source of anthocyanins and (3) bromelain, a mixture of proteases obtained from the stems and immature fruits of pineapples. The BE supplement consisted of two capsules with a total of 465, 480 and 400 mg of hawthorn extract, tart cherry powder and bromelain, respectively (CardioEffects, Fitfully LLC). This is similar to that used in previous studies and has reported no adverse side effects(Reference Levers, Dalton and Galvan29,Reference Zick, Gillespie and Aaronson30) . The anthocyanin content for this supplement was provided as two capsules containing about 68 mg of anthocyanins. The separate components include about 67 mg from tart cherry and about 2000 ng from hawthorn berry, which is consistent with previous studies(Reference Levers, Dalton and Galvan29,Reference Rodrigues, Calhelha and Barreira31) . The tart cherry anthocyanin content is similar to about 31 ml of tart cherry juice(Reference Levers, Dalton and Galvan29), while the hawthorn berry anthocyanin content is similar to about 1 g of dried hawthorn berries(Reference Rodrigues, Calhelha and Barreira31). Bromelain is primarily extracted from pineapple stems(Reference Saptarini, Rahayu and Herawati32) and, therefore, is more often given as a supplement than consumed raw. All capsules used in the present study came from the same batch as provided by the manufacturer. The placebo consisted of two tapioca powder capsules that were identical in size and appearance to the BE supplement, but did not possess any antioxidant properties. The capsules were given to each participant by a laboratory member not directly involved with the study.
Anthropometrics
A standard stadiometer was used to measure height to the nearest 0·1 cm. Body mass was measured to the nearest 0·1 kg using a standard scale. BMI was calculated as the body mass divided by the square of height (kg/m2). Percentage body fat was quantified by handheld bioelectrical impedance analysis (BIA) (model HBF-306C; Omron Healthcare, Inc.). Percentage body fat was measured in duplicate, and the average of the two was recorded as percentage body fat.
Resting heart rate and blood pressure
RHR and BP were assessed before and after BE and placebo intake. Participants rested in a seated position in a quiet room for 5 min and were informed not to talk or move during this time. RHR, systolic BP and diastolic BP were measured using an automated sphygmomanometer (Omrom Blood Pressure Monitor BP786N; Omron Healthcare, Inc.) in duplicate. The two measurements of RHR and BP were averaged and recorded as resting values.
Blood sampling
Blood samples were collected from an antecubital vein by a trained phlebotomist using EDTA tubes both before and after BE and placebo intake. Samples were centrifuged at 3500 rpm for 10 min at 4°C. Plasma samples were stored at –30°C for later analysis of TAC. TAC was assessed using a commercially available Total Antioxidant Capacity Assay Kit (catalogue no. ab65329; Abcam) according to manufacturer’s instructions. After incubating the samples at room temperature (23°C), absorbances were measured at 570 nm using a microplate reader. The average intra-assay and inter-assay CV for TAC were 6·3 and 2·9 %, respectively.
Endothelial function
Flow-mediated dilation (FMD) of the brachial artery was used to assess endothelial function before and after BE and placebo intake. FMD is an endothelium-dependent assessment that facilitates brachial artery relaxation in response to an increase in shear stress. FMD was assessed using a Terason uSmart 3300 Doppler ultrasound system (Terason Division Teratech Corporation) and EKG trigger monitor (7700 Series Trigger Monitor; IvyBiomedical Systems, Inc.). An ultrasound probe was used to locate the brachial artery on the participant’s right arm, and a rapid-inflation cuff (E20 Rapid Cuff; D.E. Hokanson) was placed on the forearm distal to the ultrasound probe. A baseline resting brachial artery diameter was recorded for 5 min using an image-capturing system (Vascular Imager; Vascular Research Tools 6, Medical Imaging Applications). The cuff was then inflated to 250 mmHg for 5 min. The cuff was released, and the reactive hyperaemic response of the artery was recorded for 5 min using the ultrasound and image-capturing system. The baseline resting diameter and post-hyperaemic stimulus were analysed using an automated edge detection software (Brachial Analyzer; Vascular Research Tools 6, Medical Imaging Applications). The most stable 30–60 s of baseline artery diameter, including at least ten cardiac cycles, was averaged as the resting diameter(Reference Harris, Nishiyama and Wray33).
