Folate is a water-soluble B vitamin, which plays an important role in the synthesis and methylation of DNA together with other B vitamins including vitamin B2, vitamin B6 and vitamin B12 as crucial cofactors in one-carbon metabolism(Reference Kim1). Dietary inadequacies of these nutrients can contribute to negative health outcomes. For example, inadequate folate intake has been linked to the risk of anaemia(Reference Carmel2), neuropsychiatric disorders(Reference Stanger, Fowler and Piertzik3) and neural tube defects(Reference Wolff, Witkop and Miller4). It has also been shown that insufficient intakes of folate, vitamin B6 or vitamin B12 are associated with elevated plasma homocysteine concentrations, a potential risk factor for CVD(5, Reference Ntaios, Savopoulos and Grekas6). Furthermore, folate deficiency leads to the disruption of DNA synthesis, repair and methylation(Reference Blount, Mack and Wehr7), which may increase the risk of developing some cancers, notably colorectal cancer(Reference Kim8).
Foods rich in folate include vegetables, especially green leafy vegetables, such as spinach and beet leaf, and dried peas and lentils as well as fruits, nuts and seeds(Reference Gnagnarella, Salvini and Parpinel9). Animal sources include offal such as beef and chicken liver(Reference Gnagnarella, Salvini and Parpinel9). The synthetic form of the vitamin, folic acid, is widely used for the purpose of food fortification and food supplements. In some countries, folic acid fortification of many cereal-based food products became mandatory, e.g. in the USA(10), Canada(Reference De Wals, Tairou and Van Allen11) and Chile(Reference Hirsch, de la Maza and Barrera12), after consideration of compelling evidence for a protective effect of periconceptional folic acid supplementation against neural tube defects(Reference Kim13). However, so far, no European countries have introduced mandatory folic acid fortification, although voluntary fortification is accepted in some countries including France and the UK(Reference Bouckaert, Slimani and Nicolas14).
Despite the suggested beneficial effects of folate on many different diseases, there has been growing concern regarding possible adverse effects of excessive levels of folate intake(Reference Kim13, Reference Osterhues, Holzgreve and Michels15–Reference Hubner, Houlston and Muir17). Increased folic acid intake has been shown to have the potential for masking the diagnosis of a vitamin B12 deficiency, particularly in the elderly(Reference Morris, Jacques and Rosenberg18, Reference Reynolds19). In addition, animal and human studies have reported that a high dose of folic acid may promote the progression of already existing premalignant and malignant lesions, particularly in colorectal cancer settings(Reference Cole, Baron and Sandler20–Reference Ulrich and Potter22). It is therefore important to monitor folate status of those groups of people who are at an increased risk of cancer and who are more likely to be exposed to higher doses of folic acid intakes(Reference Ulrich and Potter23) besides those who are at a risk of low folate intake.
Deharveng et al. (Reference Deharveng, Charrondiere and Slimani24) reported earlier a lack of clarity and consistency in the terminology and definitions used for folate information in the food composition tables available in nine countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. More recently, an evaluation of folate data in eighteen European and international databases concluded that a lack of comparability still exists between countries(Reference Bouckaert, Slimani and Nicolas14). Consequently, studies that estimated dietary folate intakes across European countries to date relied on each country's own food composition data which tend to be heterogeneous(Reference de Bree, van Dusseldorp and Brouwer25–Reference Finglas27). Thus, there is a great need to develop a more accurate and standardised database to enhance comparability and consistency of folate data across Europe.
In the absence of a reference European nutrient database, the EPIC Nutrient DataBase (ENDB) project was initiated to harmonise separate nutrient databases using common procedures and guidelines, with support from the local national compilers in ten countries in the EPIC(Reference Slimani, Deharveng and Unwin28). The ENDB was first completed for twenty-six priority components to provide a standardised reference instrument for calibrating the EPIC dietary measurements at the nutrient level(Reference Slimani and Margetts29). This work has been extended to cover other nutrients including folate, and has just been completed(Reference Nicolas and Witthöft, Vignat30). In the present descriptive study, we present and compare the levels and food sources of dietary folate intake obtained by means of a single 24 h dietary recall (24-HDR) collected from a representative sample of the EPIC cohort using standardised folate concentration data, recently compiled as an extension of the ENDB.
