Acid–base homeostasis is vital for an organism's biochemical and physiological activities. In fish, acid–base homeostasis can be challenged by changes in the environment (e.g. water salinity(Reference Genz, Taylor and Grosell1), pH(Reference Janssen and Randall2)), diet composition (e.g. mineral ions(Reference Dersjant-Li, Verreth and Schrama3)) and physical activities (e.g. burst/sustained swimming(Reference Wood, Turner and Graham4)). Despite these external and internal challenges, the acid–base homeostasis (pH) of blood is maintained within strict limits. It is regulated through one of the following: (1) instantaneous buffering capacity of blood bicarbonate and non-bicarbonate buffers; (2) respiratory compensation by the reversible CO2+H2O ↔ H++HCO3 buffer system (to a very limited extent in fish); (3) branchial compensation by the influx and efflux of ions to and from the external medium(Reference Evans, Piermarini and Choe5). Earlier studies on acid–base regulation in freshwater and seawater teleosts have generated substantial knowledge on anatomical and physiological aspects at the organ and molecular levels(Reference Claiborne, Edwards and Morrison Shetlar6–Reference Wood8). The gill has been identified as the major organ regulating pH homeostasis in fish by the passive and active exchange of ions(Reference Wood, Kajimura and Bucking9). The majority of studies on acid–base regulation as well as osmoregulation have been carried out in starved fish. However, several studies have demonstrated that feeding has a significant influence on acid–base homeostasis and postprandial alkaline tide in fish, e.g. Gulf toadfish (Opsanus beta)(Reference Taylor and Grosell10), rainbow trout (Oncorhynchus mykiss)(Reference Cooper and Wilson11, Reference Bucking, Fitzpatrick and Nadella12) and killifish (Fundulus heteroclitus)(Reference Wood, Bucking and Grosell13). However, little is known about the effects of diet composition (e.g. the dietary electrolyte balance (dEB)) on acid–base homeostasis in fish.
The dEB is defined as the sum of mineral cations minus the sum of mineral anions present in the diet. In animal nutrition, dEB is often simplified by restricting it to the difference in only the major ions of the diet (i.e. as Na+K − Cl, in mEq/kg(Reference Mongin14)). A lower dEB indicates a more acidic property of the feed and a higher dEB a more alkaline property of the feed. The dEB contents of the ingredients used in commercial feed formulations exhibit a large variation due to the differences in cation (Na, K, Ca and Mg) and anion (Cl and P) contents(Reference Patience15). The dEB of commercial fish feeds calculated using literature data(Reference Tacon and De Silva16) shows that dEB varies between 200 and 400 mEq/kg diet. The concept of an optimal dEB is well recognised in livestock nutrition and physiology (see reviews in Riond(Reference Riond17) and NRC(18)), and consequently dEB is taken into account during feed formulation. In contrast to, for example, pigs(18), the recommendations for dEB in fish are lacking(19).
In pigs, cattle and poultry, dEB values above and below the optimum have been reported to affect acid–base homeostasis and consequently energy metabolism as well as feed intake and growth performance(Reference Dersjant-Li, Schrama and Heetkamp20–Reference Borges, Fischer da Silva and Ariki23). In fish, studies on the impact of dEB are limited and have provided inconsistent outcomes among different fish species. For instance, in rainbow trout, growth rate and feed conversion were unaffected by the changes in dEB from − 90 to +638 mEq/kg diet(Reference Wilson, Cowey and Adron24), whereas the changes in dEB ( − 146 to +828 mEq/kg diet) affected the growth, feed intake and energy expenditure in African catfish (Clarias gariepinus)(Reference Dersjant-Li, Verreth and Evers25, Reference Dersjant-Li, Verreth and Tijssen26). This discrepancy in growth response to dEB in fish is possibly confounded by dietary composition, e.g. the use of purified diets(Reference Wilson, Cowey and Adron24) and the toxicity of salts used to change dEB (e.g. NH4Cl(Reference Dersjant-Li, Verreth and Evers25, Reference Dersjant-Li, Verreth and Tijssen26)). So far, a clear proof for an optimal dEB level in fish is lacking.
In mammals, about 20–35 % of total cell energy is spent on maintaining an electrochemical gradient across the plasma membrane by ATP-dependent ion transporters (Na/K-ATPase and Ca-ATPase(Reference Rolfe and Brown27)). As with mammals, osmoregulation as well as acid–base regulation in fish is generally considered to be costly in terms of energy. Suboptimal dEB levels in the feeds of fish are expected to elicit compensatory mechanisms at the gut level, either increasing or decreasing stomach acid secretion. As a consequence, this might temporarily impose systemic metabolic alkalosis or acidosis after feeding(Reference Bucking and Wood28). These disturbances of whole-fish acid–base homeostasis will then trigger compensatory mechanisms to bring back the systemic acid–base balance to the normal level, which are hypothesised to alter the energy budget (i.e. increase the amount of energy spent on maintenance processes).
Therefore, the objective of the present study was to investigate the effect of dEB on the growth, nutrient digestibility and energy budget, in particular, maintenance energy expenditure (MEm), of fish. For this purpose, Nile tilapia were fed a fixed ration with diets differing in dEB ( − 100, 200, 500 or 800 mEq/kg diet) and changes in stomach chyme and blood pH, growth and energy balance were assessed.