Skeletal muscle oxygen utility capacity
Skeletal muscle oxygen utility capacity during a single-leg extension exercise was assessed before and after BE and placebo intake. Oxygenation utility capacity of the vastus lateralis was measured during a leg extension exercise with a commercially available NIRS system (Artinis PortaMon). The PortaMon emits near-IR wavelengths of 850 and 764 nm and has a detection probe to measure returning signals, and data were recorded continuously at 10 Hz to quantify tissue saturation index (StO2, %) and concentrations of both oxygenated Hb (HbO2, in arbitrary units (a.u.)) and deoxygenated Hb (HHb, a.u.).
To determine single-leg extension strength, a one repetition maximum (1RM) test was performed using the participant’s dominant leg. Participants were familiarised with the leg extension technique before 1RM measurement, which was achieved within three attempts. During the 1RM test, the highest weight that could be lifted in good form through a full range of motions was considered. The PortaMon was secured with a commercial double-sided adhesive at one-third of the distance from the lateral femoral epicondyle and the greater trochanter, and the device was adjusted to be on the belly of the vastus lateralis muscle(Reference McLean, Kerherve and Lovell34). The device was wrapped in black, light-absorbing cloth to reduce extraneous light that may affect the signals. Participants were then asked to perform fifteen repetitions at 60 % of their 1RM, while the NIRS data were recorded continuously throughout the exercise protocol.
Fatigability index
Fatigability index was quantified during isokinetic contraction of the participant’s dominant leg before and after BE and placebo intake using a HUMAC NORM Isokinetic Dynamometer (CSMi Solutions). The participants were seated upright with the axis of rotation of the dynamometer arm oriented with the axis of rotation of the participant’s dominant knee. Belts and straps were used to secure the participant to the dynamometer, and participants were instructed to fully extend and flex their knee and to work to their maximal capacity during leg extension exercise. Before the actual test, there were four familiarisation repetitions to test resistance. The test consisted of a mild resistance at 240°/s to induce and measure muscular fatigue. HUMAC 2015 (v.15.000.0103) was used to report the data of fatigue index, which was calculated using the peak torque differences (percentages) on the first and final five repetitions of the exercise bout (fatigue index = (initial peak torque–final peak torque)/initial peak torque × 100). The data obtained from the analysis allowed for the assessment of changes in participants’ baseline measurements.
Statistical analysis
A Shapiro–Wilk test was used to determine normal distribution of the data. Independent t tests were used to evaluate baseline characteristics at BE and placebo visits. Dependent variables were assessed using a 2 × 2 repeated-measures ANOVA (group (BE and placebo) × time (before and after supplement intake)) to determine differences between pre- and post-BE and placebo intake. If a significant effect was noted, paired t-tests were used for post hoc comparisons. All statistical analyses were performed with SPSS 26.0 (IBM). Data are presented as means and standard deviations unless noted otherwise. Statistical significance was set to P < 0·05. It was calculated that a minimum of sixteen participants in a crossover design (sixteen each group, BE and placebo) would enable 80 % power to observe a 3–5 % change in FMD between the two groups(Reference Figueroa, Park and Seo35). An effect size analysis was performed using Cohen’s d; 0·2, 0·5 and 0·8 were interpreted as small, medium and large effect sizes, respectively(Reference Sullivan and Feinn36).
Results
None of the participants reported any adverse events or unfavourable symptoms as a result of BE supplement intake, and all participants (n 18) were included in the final analysis (Table 1). There were significant group × time interactions for FMD, systolic BP, StO2 and HbO2 concentration. Following BE intake, FMD significantly increased (P < 0·05, d = 1·2) from 8·2 (sem 0·8) to 11·7 (sem 0·7) % (Fig. 2), while systolic BP significantly decreased (P < 0·05, d = 0·4) from 114·1 (sem 2·3) to 110·0 (sem 2·4) mmHg (Fig. 3(a)). A time effect was noted for TAC following BE intake (P < 0·05, d = 0·3) from 14·8 (sem 4·5) to 16·3 (sem 5·1) mmol/ml (Fig. 4). Additionally, there were several changes in oxygen utility capacity following BE intake. StO2 was significantly greater at baseline and throughout leg extension exercise (P < 0·05, d = 0·5), HbO2 concentration was significantly greater for the first 10 s of leg extension exercise (P < 0·05, d = 0·5), and ΔHHb concentration was significantly less after the first 5 s of leg extension exercise (P < 0·05, d = 0·3) (Fig. 5). There were no significant differences (P > 0·05) for diastolic BP (Fig. 3(b)), RHR (Fig. 3(c)) or muscle fatigability index (Fig. 6). There were no significant differences between sexes on any study measurement in response to BE intake (P > 0·05).