Materials and methods
Study population, design and dietary assessment
The rationale and methods of the EPIC study have previously been described in detail(Reference Riboli, Hunt and Slimani31–Reference Bingham and Riboli33). The EPIC cohort consists of twenty-three subcohorts in ten European countries (Denmark, France, Greece, Germany, Italy, The Netherlands, Norway, Spain, Sweden and UK), providing a wide range of cancer occurrence rates, lifestyle and dietary habits. EPIC participants were mostly recruited from the general population residing within defined geographical areas between 1992 and 2000, with some exceptions: women members of a health insurance for school employees (France); women attending breast cancer screening (Utrecht, The Netherlands); blood donors (some centres in Italy and Spain) and a cohort consisting predominantly of vegetarians (‘health conscious’ cohort in Oxford, UK). In France, Norway, the Utrecht centre of The Netherlands and in the Naples centre of Italy, all participants were women. For the purposes of the present study, the initial twenty-three EPIC administrative centres have been redefined into twenty-seven geographical regions relevant to the analysis of dietary consumption patterns(Reference Slimani, Kaaks and Ferrari34). Approval for the study was obtained from the ethical review boards of the International Agency for Research on Cancer (Lyon, France) and from all local recruiting institutes. All EPIC participants provided informed consent.
Within the design of the EPIC study, a subsample of each study centre was randomly (age, sex stratified) chosen for the application of a standardised 24-HDR assessment gathered by means of a computerised software (EPIC-Soft®; International Agency for Research on Cancer, Lyon, France)(Reference Slimani, Deharveng and Charrondiere35, Reference Slimani, Ferrari and Ocke36). This subcohort is referred to as the EPIC Calibration substudy and was undertaken between 1995 and 2000. Each participant provided a single 24-HDR in a face-to-face interview(Reference Slimani, Deharveng and Charrondiere35), except in Norway where it was obtained by a telephone interview(Reference Brustad, Skeie and Braaten37). In total, complete 24-HDR information was available from 36 994 participants (13 486 men and 23 508 women), representing approximately 8 % of the entire EPIC cohort. This sample has been shown to be a reasonably representative sample of the entire EPIC cohort(Reference Slimani, Kaaks and Ferrari34). A total of 36 034 participants with 24-HDR data were included in the present analysis, after exclusion of 960 participants under 35 or over 74 years of age because of low participation in these age categories. Using the EPIC-Soft®, information on the intake of all foods and beverages was collected, described, quantified and coded according to common rules. The classification of the EPIC-Soft® food groups and food subgroups used in the calibration study is derived from a system described in detail elsewhere(Reference Slimani, Kaaks and Ferrari34).
Dietary folate intake was estimated using the updated ENDB(Reference Nicolas and Witthöft, Vignat30). Although the ENDB values were obtained from country-specific food composition tables, they were standardised as much as possible across the EPIC countries by matching of the EPIC foods to the national databases according to the recommendation from the recent review(Reference Bouckaert, Slimani and Nicolas14). In particular, a microbiological assay was chosen as the reference analytical method for folate values in the ENDB. Folate values of unavailable foods were derived by values either from recipe calculation or borrowed from similar foods(Reference Nicolas and Witthöft, Vignat30). During the ENDB compilation for folate, particular attention has been given to the issue of fortification of breakfast cereals, particularly in the UK and France as their cereal consumption was substantially higher compared with other EPIC countries. In Scandinavian countries and in The Netherlands, fortification was not allowed at the time of data collection. In other EPIC countries, breakfast cereal consumption was very low and the information on folic acid-fortified foods was not always available(Reference Bouckaert, Slimani and Nicolas14). It was therefore decided not to adopt the dietary folate equivalent conversion, which considers different bioavailability of naturally occurring folate and synthetic folic acid.
Data on age as well as body weight and height in most centres were self-reported by the participants during the 24-HDR interview. Data on other lifestyle factors, including total physical activity, educational level, smoking history and alcohol intake, considered in the present analysis were collected at baseline through standardised questionnaires and clinical examinations, and have been described elsewhere(Reference Riboli, Hunt and Slimani31, Reference Slimani, Kaaks and Ferrari34, Reference Friedenreich, Cust and Lahmann38–Reference Haftenberger, Schuit and Tormo40). The physical activity questions being asked in the Umeå (Sweden) and Norwegian centres were different from the EPIC core questions(Reference Haftenberger, Schuit and Tormo40) and the information was omitted from the analyses of the present study. The mean time interval between these baseline questionnaire measures and the 24-HDR interview varied by country, from 1 d to 3 years after(Reference Slimani, Kaaks and Ferrari34).