Experimental methods
The present experiment was approved by the Ethical Committee Judging Animal Experiments (DEC) of Wageningen University (DEC code: 2009121.b) in accordance with the Dutch law on animal experiments and conducted at the aquatic research facility ‘De Haar Vissen’ of Wageningen University.
Diets, fish and experimental procedures
In the present study, four diets were formulated to have different dietary electrolyte balance (dEB) levels ( − 100, 200, 500 and 800 mEq/kg) with a similar basal nutrient and ingredient composition (Table 1). The contrast in dEB (i.e. Na+K − Cl mEq/kg) between the diets was achieved by adding different amounts of Na+ (as Na2CO3) and Cl− (as CaCl2.2H2O) to the basal ingredient while keeping the K+ ion constant. The addition of CaCO3 and Diamol (acid-insoluble ash, inert filler) ensured, respectively, an identical Ca2+ content and a similar volume of the test ingredients among the diets. The diets were prepared by an extrusion process, resulting in a pellet size of about 3 mm (Research Diet Services).
Table 1 Ingredient and nutrient contents of the experimental diets
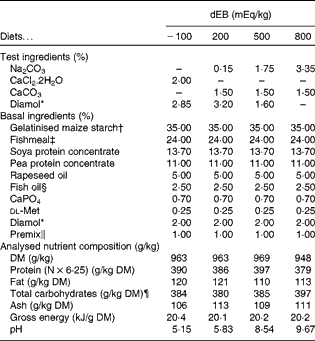
dEB, dietary electrolyte balance.
* Diamol GM; Franz Bertram.
† Merigel 100, Tate & Lyle; Amylum Europe NV.
‡ Fishmeal LT (90 % blue whiting and 10 % sprat; crude protein content 72 %) Triple Nine Fish protein.
§ Triple Nine Fish oil.
∥ Mineral premix composition (mg/kg diet): Fe (as FeSO4.7H2O), 50; Zn (as ZnSO4.7H2O), 100; Co (as CoSO4.7H2O), 2·4; Cu (as CuSO4.5H2O), 5; Se (as Na2SeO3), 1; Mn (as MnSO4.4H2O), 25; Mg (as MgSO4.7H2O), 300; Cr (as CrCl3.6H2O), 1; I (as CaIO3.6H2O), 5. Vitamin premix composition (mg/kg diet): thiamin, 30; riboflavin, 30; nicotine acid, 200; pantothenic acid, 100; pyridoxine, 30; cyanocobalamin, 0·05; ascorbyl phosphate, 500; α-tocopheryl acetate, 200; folic acid, 15; retinylacetate, 15; cholecalciferol, 4; menadione nicotinamide bisulphite (51 %), 8; inositol, 400; choline (as choline chloride), 1000; antioxidant butylated hydroxytoluene (E300-321), 100; calcium propionate, 1000.
¶ Calculated as follows: Total carbohydrates = 1000 − (protein+fat+ash).
Juvenile male Nile tilapia (Oreochromis niloticus) of ‘Red, Manzala and Manila’ strains were obtained from Til-Aqua International. After the acclimatisation of the fish to the experimental facility, 320 fish were weighed individually and randomly allocated among sixteen rectangular glass tanks (120 litres), twenty fish (initial body weight (BW) 92·2 (sd 3·8) g) per tank. The experimental tanks were connected to a common recirculating water system, which shared similar water. Each diet was randomly assigned to these tanks in quadruplicate. The experiment lasted for 6 weeks, which consisted of 5 weeks of a energy balance period followed by 1 week of a recovery period before postprandial sampling for blood and stomach chyme pH. Throughout the study, the fish were hand-fed once a day (09.00 hours) with a daily fixed amount of feed per total biomass per tank in all the diet groups. This was done to avoid variation within and between the dietary treatments. When mortality occurred in a tank, the amount of feed given was reduced accordingly. In addition, after each feeding, the uneaten feed was collected from the tanks and the number of uneaten feed pellets was counted to accurately determine the mean feed intake of the fish. In addition, faeces were collected using a swirl separator (17 litres; AquaOptima AS) during the energy balance period for assessing nutrient digestibility. The fish (Nile tilapia) were reared in freshwater throughout the experiment with optimal water quality parameters (photoperiod, 12 h dark–12 h light; temperature, 27 ± 0·09°C; conductivity, 2·30 (sd 0·46) μS/cm; pH, 7·5 (sd 0·15); dissolved oxygen, 5·6 (sd 0·74) mg/l; NH4+, 0·28 (sd 0·83) mg N/l; NO2−, 0·07 (sd 0·05) mg N/l).