Table 1. Participant characteristics
(Mean values and standard deviations)
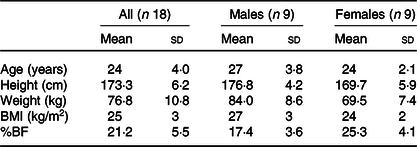
BF, body fat.
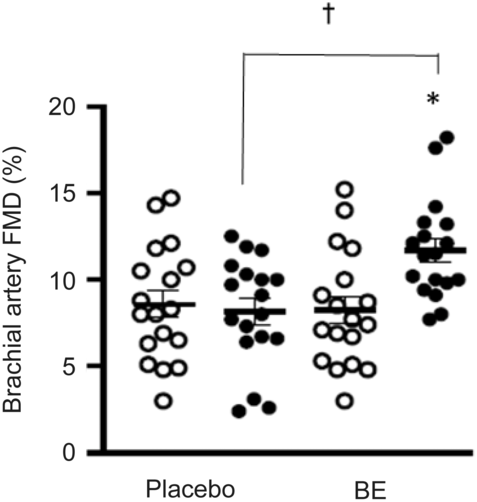
Fig. 2. Flow-mediated dilation (FMD, %) of the brachial artery before and after placebo and berry extract supplement (BE) intake. Brachial artery FMD dilation significantly increased post-BE and was significantly greater than post-placebo, d = 1·2. Values are means with their standard errors. *P < 0·05 v. pre (![]() ). † P < 0·05 v. placebo (
). † P < 0·05 v. placebo (![]() ).
).
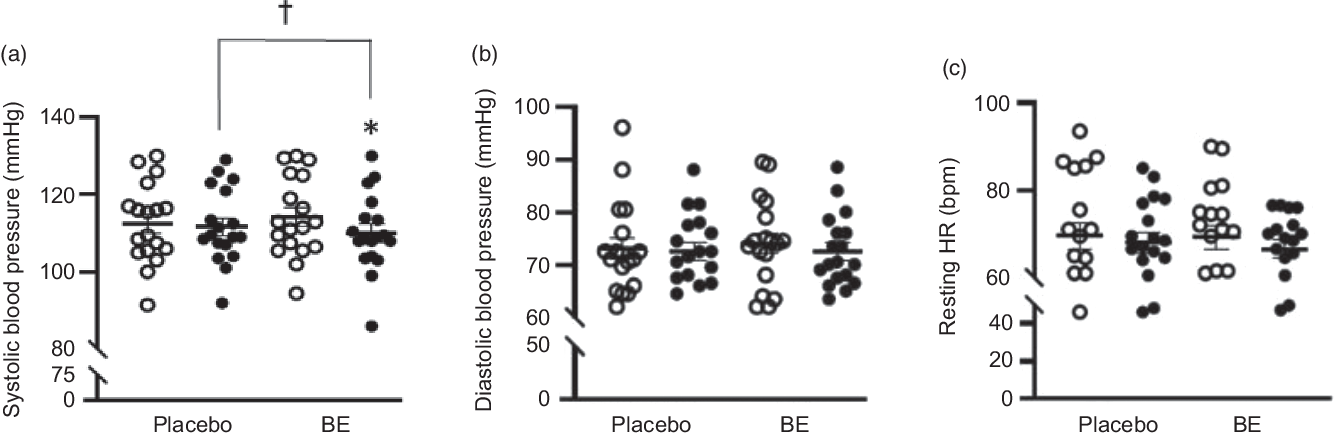
Fig. 3. Systolic blood pressure (mmHg), diastolic blood pressure (mmHg) and resting heart rate (RHR, bpm) before and after placebo and berry extract supplement (BE) intake. (a) Systolic blood pressure reduced post-BE intake, d = 0·4. (b) Diastolic blood pressure showed no changes between pre- and post-placebo and BE intake, d = 0·1. (c) RHR showed no changes between pre- and post-placebo and BE intake, d = 0·1. Values are means with their standard errors. *P < 0·05 v. pre (![]() ). † P < 0·05 v. placebo (
). † P < 0·05 v. placebo (![]() ).
).