The folate data described here come from food intakes only, as our main interest is in dietary folate levels in European populations (not from dietary supplements). However, we present the level of dietary folate intake of those who reported being dietary supplement users compared with those who did not, as they may differ from each other with regard to dietary characteristics(Reference Kirk, Cade and Barrett41). Dietary supplement information was obtained at the end of the 24-HDR with a question, ‘Did you take any dietary supplements?’ with yes or no answers, followed by further questions on the name of the supplements, the frequency of use on the recalled day and the number of units taken per consumption occasion(Reference Skeie, Braaten and Hjartaker42). For the present study, a dietary supplement user was defined as any subject who reported taking at least one dietary supplement on the recalled day. A folic acid-containing supplement user was defined as any subject who reported taking at least one dietary supplement containing folic acid on the recalled day. Nevertheless, it should be noted that quantitative nutrient data derived from food supplements were not available for the present study.
Statistical methods
Folate intake (μg/d) was calculated as least square means and standard errors by sex, age (10-year categories from 35–74 years) and EPIC centre (ordered according to a geographical south–north gradient). The mean intake was adjusted for age, total energy intake, weight and height and weighted by season of the 24-HDR collection (spring, summer, autumn and winter) and day of the week of recall (Monday to Thursday; Friday to Sunday) to control for different sampling procedures of the 24-HDR interviews across seasons and days of the week.
Stratified analyses were performed to describe differences in folate intake levels according to level of physical activity (inactive, moderately inactive, moderately active and active; data completeness>86 %), smoking status (never smoker, former smoker and current smoker; completeness>98 %), daily alcohol intake (abstainers, < 12 g/d, 12 to < 24 g/d and ≥ 24 g/d, as presented in an earlier report(Reference Sieri, Agudo and Kesse43); no missing data), educational level (none/primary, technical/secondary, university or higher; completeness>98 %) and season (spring, summer, autumn and winter; no missing data). When analyses were stratified by season, the mean intake of folate was not weighted by season. Further analyses were stratified by dietary supplement use (yes, no). In the stratified analysis, sex- and centre-specific mean intakes are presented across variables of interest in main tables. Tests for trend were performed by allocating scores for participants in each category of lifestyle factors. P values were derived from t tests for continuous variables. The main food groups contributing to folate intake are presented as the mean percentage of intake (percentage of total intake, derived from the unadjusted 24-HDR data); the contribution of a subgroup was given as a percentage of the food group. The categorisation into food groups and food subgroups is common across the centres and is adapted from the EPIC-Soft® food classification system as described elsewhere(Reference Slimani, Ferrari and Ocke36, Reference Slimani, Fahey and Welch44). All analyses were conducted using SAS statistical software (version 9.1, SAS Institute, Cary, NC, USA).
Results
Intake of folate
Table 1 shows the adjusted mean folate intake (μg/d) presented by each centre and age categories at recruitment in men and women. Daily folate intake in all centres, except in the UK health conscious group, ranged from 250 to 350 μg/d in men and 200 to 300 μg/d in women. Intake was higher in centres in Spain, France and in the UK general population and was relatively low in the Swedish centres, especially in Malmö in both men and women, but with no obvious geographical gradient (Fig. 1). In the UK health conscious group, where participants are mainly vegetarians or vegans, folate intake was markedly higher, averaging approximately 480 μg/d in men and approximately 360 μg/d in women. There was neither a consistent nor a strong trend observed in folate intake across age categories, although it tended to increase with age in men from Greece, Asturias (Spain) and Heidelberg (Germany) and women from Ragusa (Italy), The Netherlands and South and East Norway. Additional adjustment for smoking status and alcohol consumption did not materially alter the results in men and women (data not shown).
Table 1 Mean intake of folate (μg/d)* by centre ordered from south to north, sex and age group†
(Number of participants, mean values with their standard errors)

* Adjusted for age (when not stratified for age), total energy intake, weight and height and weighted by season and day of recall.
† P trend was derived from allocating scores 1, 2, 3 and 4 for all participants in the age group of 35–44, 45–54, 55–64 and 65–75 years, respectively.
‡ If fewer than twenty persons are present in a certain age group, mean intake is not presented.