Sampling and measurements
At the start of the experiment, a sample of twenty fish was taken for the analysis of initial body composition. At the end of the energy balance period (5 weeks), the fish were weighed individually to determine their growth. In addition, ten fish per tank were sampled to analyse the final body composition. The fish sampled for body composition were euthanised using an overdose of anaesthesia (2-phenoxyethanol) and were stored at − 20°C until further analysis. At 1 week after the end of the energy balance period, the fish were sampled at 3 and 7 h postprandially to measure blood pH, stomach chyme pH and DM. At each postprandial time point (3 and 7 h), ten fish per treatment were sampled from duplicate tanks (five fish/tank). Under anaesthesia (0·25 parts per million, 2-phenoxyethanol), blood was drawn from the heart and caudal vein (below the vertebrae) of the fish using a 1 ml heparinised syringe and the pH was measured (pH meter, WTW pH 320; pH electrode, WTW SenTix Sp). It should be noted that blood collected from the caudal vein in some cases might have been mixed with the arterial blood. The duration that the fish were under anaesthesia (2 min) and the fish-handling period were strictly standardised for all fish to minimise the variation in blood pH due to sampling. After blood collection, the fish were individually weighed, euthanised and dissected to collect chyme from their stomach and the amount of chyme and chyme pH (pH meter, WTW pH 320; pH electrode, WTW SenTix Sp) were measured. The collected chyme was pooled per tank and analysed for DM. The pH of the experimental diets was measured by dissolving 2·0 g of a ground sample in 10 ml of distilled water. The samples used for the analysis of final body composition and faeces were pooled per tank to analyse the proximate composition. A chemical analysis of the feed, faeces and fish carcasses was carried out in triplicate. DM and ash were determined gravimetrically: DM after drying at 103°C for 4, 8 and 24 h until a constant weight was obtained, respectively, for the feed, freeze-dried faecal and fish samples (ISO 6496, 1983); ash after incineration at 550°C for 4 h (ISO 5984, 1978). Acid-insoluble ash was measured by dissolving the residues obtained after ash determination in HCl (ISO 5985, 1981). Protein (N × 6·25) was determined by the Kjeldahl method (ISO 5983, 1979) and fat by petroleum–diethyl ether extraction (ISO, 6492, 1999). Before fat analysis, the feed and faecal samples were hydrolysed by boiling for 1 h with 3 m-HCl. Energy was measured using a bomb calorimeter (IKA-C-7000; IKA-Analysentechnik).
Calculations
The percentage of fish survival was calculated as (N f/N i) × 100, where N f is the final number of fish and N i is the initial number of fish. Growth (in g/d) was calculated as (W f− W i)/T, where W f is the mean final BW (in g), W i is the mean initial BW (in g) and T is the experimental period. Actual feed intake (g DM/fish per d) was calculated as FItot/T, where FItot is the sum of daily feed intake per fish corrected for fish mortality and uneaten feed (in g DM) and T is the length of the experimental period (in d). The apparent digestibility coefficients (ADC, in %) of DM, protein, fat, total carbohydrates and energy were calculated by comparing the amount of acid-insoluble ash (as an inert marker) in the feed and faeces in relation to the content of the nutrient in the feed and faeces as described previously(Reference Saravanan, Schrama and Figueiredo-Silva29). The gross N intake (mg N/fish per d) was calculated as the product of total feed intake (g DM/fish per d) and N content of the feed (mg/g). The digestible N intake (mg N/fish per d) was calculated as the product of gross N intake and ADC of N (%). Faecal N loss (mg N/fish per d) was calculated as the difference between gross N intake and digestible N intake. The retained N (mg N/fish per d) was calculated as the difference between the N content of the final fish carcass and that of the initial fish carcass. Branchial and urinary N loss (BUN) was calculated as the difference between digestible N intake and retained N. The parameters of energy balance (kJ/fish per d) were calculated as follows: gross energy intake as the product of feed intake (g DM/fish per d) and energy content of the diet; digestible energy intake as the product of gross energy intake and ADC of energy; metabolisable energy intake (MEI) as the difference between digestible energy intake and branchial and urinary energy loss (BUE), which was estimated as BUE = (BUN × 24·85)/1000, where 24·85 is the amount of energy (in kJ) equivalent to 1 g excreted N, assuming that all N is excreted as NH3–N; retained energy (RE) as the difference between the energy content of the final fish carcass and that of the initial fish carcass. Heat production was calculated as the difference between MEI and RE. Metabolisable MEm was estimated as (MEI − (RE/0·65)), where 0·65 is the efficiency of energy utilisation for growth(Reference Schrama, Saravanan and Geurden30). MEm was divided by the geometric mean metabolic BW of the fish to be expressed in kJ/kg0·8 per d.
Statistical analyses
Statistical analyses were performed using SAS 9.2 (SAS Institute). In the 500 dEB group, at the end of the experiment, one fish was found to be missing from one of the replicate tanks, so data from this tank were excluded from the statistical analyses. The data were tested for linear and quadratic relations with dEB levels using PROC GLM. The normal distribution of the residuals was verified with the Kolmogorov–Smirnov test using PROC UNIVARIATE. The residuals of the ADC of DM and fat, RE as protein, and stomach chyme pH at 7 h were not normally distributed, and log transformation fulfilled the assumptions to obtain the statistical inference. The level of significance was set at P< 0·05.
Results
Fish performance
The percentage of fish survival was 94 % (averaged over the diet groups) during the experimental period and was unaffected by dEB (P>0·05). No significant differences were observed in the absolute feed intake between the diet groups (approximately 1·7 g DM/fish per d) since the fish were fed restrictively. Despite a similar feed intake, the growth (g/d) and final BW (g) of the fish were affected by dEB (Tables 2 and 3). Growth decreased linearly with increasing dEB levels (Table 3). Similarly, the final body fat content of the fish decreased linearly with increasing dEB levels (P< 0·05; Table 3). The other constituents of the final body composition were unaffected by dEB (P>0·05): present, on average, across the experimental diets 260 (sd 7·4) g/kg for DM, 155 (sd 2·5) g/kg for protein, 59 (sd 13·4) g/kg for ash and 6·0 (sd 0·3) kJ/kg for energy.