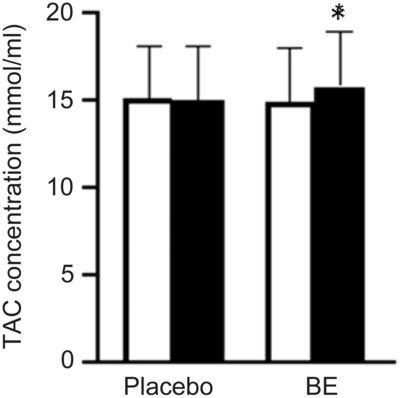
Fig. 4. Group mean changes in total antioxidant capacity (TAC, mmol/ml) before and after placebo and berry extract supplement (BE) intake. TAC was significantly higher post-BE intake compared with pre-BE, d = 0·3. Values are means with their standard errors. *P < 0·05 v. pre (![]() ).
).
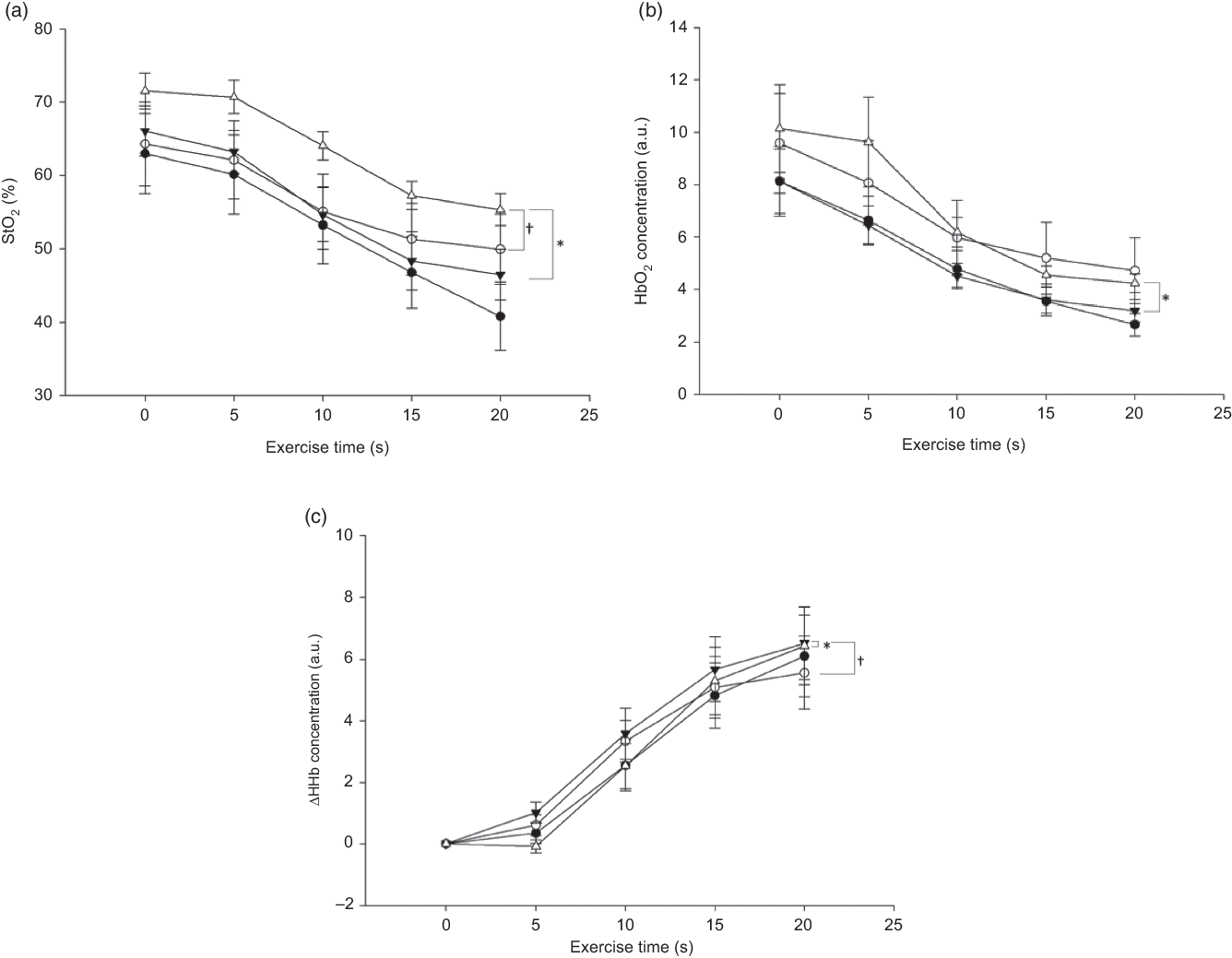
Fig. 5. Group mean changes in tissue saturation index (StO2, %), oxygenated Hb (HbO2) concentration (arbitrary units; a.u.) and change in deoxygenated Hb (ΔHHb) concentration (a.u.) pre- and post-berry extract supplement (BE) and placebo intake every 5 s during leg extension exercise. Values are means with their standard errors. (a) * Pre-BE (![]() ) and post-BE (
) and post-BE (![]() ) significantly different (P < 0·05) at all time points. † Post-placebo (
) significantly different (P < 0·05) at all time points. † Post-placebo (![]() ) and post-BE significantly different (P < 0·05) only at 0, 5, 10 and 20 s, d = 0·5. (b) * Pre-BE and post-BE significantly different (P < 0·05) only at 5 and 10 s, d = 0·5. (c) * Pre-BE and post-BE significantly different (P < 0·05) only at 5 s, d = 0·1. † Post-placebo and post-BE significantly different (P < 0·05) only at 15 and 20 s.