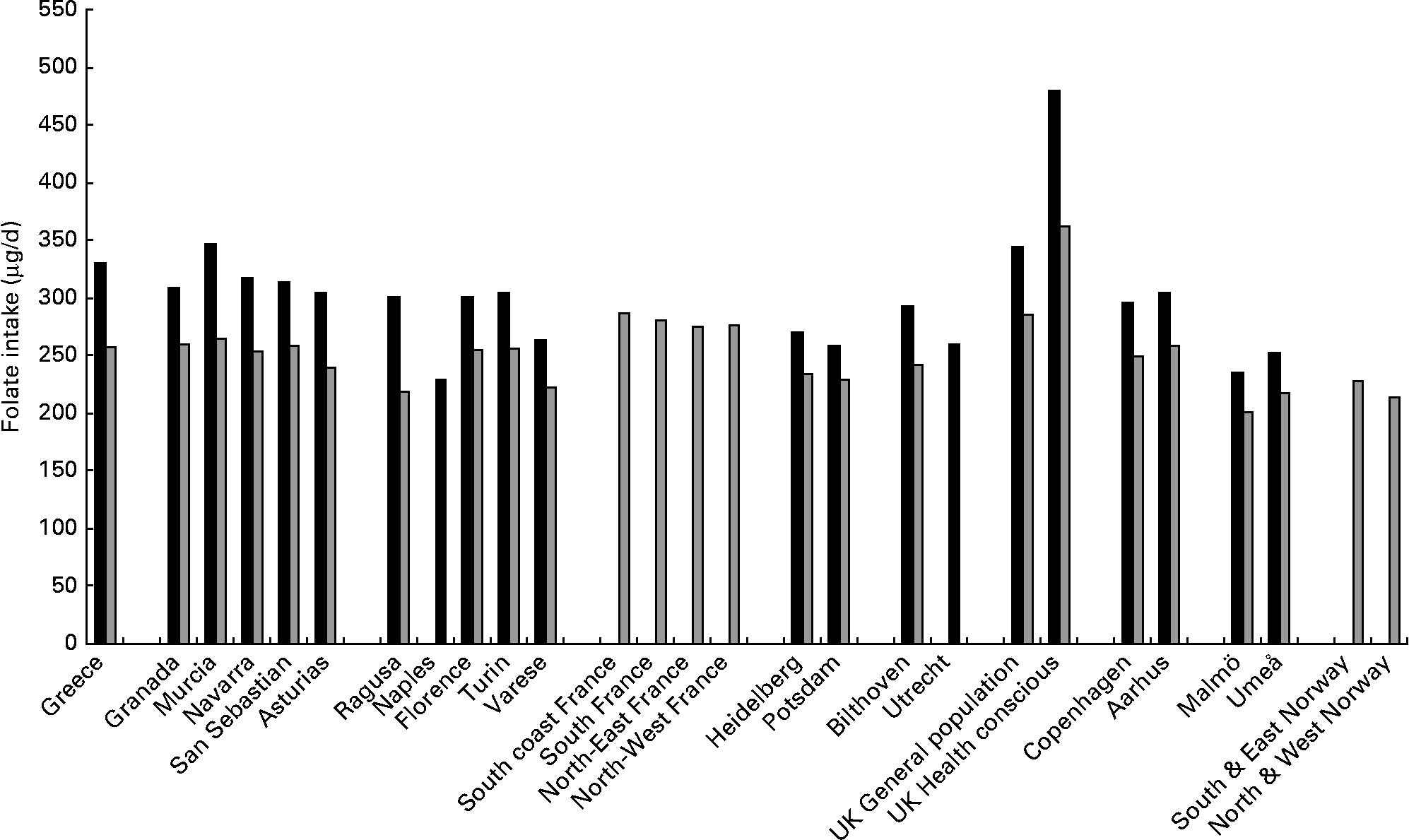
Fig. 1 Mean intake of folate (μg/d) in men (■) and women (![]() ), stratified by centre ordered from south to north, adjusted for age, total energy intake, weight and height and weighted by season and day of recall.
), stratified by centre ordered from south to north, adjusted for age, total energy intake, weight and height and weighted by season and day of recall.
Intake of folate stratified by lifestyle factors, educational level and season
Tables 2 and 3 present adjusted folate intake in each centre according to lifestyle factors in men and women. There were no systematic differences observed in the levels of folate intake when participants were stratified according to different physical activity levels (Table 2). Compared with those with lower educational level, participants with university or higher degree were likely to report higher folate intake, with the tendency being clearer in women (Table 2). Folate intake varied according to smoking status and level of alcohol intake (Table 3). Though not entirely consistent in all centres, the mean intake of folate was lower among current smokers and among heavier alcohol drinkers. No systematic variations were observed for folate intake according to the season of 24-HDR collection, although folate intake in southern European countries seemed lowest in summer (see Table S1 of the supplementary material, available online at http://www.journals.cambridge.org/bjn).