Table 2 Effect of different dietary electrolyte balance (dEB) levels on the growth performance, fish body composition, nutrient digestibility and energy balance of Nile tilapia* (Mean values and standard deviations)
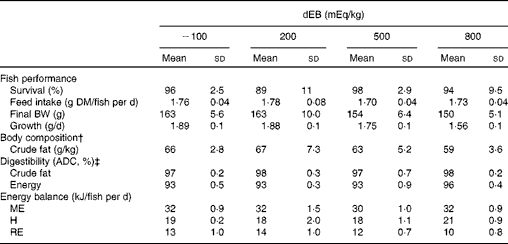
BW, body weight; ADC, apparent digestibility coefficient; ME, metabolisable energy intake; H, heat production; RE, retained energy.
* Number of observations (n) used: n 4 for variables of fish performance, body composition, digestibility and energy balance (except for the 500 dEB group, n 3).
† Mean DM, protein, ash and energy of the final body composition are mentioned in the text (refer under ‘Fish performance’ in the Results section).
‡ ADC of nutrients.
Table 3 Relationship between the dependent variables and dietary electrolyte balance (dEB) (−100, 200, 500 and 800 mEq/kg) in Nile tilapia

BW, body weight; L, linear effect; ADC, apparent digestibility coefficient; Q, quadratic effect; ME, metabolisable energy intake; H, heat production; RE, retained energy; REf, retained energy as fat; REp, retained energy as protein; MEm, maintenance energy expenditure.
* P< 0·05, ** P< 0·01, *** P< 0·001.
† Number of observations (n) used in the statistical procedure: n 4 for variables of fish performance, body composition, digestibility and energy balance; for chyme DM at 3 and 7 h, n 2; for chyme pH and blood pH in the heart and caudal vein, n 10 at 3 h and 7 h.
‡ ADC of nutrients.
Nutrient digestibility
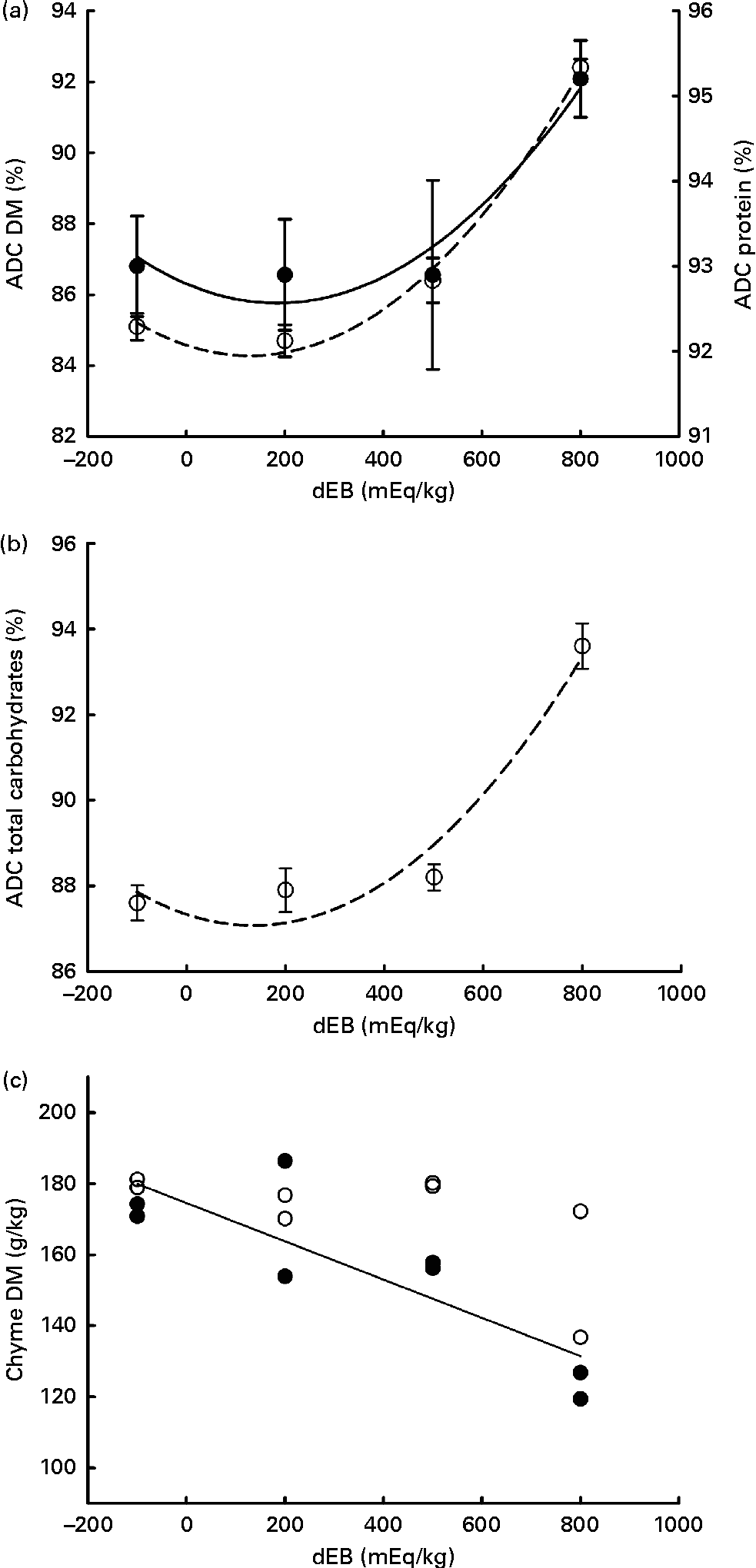
The ADC of DM, protein, total carbohydrates and energy were curvilinearly related to the dEB levels (P< 0·05). For these nutrients, the fish fed the 800 dEB diet had a higher ADC compared with those fed the other experimental diets (Table 3; Fig. 1(a) and (b)). In contrast to that of the other nutrients, the ADC of fat was unaffected by dEB (Table 3).