) and post-BE significantly different (P < 0·05) only at 0, 5, 10 and 20 s, d = 0·5. (b) * Pre-BE and post-BE significantly different (P < 0·05) only at 5 and 10 s, d = 0·5. (c) * Pre-BE and post-BE significantly different (P < 0·05) only at 5 s, d = 0·1. † Post-placebo and post-BE significantly different (P < 0·05) only at 15 and 20 s.
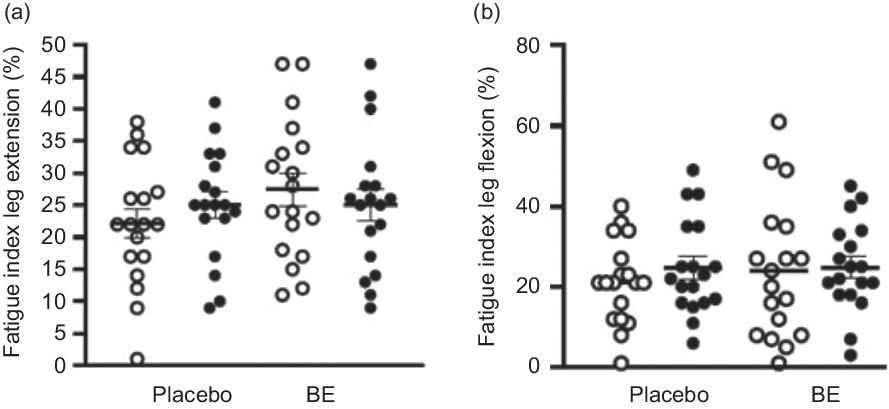
Fig. 6. Muscular fatigue index (%) during leg extension and leg flexion pre- and post-placebo and berry extract supplement (BE) intake. Values are means with their standard errors. (a) Fatigue index during leg extension showed no changes between pre- and post-placebo and BE intake, d = 0·2. (b) Fatigue index during leg flexion showed no changes between pre- and post-placebo and BE intake, d = 0·1. ![]() , Pre;
, Pre; ![]() , post.
, post.
Discussion
The present study was conducted to investigate the effects of a combined anthocyanin and bromelain-rich supplement on endothelial function, BP, TAC, skeletal muscle oxygen utility capacity and muscular fatigability in healthy young adults. The results of this study revealed several novel findings of considerable benefit to this population: (1) brachial artery FMD significantly improved post-BE intake, (2) there was a significant reduction in systolic BP following BE ingestion, (3) TAC increased following BE ingestion and (4) skeletal muscle StO2 and HbO2 concentration significantly increased and ΔHHb concentration decreased during exercise following BE intake. To our knowledge, this is the first study to examine the beneficial effects of BE on endothelial function, BP, TAC and oxygen utility capacity during exercise in humans.
Endothelial function
ROS acts as signalling molecules for cell function regulation(Reference Finkel37); however, excessive ROS could induce oxidative stress damage, thereby contributing to vascular endothelial dysfunction and vascular disease development(Reference Steven, Frenis and Oelze3,Reference Guzik, Mussa and Gastaldi7,Reference Rodrigo, Gonzalez and Paoletto8,Reference Incalza, D’Oria and Natalicchio38) . Excessive ROS production has been reported to negatively affect the endogenous antioxidant defence system and NO bioavailability. The endogenous antioxidant system works to protect against oxidative damage by scavenging ROS and reducing excessive ROS formation(Reference Liu, Ren and Zhang39), while excessive ROS has been reported to uncouple eNOS and also directly scavenge NO, resulting in attenuated endothelial function and reduced NO bioavailability(Reference Wink and Mitchell40). Therefore, upregulating the antioxidant defence system and scavenging excessive ROS may help protect against oxidative damage and NO bioavailability, which may collectively play a role in preserving intact endothelial function and reducing CVD risk.