Table 2 Mean intake of folate (μg/d)* by centre, according to levels of physical activity and education†
(Mean values with their standard errors)

* Adjusted for age, total energy intake, weight and height and weighted by season and day of recall.
† P trend was derived by allocating scores for participants in each category of lifestyle factors.
Table 3 Mean intake of folate (μg/d)* by centre, according to smoking and alcohol status†
(Mean values with their standard errors)

* Adjusted for age, total energy intake, weight and height and weighted by season and day of recall.
† P trend was derived by allocating scores for participants in each category of lifestyle factors.
Dietary supplement use and folate intake
On average, approximately 22 % of men and 34 % of women reported using any type of dietary supplements during the recalled day, of which 33 and 25 %, respectively, reported using folic acid-containing supplements (Table 4). Total dietary folate intake was generally higher in users of any type of supplement in most centres. An exception to this was men in the UK health conscious group, where more than 50 % of the participants were supplement users. The proportion of folic acid-containing supplement users was low, especially in participants from the southern European countries (Table 4). Dietary folate intake among those who consumed folic acid-containing supplements varied across the countries.
Table 4 Mean intake of folate (μg/d)* by country, according to use of supplements (any types† and folic acid-containing supplement‡)§
(Number of participations, mean values with their standard errors)

* Adjusted for age, total energy intake, weight and height and weighted by season and day of recall.
† Any type of supplement user was defined as any subject who reported taking at least one dietary supplement on the recalled day.
‡ Folic acid-containing supplement user was defined as any subject who reported taking at least one dietary supplement containing folic acid on the recalled day.
§ P value was derived from t tests and is not presented for comparisons with fewer than ten persons.
Common dietary sources of folate intake
Table 5 shows the main food groups contributing to the intake of folate in men and women in each country. Among all the food groups, vegetables, cereals and fruits, nuts and seeds were the main contributors of dietary folate intake in all centres (Fig. 2 and see Table S2 of the supplementary material, available online at http://www.journals.cambridge.org/bjn). On the one hand, vegetables in Greece and Italy accounted for approximately 30 % of folate intake in men and more than 30 % in women. There was a clear north–south difference in the source of vegetable contribution to folate intake, with leafy and fruiting vegetables being the major vegetable source of folate in the southern countries and cabbages and root vegetables being the major vegetable source in the northern countries (Fig. 3).
Table 5 Percentage contribution of main food groups to the intake of folate by country in men and women


Fig. 2 Percentage contribution of all food groups (![]() , miscellaneous;
, miscellaneous; ![]() , soups, bouillons;
, soups, bouillons; ![]() , sugar and confectionery;
, sugar and confectionery; ![]() , cakes and biscuits;
, cakes and biscuits; ![]() , condiments and sauces;
, condiments and sauces; ![]() , egg and egg products; □, fish and shellfish;
, egg and egg products; □, fish and shellfish; ![]() , alcoholic beverages;
, alcoholic beverages; ![]() , non-alcoholic beverages;
, non-alcoholic beverages; ![]() , potatoes and other tubers;
, potatoes and other tubers; ![]() , meat and meat products;
, meat and meat products; ![]() , legumes;
, legumes; ![]() , dairy products;
, dairy products; ![]() , fruits, nuts and seeds;
, fruits, nuts and seeds; ![]() , cereal and cereal products; ■, vegetables) to the intake of folate by centre ordered from south to north in (a) men and (b) women.
, cereal and cereal products; ■, vegetables) to the intake of folate by centre ordered from south to north in (a) men and (b) women.

Fig. 3 Percentage contribution of vegetable sources (![]() , mixed salad, mixed vegetables;
, mixed salad, mixed vegetables; ![]() , stalk vegetables, sprouts; □, grain and pod vegetables;
, stalk vegetables, sprouts; □, grain and pod vegetables; ![]() , mushrooms;
, mushrooms; ![]() , onion, garlic;
, onion, garlic; ![]() , root vegetables;
, root vegetables; ![]() , cabbages;
, cabbages; ![]() , fruiting vegetables; ■, leafy vegetables) to the intake of folate by country ordered from south to north in (a) men and (b) women.
, fruiting vegetables; ■, leafy vegetables) to the intake of folate by country ordered from south to north in (a) men and (b) women.