Fig. 1 Apparent digestibility coefficient (ADC, %) and chyme DM of Nile tilapia fed diets with varying dietary electrolyte balance (dEB) levels. (a) Quadratic relation between the ADC of protein (●), DM (○) and dEB levels. (b) Quadratic response of total carbohydrates (○) to the dEB levels. (c) Relationship between the DM of chyme at 3 h (○) and 7 h (●) and the dEB levels; at 7 h, the DM of chyme decreased linearly with increasing dEB levels. Values are means, with standard deviations represented by vertical bars. See Table 3 for equation and significance level.
Energy balance
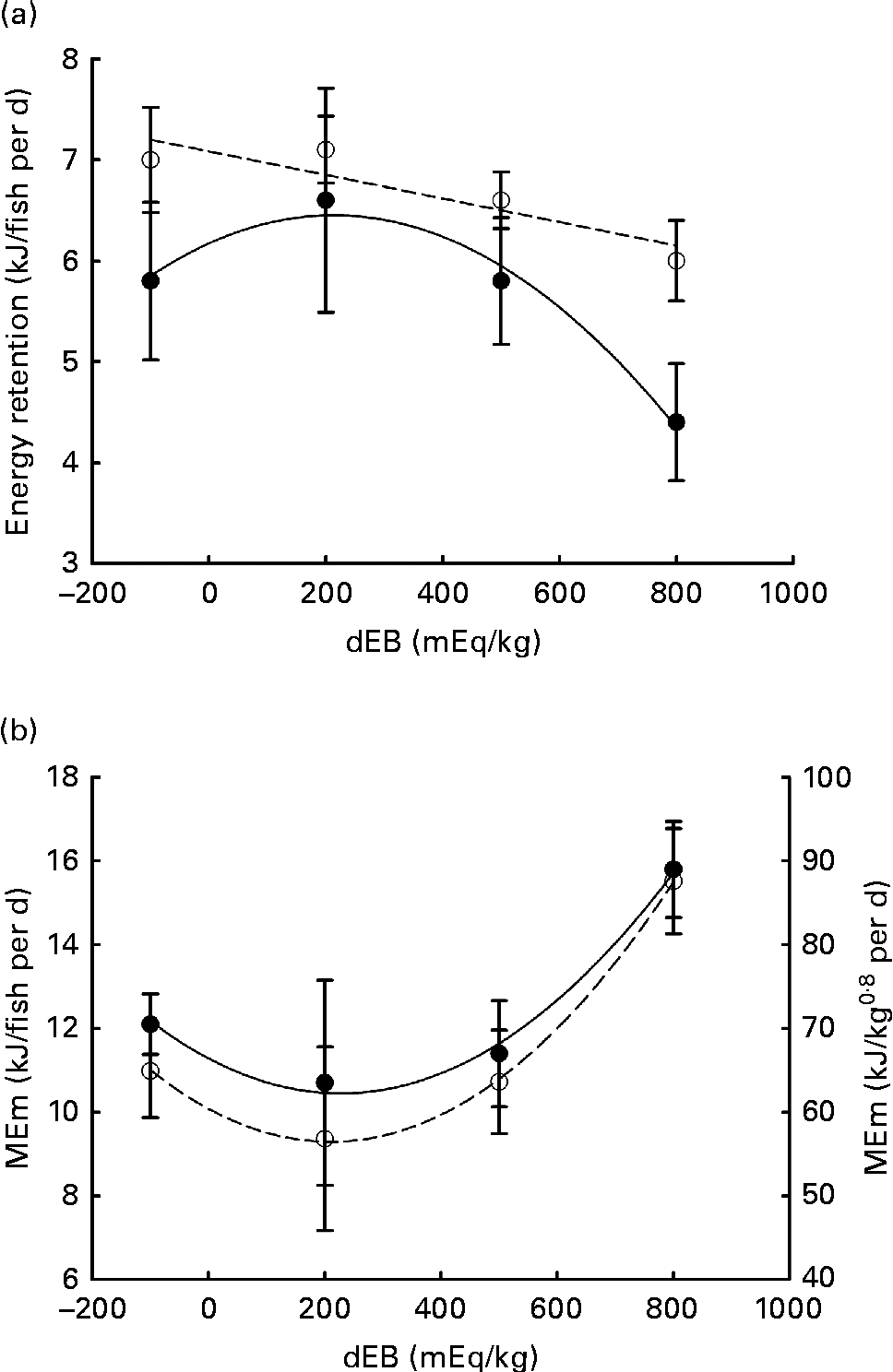
Gross energy intake, digestible energy intake and MEI of the fish were unaffected by the dEB levels (P>0·05), being 35·3, 33·0 and 31·7 kJ/fish per d averaged over the diets, respectively. Heat production was quadratically affected by the dEB levels (P< 0·05; Table 3). RE as protein was linearly related to the dEB levels (Fig. 2(a)). However, RE, RE as fat and MEm were curvilinearly related to the dEB levels, with the lowest MEm and the highest RE and RE as fat being observed at approximately 200 mEq/kg diet. MEm increased as the dEB level either increased or decreased from approximately 200 mEq/kg diet (Fig. 2(b); Table 3). The fish fed the 800 dEB diet spent about 54 % more energy (kJ/kg0·8 per d) on maintenance than those fed the 200 dEB diet.