Several studies have demonstrated that an intake of anthocyanins improves endothelial function in young adults(Reference Rodriguez-Mateos, Feliciano and Boeres41–Reference Istas, Feliciano and Weber43). Our findings are consistent with these studies, as endothelial-dependent vasodilation measured by brachial artery FMD significantly improved following BE intake (Δ3·5 %, d = 1·2) (Fig. 2). Interestingly, the improvement in FMD reported by the present study may be clinically relevant. A study has reported that there is an estimated 13 % decrease in the future risk for cardiovascular events for every 1 % increase in FMD(Reference Inaba, Chen and Bergmann44,Reference Green, Jones and Thijssen45) . Our results indicated an improvement in FMD of about 3·5 % with a large effect size (d = 1·2) following BE intake, suggesting that this increase in FMD might reduce future risks of cardiovascular events by nearly 50 %. It is important to note that this study only examined an acute dose of this supplement. Chronic supplementation studies are needed to fully assess the potential long-term improvements in FMD, cardiovascular risk reduction and clinical relevance in this population.
Previous studies have proposed potential mechanisms underlying improvements in endothelial function, such as scavenging of ROS and improvements in eNOS and NO bioactivity after anthocyanin intake(Reference Anselm, Socorro and Dal-Ros13,Reference Brixius, Willms and Napp14,Reference Howatson, McHugh and Hill20,Reference Chai, Davis and Zhang21,Reference Rodriguez-Mateos, Feliciano and Boeres41–Reference Istas, Feliciano and Weber43,Reference Akila and Devaraj46,Reference Elango, Jayacharan and Niranjali Devaraj47) . Several in vivo and animal studies have previously demonstrated that anthocyanins can improve TAC(Reference Howatson, McHugh and Hill20), restore the activity of antioxidant enzymes(Reference Akila and Devaraj46) and reduce oxidative stress markers(Reference Chai, Davis and Zhang21,Reference Elango, Jayacharan and Niranjali Devaraj47) . In the present study, there was a significant increase in TAC after BE intake (Δ1·5 mmol/ml, d = 0·3) (Fig. 4), which is consistent with previous findings(Reference Howatson, McHugh and Hill20) and may have contributed to the improvement in endothelial function reported by the present study. Improved TAC may serve to reduce excessive ROS, which may in turn reduce ROS-mediated NO scavenging and thereby increase NO bioavailability in the vasculature(Reference Wink and Mitchell40,Reference Endemann and Schiffrin48) . In addition to scavenging ROS, anthocyanins have been reported to mediate endothelial-dependent vasodilation by increasing eNOS activation, which subsequently improves NO bioavailability(Reference Anselm, Socorro and Dal-Ros13,Reference Brixius, Willms and Napp14) . Collectively, these improvements in TAC and potential improvements in ROS, NO bioavailability and eNOS activation may have played a role in improved FMD in the present study. However, further research is needed to clarify these potential mechanisms.
Blood pressure
Previous studies have demonstrated beneficial impacts of anthocyanins derived from berries and cherries on BP. Specifically, acute and chronic intake of tart cherry has been shown to reduce systolic BP in young, middle-aged and older adults, while hawthorn berry extract has demonstrated BP-lowering effects in several clinical populations, such as in patients with heart failure, hypertension and diabetes(Reference Keane, Haskell-Ramsay and Veasey49–Reference Schmidt, Kuhn and Ploch54). Our results are consistent with these studies as there was a significant reduction in systolic BP (Δ –4 mmHg, d = 0·4) following BE intake (Fig. 3(a)). This reduction in BP has clinical relevance. A reduction as little as 2 mmHg in systolic BP might reduce mortality from stroke and coronary heart disease by 6 and 4 %, respectively(Reference Chobanian, Bakris and Black55). A reduction by about 4 mmHg in systolic BP following BE intake suggests a significant potential in preventing cardiovascular complications.