On the other hand, cereals were the most important dietary source of folate intake in both men and women in the UK general population, where breakfast cereals accounted for 43 % of cereal and cereal products as source of folate (Fig. 4). This was quite distinctive from other centres where bread was the main source of folate intake among cereal products, contributing more than 80 %. An exception to this was Italy, where pasta, rice and dough such as pizza together with bread were the major cereal-based source of folate intake.

Fig. 4 Percentage contribution of cereal sources (![]() , salty biscuits, aperitif biscuits, crackers;
, salty biscuits, aperitif biscuits, crackers; ![]() , flour, flakes, starches, semolina;
, flour, flakes, starches, semolina; ![]() , dough and pastry (puff, short-crust, pizza);
, dough and pastry (puff, short-crust, pizza); ![]() , pasta, rice, other grains;
, pasta, rice, other grains; ![]() , breakfast cereals; ■, bread, crispbread, rusks) to the intake of folate by country ordered from south to north in (a) men and (b) women.
, breakfast cereals; ■, bread, crispbread, rusks) to the intake of folate by country ordered from south to north in (a) men and (b) women.
Both non-alcoholic (e.g. fruit and vegetable juices) and alcoholic beverages (e.g. wine and beer) were also some of the main sources of folate intake in the northern countries (Table 5). In the UK health conscious group, the condiments and sauces and miscellaneous food groups (consisting primarily of special vegan/vegetarian food items including soya-based products and non-dairy cheeses) were found to account for 15 % of folate in men and 13 % in women while these food groups contribute less than 2 % of intake in all other centres (Fig. 2).
Discussion
In the present study, we compared standardised dietary folate intake across ten European countries according to selected lifestyle factors and major contributing food sources. Dietary folate intake did not differ greatly between centres apart from the UK centres, especially the UK health conscious group where a much higher intake was observed compared with other centres. The average dietary folate intake observed in the present study without the UK health conscious group was 307 μg/d for men and 252 μg/d for women, after adjusting for participants' age, total energy intake, weight and height and the effect of season and day of recall.
There have been few published data that investigated the level of dietary folate intake at the national level across European countries. A study by de de Bree et al. (Reference de Bree, van Dusseldorp and Brouwer25) reported mean dietary folate intake in 18–64-year-old adults of 291 μg/d (range 197–326) for men and 247 μg/d (range 168–320) for women in nine European countries by reviewing their national food consumption surveys. In 2005, a final report on the European project on folate estimated that average dietary folate intakes in participants aged 18–90 years were 283 μg/d (range 218–352) for men and 238 μg/d (range 207–284) for women in eight European countries on the basis of each country's food consumption and food composition data(Reference Finglas27). It is not clear in these studies whether folate values were adjusted for energy intake and other covariates. More recently, the European Nutrition and Health Report has summarised that dietary folate equivalent (i.e. 1 μg food folate = 0·5 μg folic acid = 0·6 μg folic acid taken with meals, values unadjusted) ranged from 203 to 494 μg/d in men and 131 to 392 μg/d in women aged 19–64 years in twenty-one participating countries(Reference Elmadfa26).
While these studies provide an overview on the folate status in European countries attempting to use nationally representative data, none of these used a standardised nutrient database across countries, limiting the comparability of the studies included in each report due to the use of different dietary assessment methods, different years and periods of data collection and different age classifications. It has also been pointed out that quantification methods, terminologies and mode of expression used in folate data varied across food composition tables in different countries in the absence of a reference European nutrient database(Reference Bouckaert, Slimani and Nicolas14). We describe for the first time the dietary folate intake in ten European countries using a folate database that has been standardised across those countries.
In the present study, we observed a relatively high dietary folate intake in both of the UK centres. In the UK, folic acid has been added to most breakfast cereals since 1987, with the amount of added folic acid being further increased in 1994, followed by a considerable increase in dietary folate intake(Reference Henderson, Gregory and Irving45) as well as in blood folate levels(Reference Clarke, Sherliker and Hin46). Considering that cereal and cereal products, notably breakfast cereals, were the main contributor of folate intake, high folate intake in the UK centres may be partly explained by voluntary fortification of breakfast cereals. Indeed, in the other EPIC countries, breakfast cereals were not a big contributor of folate intake, as they are neither much consumed nor widely fortified. In addition to the relatively high cereal consumption, the vegetarian diet practised in the UK health conscious group resulted in an exceptionally high dietary folate intake compared with the other centres included in the present study. Vegetarian (legume/soya-based) dishes are a good source of folate and the consumption of these food items (categorised as miscellaneous foods in the present study) in this health conscious group was four (e.g. compared with The Netherlands or France) to forty times (compared with Greece) as high as the one in other EPIC countries (data not shown).