Fig. 2 Parameters of energy balance as affected by the dietary electrolyte balance (dEB) levels in Nile tilapia. (a) Linear response of retained energy as protein (○) and quadratic response of retained energy as fat (●) to increasing levels of dEB. (b) Curvilinear response of maintenance energy expenditure (MEm) to the dEB levels in Nile tilapia, MEm in kJ/fish per d (●) and MEm in kJ/kg0·8 per d (○). Values are means, with standard deviations represented by vertical bars. Level of significance and equation are given in Table 3.
Chyme characteristics and blood pH
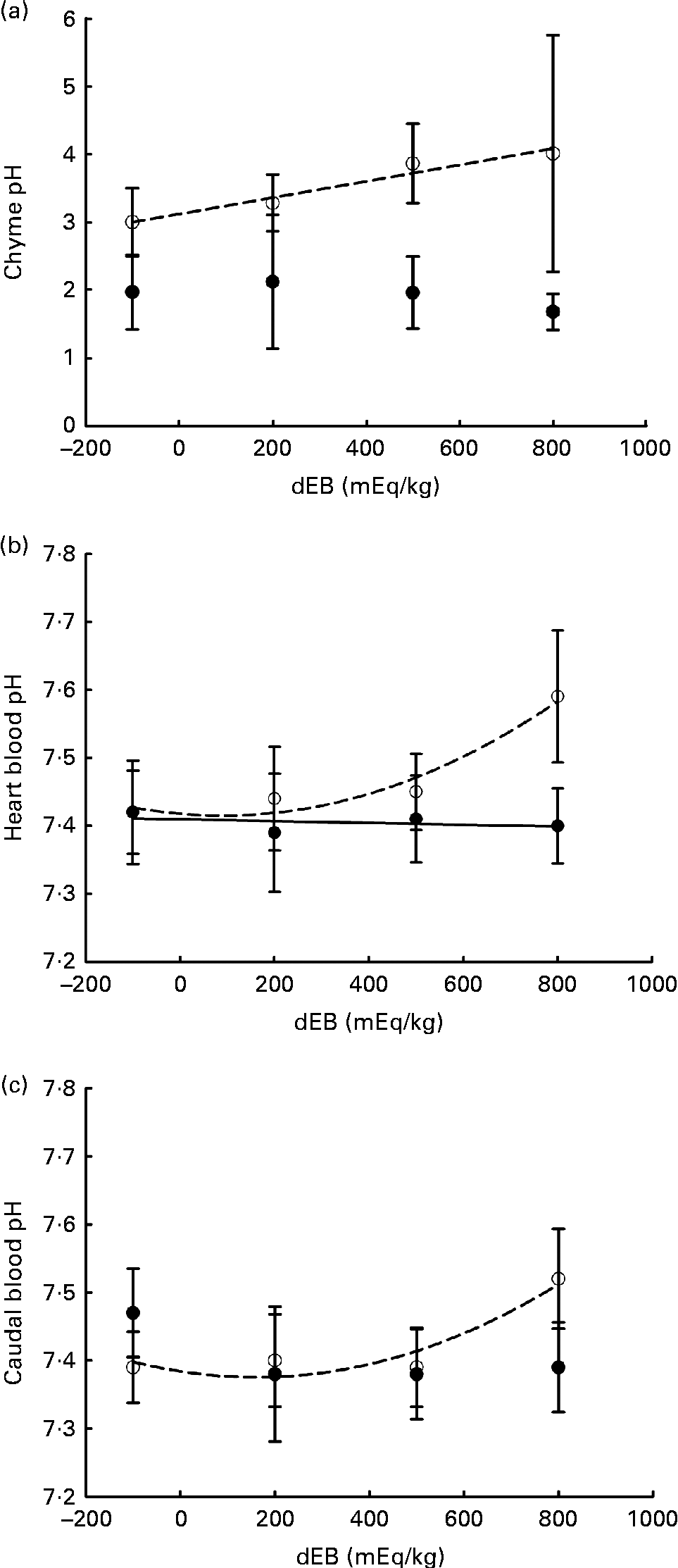
Averaged over the diets, chyme DM contents in the stomach were 172 and 156 g/kg at, respectively, 3 and 7 h postprandially. At 3 h after feeding, stomach chyme DM was unaffected by dEB (P>0·05), but at 7 h after feeding, it decreased linearly with increasing dEB (P< 0·01; Table 3 and Fig. 1(c)). At 3 h postprandially, stomach chyme pH increased linearly with increasing dEB levels (P< 0·05; Table 3 and Fig. 3(a)). At 3 h postprandially, stomach chyme pH was 2·9 and 3·9 in the fish fed, respectively, the − 100 and 800 dEB diets. At 7 h postprandially, stomach chyme pH was not influenced anymore by dEB; averaged over all the diets, stomach chyme pH was 1·9. At 3 h after feeding, the pH of the blood drawn from the heart as well as from the caudal vein was curvilinearly related to the dEB levels (P< 0·05; Table 3). Above a dEB level of 200 mEq/kg, blood pH at both locations increased with increasing dEB levels; however, this increase in blood pH with dEB was larger in the heart than in the caudal vein (Fig. 3(b) and (c)). At 7 h postprandially compared with 3 h postprandially, the differences in blood pH between the fish fed the experimental diets were much smaller (Fig. 3(b) and (c)). At 7 h post-feeding, the heart blood pH decreased linearly with increasing dEB levels (P< 0·05; Fig. 3(b)) and the caudal vein blood pH was unaffected by dEB (P>0·05; Fig. 3(c)). In addition, the variability in chyme and blood pH was significantly explained by the differences in the stomach chyme content (i.e. amount of feed eaten) of the fish; however, including stomach chyme content as a covariate in the statistical model did not alter the impact of the dEB levels (data not shown).