Increases in BP seem to be mediated, at least in part, by reduced NO bioavailability and eNOS dysfunction(Reference Li, Youn and Cai56). Anthocyanins have been reported to scavenge ROS and induce eNOS phosphorylation, both of which may serve to improve NO bioavailability(Reference Brixius, Willms and Napp14,Reference Ljubuncic, Portnaya and Cogan57–Reference Kim, Kang and Kim59) . Although the present study did not investigate the mechanism(s) underlying the reduction in systolic BP, it was likely that an improvement in NO bioavailability after BE intake contributed to this response. Improvement in TAC following BE intake prompted a reduction in excessive ROS within the vasculature, which may have contributed to a reduction in and/or protection against ROS-mediated increase in peripheral vascular resistance and BP reduction(Reference Wink and Mitchell40,Reference Endemann and Schiffrin48) . However, improvements in NO bioavailability and excessive ROS-mediated increases in peripheral vascular resistance warrant further study.
Additionally, there were no changes in diastolic BP after BE intake (Fig. 3(b)). Even though reductions in diastolic BP may be desirable under CVD conditions, abnormally low diastolic BP has been reported to be associated with CHD and mortality(Reference Franklin, Larson and Khan60). Our study population consisted of healthy adults, and therefore their homeostatic regulation may have prevented diastolic BP from reducing beyond the normal range. In the absence of this regulatory mechanism, anthocyanins may potentially exert similar side effects to anti-hypertensive drugs, such as dizziness and headache.
Oxygen utility capacity
ROS and inflammation are indicated as important players in controlling peripheral vascular resistance, skeletal muscle blood flow and thus oxygen delivery and utility capacity(Reference Trinity, Broxterman and Richardson61–Reference Durand, Dharmashankar and Bian63). When pro-oxidative conditions prevail and the redox environment is imbalanced, this excessive ROS can blunt blood flow and attenuate vasodilatory function, which dictates vascular resistance(Reference Donato, Uberoi and Bailey64). Blood flow is considered a key factor for tissue perfusion, and therefore targeting improvements in blood flow may serve to increase tissue oxygen utility capacity(Reference Mortensen, Damsgaard and Dawson65). Antioxidant and anti-inflammatory agents such as anthocyanins and bromelain may stabilise the redox environment by scavenging excessive ROS and reducing inflammation, which will improve blood flow and oxygen utility capacity of the skeletal muscle(Reference Wu, Gao and Guo15,Reference Romano, Fasolino and Pagano18–Reference Chai, Davis and Zhang21) .
There were observed improvements in StO2, HbO2 concentration and ΔHHb concentration at baseline and at several time points throughout the leg extension exercise in the present study (Fig. 5), and these results may suggest that oxygen utility capacity improved following BE intake at baseline and during exercise, further supporting other recent findings regarding anthocyanin intake and skeletal muscle oxygen utility capacity. Morgan et al. demonstrated increases in baseline StO2 following anthocyanin intake(Reference Morgan, Barton and Bowtell66). Notably, in addition to a significant difference in StO2 at baseline, we also found significantly higher StO2 during leg extension exercise. However, it is important to note that our study incorporated single leg extension exercise, while Morgan et al. investigated oxygen utility capacity with cycling exercise(Reference Morgan, Barton and Bowtell66). Therefore, it may be of interest to investigate oxygen utility capacity during exercise requiring greater muscle recruitment (e.g. cycling, running) and at a higher intensity to fully elucidate these oxygen utility changes in response to BE intake.
Additionally, this improvement in oxygen transfer and utility capacity in the skeletal muscle may be relevant to CVD populations. In diseases involving oxidative stress and chronic inflammation, impaired vasodilation, blood flow and oxygen utility capacity has been reported(Reference Trinity, Broxterman and Richardson61,Reference Payne62) , which can negatively affect physical capacity and the quality of life(Reference Kalogeris, Baines and Krenz67,Reference Taylor68) . Intake of this combined supplement may potentially improve the oxygen transport and utility capacity, which may help improve exercise tolerance and the quality of life of these populations.
Fatigability index
Although improvements in oxygen transport and utility capacity following BE intake were found, such improvements were not transferrable to muscle fatigue index improvements during leg extension exercise (Fig. 6). Interestingly, previous studies have demonstrated that a long-term intake of anthocyanins (about 7 d) improved endurance exercise performance and reduced fatigability in highly trained triathletes and cyclists(Reference Morgan, Barton and Bowtell66,Reference Willems, Myers and Gault69,Reference Cook, Myers and Blacker70) , while an acute intake of anthocyanins demonstrated no effect on time-to-exhaustion during high-intensity cycling exercise(Reference Keane, Bailey and Vanhatalo50). Our supplement regimen could not improve fatigability, and these results are consistent with previous literature. Longer-term supplementation (i.e. ≥7 days) may be warranted to determine the impacts of this BE supplement on fatigue index.