We also showed some different patterns in the dietary folate intake between the southern and northern European countries. While the intake of folate did not differ statistically significantly according to the season that the 24-HDR was collected, folate intake in Greece, Spain and Italy appeared to be lower in summer compared with the northern countries. This may be partly due to the fact that the green leafy vegetables, one of the major dietary source of folate in those countries, are broadly known as an early-spring or a late-autumn crop(Reference Caron and Walker47). Furthermore, both alcoholic and non-alcoholic beverages contributed to a greater proportion of folate intake in the northern countries. It should be noted that there was an approximately 10-fold difference in non-alcoholic beverage consumption between the southern and northern countries (data not shown). A higher proportion of folate intake was accounted for by alcoholic beverages in countries such as Germany, The Netherlands, the UK and Denmark, where beer, a source of folate, is the main source of alcohol consumption(Reference Sieri, Agudo and Kesse43).
Men, in general, in the present study had a substantially higher folate intake than did women. This may be attributed to the fact that men tend to have larger body size and to consume more food compared with women. These differences in folate intake between men and women seemed slightly larger (approximately 45 %) in the southern European centres compared with those in the northern centres. This may reflect to some extent a different dietary pattern which is specific to each sex and to each centre. For example, consumption of vegetables, especially leafy vegetables, one of the biggest source of dietary folate, was much higher in men compared with women in the southern countries such as Greece, Spain and Italy, while little difference by sex was observed in northern countries(Reference Agudo, Slimani and Ocke48). Similarly, consumption of cereal, meat and certain alcoholic beverages, which were also the sources of folate intake, was considerably higher in men from the southern countries compared with women (data not shown). In the case of beer and wine consumption, there was up to a 5-fold difference in the level of consumption in men and women in those countries(Reference Sieri, Agudo and Kesse43).
The present results also showed that folate intake tended to be lower among current smokers and heavier alcohol drinkers. Previous studies have found that smokers have lower plasma and erythrocyte folate concentrations compared with non-smokers(Reference Piyathilake, Macaluso and Hine49–Reference Walmsley, Bates and Prentice52) even after adjusting for dietary folate intake(Reference Mannino, Mulinare and Ford50, Reference Walmsley, Bates and Prentice52). Similarly, heavy alcohol drinkers have been observed to have lower circulating concentration of folate(Reference Gloria, Cravo and Camilo53, Reference Cravo, Gloria and Selhub54) as well as lower dietary folate intake(Reference Manari, Preedy and Peters55). Chronic ethanol intake has been shown to interfere with folate metabolism(Reference Cravo, Gloria and Selhub54, Reference Mason and Choi56). This indicates that smokers and heavy alcohol drinkers whose folate bioavailability is low and who may have additionally lower dietary folate intake are at an increased risk of suboptimal folate status and therefore need to be carefully monitored.
To our knowledge, this is the first and largest study that evaluated dietary folate intake using standardised methods across European countries. Dietary folate intake in the present study was derived from the ENDB, a nutrient database that has been standardised across all the countries participating in the EPIC study(Reference Slimani, Deharveng and Unwin28). In addition, the 24-HDR was validated against independent biomarkers in the EPIC(Reference Slimani, Bingham and Runswick57, Reference Al-Delaimy, Ferrari and Slimani58) and the interview procedures were standardised, with interviewers receiving substantial training in the use of the software(Reference Slimani, Ferrari and Ocke36). This comparison also benefits from the unique setting offered by the EPIC, a Europe-wide study with heterogeneity in dietary habits and in other lifestyle factors across countries(Reference Bingham and Riboli33).
However, some limitations of the present study need to be discussed. First, in the present study, only one set of 24-HDR was collected from a large population sample with the ultimate purpose to have a good and sufficiently valid estimate of mean population intakes, and this approach was not designed to provide true long-term individual intakes due to the lack of information on intra-individual variability(Reference Willett59) or intake distribution (several repeated 24-HDR would have been required for this aim). Second, the 24-HDR was undertaken between 1995 and 2000 and may not reflect the most up-to-date information on folate intake in Europe. For example, voluntary fortification of foods with folic acid has been more widely practised in the European Union since 2006 under the regulation on the addition of vitamins and minerals to foods(60). Nonetheless, the present study still provides valid and important information on the level of dietary folate intake in European countries before voluntary fortification was widespread. Further, this standardised information across European countries improves our understanding on the differences in dietary folate exposure across European countries and its relationship to cancer and other chronic diseases through studies carried out within the EPIC.