Fig. 3 Postprandial changes (at 3 and 7 h post-feeding) in the pH of chyme and blood in Nile tilapia fed diets with varying levels of dietary electrolyte balance (dEB) levels. (a) Chyme pH at 3 h (○) increased linearly with increasing dEB levels and chyme pH at 7 h (●) did not show any significant relationship. (b) pH of the blood drawn from the heart at 3 h (○) showed a curvilinear response to the dEB levels and at 7 h (●) decreased linearly with the dEB levels. (c) pH of the blood drawn from the caudal vein at 3 h (○) showed a significant curvilinear response to the dEB levels and at 7 h (●) no significant relationship was found. Values are means, with standard deviations represented by vertical bars.
Discussion
In the present study, the growth of Nile tilapia was affected by dEB, as has been observed in studies carried out in broiler chickens(Reference Borges, Fischer da Silva and Ariki23), pigs(Reference Dersjant-Li, Schulze and Schrama31, Reference Golz and Crenshaw32), lambs(Reference Fauchon, Seoane and Bernier33) and African catfish(Reference Dersjant-Li, Verreth and Evers25). However, growth observed in the latter studies was positively related to the dEB levels, which contrasts with the present finding of the decreased growth of tilapia fed diets with increasing dEB levels. This difference in growth response reported in the literature and that observed in the present study is probably related to the different feeding strategies. In the present study, tilapia were fed a restricted amount of feed, being equal for all treatments, whereas animals in the aforementioned studies were fed to satiation. Importantly, ad libitum feed intake in these studies increased linearly with dEB, which may explain the better growth. The present finding of decreased growth in tilapia fed the high-dEB v. low-dEB diets, despite similar feed intakes, demonstrates a direct impact of dEB on fish growth. As such, it is important to know how the applied changes in dEB affect the growth of fish by looking at the energy balance parameters, particularly the MEm.
In the present study, fish were kept under similar rearing conditions and given equal amounts of identical nutrients, except dEB. Consequently, the observed changes in the MEm of tilapia can be attributed to the applied changes in dEB. This is probably related to the mechanisms counteracting the effect of dEB on acid–base homeostasis (pH) in both the stomach and blood of tilapia, i.e. acid/base secretion in the gut and base excretion from the blood via gills into the water. These mechanisms, which are part of the energy requirements for maintenance, demand extra energy (ATP) to maintain the overall acid–base homeostasis(Reference Tresguerres, Parks and Wood34). In this respect, tilapia showed a curvilinear response both in blood pH at 3 h post-feeding and in MEm, suggesting a link between acid–base disturbance and MEm.
The energy expenditure towards maintenance has prominence over growth in fish(Reference Brett, Groves, Hoar, Randall and Brett35). Therefore, under circumstances of increased MEm, energy available for growth will be reduced. It is evident from the present results that the growth of tilapia reduced correspondingly with higher MEm due to dEB. For instance, the fish fed the 800 dEB diet showed approximately 15 % lower growth and approximately 54 % higher MEm compared with those fed the 200 dEB diet. Thus, the reduction in the growth of tilapia in the present study is predominantly related to their correspondingly higher MEm.
The MEm of tilapia showed a dose-dependent response to the dEB levels, with the highest MEm being observed in the 800 diet group and the lowest in the 200 dEB diet group. The MEm value of 57 kJ/kg0·8 per d observed in tilapia fed the 200 dEB diet complies with the literature value reported for Nile tilapia reared at a similar (26°C) water temperature (57 kJ/kg0·8 per d(Reference Meyer-Burgdorff, Osman and Günther36)). Besides, the 200 mEq/kg dEB level resulting in the lowest MEm and the highest energy retention suggests this level to be optimal for Nile tilapia, which is also in line with the optimal range of dEB for broiler chickens (200–250 mEq/kg(Reference Mongin14, Reference Borges, Fischer da Silva and Ariki23)) and pigs (175 mEq/kg(Reference Patience, Austic and Boyd37); 200 mEq/kg(Reference Dersjant-Li, Schulze and Schrama31)).