During exercise, ROS is critical for cell signalling and plays a role in promoting exercise adaptations(Reference Trinity, Broxterman and Richardson61,Reference Sachdev and Davies71,Reference Thannickal and Fanburg72) . In healthy young individuals, exercise-induced ROS production assists with proper vasodilation and blood flow, and the endogenous antioxidant defence system is able to sufficiently clear exercise-induced ROS(Reference Trinity, Broxterman and Richardson61). Additionally, an exogenous intake of antioxidants to further reduce ROS in healthy individuals might negatively impact this regulatory function, potentially causing the redox environment to become more pro-oxidative in nature, which would impair blood flow and vasodilatory function(Reference Trinity, Broxterman and Richardson61). Therefore, an acute intake of BE before leg endurance exercise may not have significantly affected ROS signalling associated with skeletal muscle fatigability and performance in these healthy individuals.
Sex differences
There were no significant differences in any study measures based on sex. Therefore, it may be inferred that this BE supplement might similarly affect both sexes. However, the case of older adults may be different. Men and women demonstrate different rates of vascular function decline, with men experiencing it earlier in life compared to women, and women specifically experiencing accelerated vascular decline following menopause(Reference Celermajer, Sorensen and Spiegelhalter73,Reference Taddei, Virdis and Ghiadoni74) . Sex differences in vascular reactivity and oxygen utility capacity following BE intake may be more detectable in these older adults. Investigation on older populations’ response to BE intake is warranted.
Limitations
This study has several limitations. First, although our study examined a novel combination of anthocyanins and bromelain on vascular function and oxygen utility capacity, we did not investigate their synergistic effects. Future studies should include an experimental group with anthocyanin intake only and a control group with bromelain intake only to better understand the synergistic effects. Second, we did not apply strict control over diet or exercise. Participants were asked to avoid antioxidant supplements and antioxidant-rich foods for 48 h prior to visits and to not change their diet during the study period, which was based on self-report, and dietary logs were not maintained by participants. More intakes of some foods and minerals over others may impact measurements, as an increased intake of dietary fat may negatively impact the redox environment(Reference Das, Mandala and Bhattacharjee75), while an increased intake of sodium may have undesirable effects on BP and endothelial function(Reference Dickinson, Clifton and Keogh76). Participants were also asked to avoid excessive exercise for at least 48 h prior to both visits, as excessive strenuous exercise may negatively impact redox balance. However, our study population likely had adequate endogenous antioxidant defence mechanisms to alleviate the ROS induced by exercise(Reference Trinity, Broxterman and Richardson61,Reference Sachdev and Davies71) . Lastly, although we demonstrated improvements in oxygen utility during leg extension exercise, it did not inform us about oxygen utility under activities of greater muscle recruitment (e.g. running) and higher-intensity exercise, which requires further investigation.
Conclusion
Our results revealed for the first time that a combined anthocyanin and bromelain-rich antioxidant supplement showed acute improvements in endothelial function, systolic BP, TAC and skeletal muscle oxygen transport and utility capacity both at rest and during exercise. This BE supplement has a potential to help improve and support vascular health in healthy populations. Further research is needed to demonstrate whether these results may translate into clinical benefits and if this supplement may offer a potential therapeutic approach for CVD populations.
Acknowledgements
We thank Jiwon Song (University of Oklahoma) for his help with data analysis. We are also grateful to the participants.
This work was supported by the University of Nebraska at Omaha Graduate Research and Creative Activity (GRACA) grant. The GRACA grant had no role in the design, analysis or writing of this article. The supplement was provided by Fitfully, LLC, Omaha, NE.
The contributions of the authors were as follows: formulating the research question: E. J. P., J. S. and S.-Y. P.; designing the study: E. J. P. and S.-Y. P.; carrying out the study: E. J. P., J. S., R. J. H., G. L., S. K. Y., S. D. S. and S.-Y. P; analysing the data: E. J. P., J. S., R. J. H., G. L., S. K. Y., S. D. S. and S.-Y. P.; draft preparation: E. J. P. and S.-Y. P.; revising the article: E. J. P., J. S., R. J. H., G. L., S. K. Y., S. D. S. and S.-Y. P.; approval of the final version submitted: E. J. P., J. S., R. J. H., G. L., S. K. Y., S. D. S. and S.-Y. P.
The authors declare that there are no conflicts of interest.