Another limitation is that not all of the EPIC centres applied population-based sampling, and thus a direct comparison of the present results to the general population of each region should be made with caution(Reference Riboli, Hunt and Slimani31). Nevertheless, the subsample used for the present study has been shown to be representative of the entire EPIC cohort(Reference Slimani, Kaaks and Ferrari34).
In the present study, we did not consider the differential bioavailability of food folate and folic acid added to fortified foods using the dietary folate equivalent conversion. However, apart from the UK and France, folic acid fortification was not a common practice in Europe at the time of the data collection, resulting in considerably low consumption of the folic acid-fortified foods in those countries. Similarly, as mentioned earlier, the contribution of folic acid added to supplements has not been taken into account in estimating total folate intakes because although we had data for types of supplements consumed, quantitative data on dietary supplements were not available(Reference Skeie, Braaten and Hjartaker42). Nonetheless, folic acid-containing supplements were not one of the most frequently consumed types of supplements in this study(Reference Skeie, Braaten and Hjartaker42). Moreover, the primary aim of the present study was to investigate dietary folate intake from food sources in European populations with diverse dietary practices. Given the controversial role of high folic acid intakes, older adults, especially those who may have pre-existing lesions or be at risk for a marginal vitamin B12, should be carefully examined in total amounts of folate uptake and should be encouraged to meet the folate requirement by food sources.
In summary, we described dietary folate intake across the EPIC cohorts in ten European countries using a recently developed standardised nutrient database. The present results showing sex- and region-specific differences in folate intake may serve as a good basis for epidemiological research of cancer and other chronic diseases in relation to folate status. Considering that any dietary intake assessment methods are associated with measurement errors to some extent(Reference Bingham61), future priorities should be given to the use of biomarker data of folate status, in addition to the standardised dietary data, in the beneficial context of international settings.
Acknowledgements
We thank all of the study participants for their cooperation and all of the persons who participated in the fieldwork studies in each EPIC centre. We particularly thank Jérôme Vignat for his expertise in the compilation of the folate nutrient database. This study was undertaken during a tenure of a Postdoctoral Fellowship from the International Agency for Research on Cancer, partially supported by the European Commission FP7 Marie Curie Actions – People – Co-funding of regional, national and international programmes (J. Y. P.), and was supported by the World Cancer Research Fund (grant no. 2008/51), and by the ‘Europe Against Cancer’ Programme of the European Commission. The cohort studies included in the EPIC study received funding from the following: Public Health and Consumer Protection Directorate 1993–2004; Research Directorate-General 2005; Ligue contre le Cancer (France); Société 3M (France); Mutuelle Générale de l'Education Nationale; Institut National de la Santé et de la Recherche Médicale (INSERM); Institut Gustave Roussy; German Cancer Aid; German Cancer Research Centre; German Federal Ministry of Education and Research; Danish Cancer Society; Health Research Fund of the Spanish Ministry of Health; Spanish Regional Governments of Andalucía, Asturias, Basque Country, Murcia and Navarra and the Catalan Institute of Oncology; ISCIII RETIC (RD06/0020), Spain; Cancer Research UK; Medical Research Council, UK; Stavros Niarchos Foundation and the Hellenic Health Foundation (Greece); Italian Association for Research on Cancer; Italian National Research Council, Regione Sicilia (Sicilian government); Associazione Iblea per la Ricerca Epidemiologica – ONLUS (Hyblean association for epidemiological research, NPO); Dutch Ministry of Public Health, Welfare and Sports; Dutch Prevention Funds; LK Research Funds; Dutch ZON (Zorg Onderzoek Nederland); World Cancer Research Fund (WCRF); Swedish Cancer Society; Swedish Research Council; Regional Government of Skane and the County Council of Vasterbotten, Sweden; Norwegian Cancer Society; the Norwegian Research Council and the Norwegian Foundation for Health and Rehabilitation. The authors' responsibilities were as follows: J. Y. P., G. N., H. F., I. R., A. S., C. B., V. C., S.-C. C., P. V. and N. S. constituted the writing group, conducted the statistical analysis and prepared the manuscript; G. N. compiled the folate nutrient database; N. S. supervised the preparation of the manuscript; all other authors contributed to and/or supervised the collection and analysis of dietary data and/or provided comments and suggestions on the intermediate and final manuscripts. All authors read and approved the final manuscript. None of the authors had a personal or financial conflict of interest.