The present results obtained for tilapia confirm previous findings in African catfish on the effect of dEB on MEm(Reference Dersjant-Li, Verreth and Tijssen26). However, the response of MEm to changes in dEB levels is contradictory in both studies. In African catfish, MEm increased with decreasing dEB levels from 713 to − 146 mEq/kg(Reference Dersjant-Li, Verreth and Tijssen26). In contrast, in the present study, MEm was highest at 800 mEq/kg. The increased MEm at a low dEB level in catfish is probably caused by the increased dietary inclusion of NH4Cl used to lower dEB compared with CaCl2 used in the present study. We speculate that the higher MEm in catfish was related to the inherent toxicity of NH4Cl to fish(Reference Felista Rani, Elumalai and Balasubramanian38). Together, these results suggest the negative effect of dEB on the energy balance and growth of tilapia caused by the increased MEm.
Despite the increase in MEm causing the reduced growth, increasing dEB levels from 200 to 800 mEq/kg had a positive effect on the ADC of the measured nutrients (except for fat) in Nile tilapia. This increased digestibility of nutrients in the 800 dEB treatment, thus, partially compensated for the increased maintenance requirements. The influence of dEB on the digestibility of nutrients (DM, protein and total carbohydrates) in tilapia reiterates observations in pigs(Reference Dersjant-Li, Schulze and Schrama31, Reference Haydon, West and McCarter39, Reference Patience and Chaplin40), where the digestibility of DM and protein increased with increasing dEB. The mechanism underlying the effect of dEB on nutrient digestibility was not explained previously. Nutrient digestibility as determined here, which indicates that the relative proportion of nutrients disappeared from the ingested food, depends on (1) the enzymatic hydrolysis of dietary nutrients and (2) the absorption of (hydrolysed) nutrients from the gastrointestinal tract.
The liquefaction of the ingested feed inside the stomach is a prerequisite for effective enzymatic hydrolysis. It can be modified by water intake (drinking) or through the secretion of digestive fluids. Pigs fed increasing dEB levels display increased drinking of water together with reduced faecal DM content(Reference Dersjant-Li, Schulze and Schrama31, Reference Patience and Chaplin40), which may contribute to the enhanced digestion observed in pigs fed a high-dEB diet. Freshwater fish (hyperosmotic), such as Nile tilapia, exhibit a minimal intake of water by drinking in order to avoid the excess intake of water from the (hypo-osmotic) environment(Reference Fuentes and Eddy41). Nevertheless, at 7 h after feeding, chyme DM content decreased with increasing dEB levels. This implies a higher liquefaction of chyme in the stomach with increasing dEB. It can be postulated that this increased liquefaction of chyme is related to (1) an altered osmolarity in the stomach with the dEB levels, which promotes hydration through osmosis with endogenous water(Reference Grosell42, Reference Bucking and Wood43), or (2) an increased secretion of digestive fluids in the stomach. The liquefaction of a dry feed in the stomach, by the secretion of either endogenous water and/or digestive fluid, creates a feed consistency much closer to that of a natural feed of fish, believed to facilitate digestive processes. In support, Amirkolaie et al. (Reference Amirkolaie, Verreth and Schrama44) found a negative relationship between the DM content of chyme and nutrient digestibility in Nile tilapia.
Changes in dEB may also affect nutrient digestibility by affecting the gut pH(Reference Patience, Austic and Boyd37). In stomach, a low pH enhances the activity of proteolytic enzymes and increases protein denaturation(Reference Bakke, Glover and Krogdahl45). Acid addition improves the digestibility of not only protein, but also of other nutrients as demonstrated for P following a dietary acid (e.g. HCl, formic acid) supplementation in rainbow trout(Reference Sugiura, Roy and Ferraris46, Reference Vielma and Lall47). In the present study with increasing dEB levels, chyme pH in the stomach increased at 3 h postprandially. This suggests that the increased digestibility of nutrients with increasing dEB is not closely related to the pH in the stomach, but rather related to the liquefaction of stomach contents. The effects of dEB on the activity of digestive enzymes and nutrient absorption were not measured in the present study; however, they cannot be excluded and deserve further investigation. Despite the influence of dEB on protein and carbohydrate digestibility, fat digestibility was unaffected by the dEB levels in Nile tilapia. This implies that changes in stomach conditions brought about by dEB are less likely to affect fat digestion as it occurs primarily in the intestine(Reference Austreng48).
Freshwater fish fed a commercial feed undergo postprandial metabolic alkalosis (alkaline tide) during peak hours of digestion(Reference Bucking and Wood28). The surge in blood base load (basolateral HCO3− excretion) occurs in order to counteract the increased acid secretion (apical H+ extrusion) in the stomach, mediated by the Cl−/HCO3− exchanger(Reference Bucking, Fitzpatrick and Nadella12). The dEB (i.e. dietary mineral composition) modified the postprandial blood pH in the heart and the caudal vein at 3 h in Nile tilapia, suggesting the influence of dEB on metabolic alkalosis. The pH of the blood drawn from the heart at 3 h was almost 0·20 units greater in the 800 fish group than in the − 100 dEB fish group. Similarly, increasing dEB levels increased the pH of portal venous blood in dairy cows (140–450 mEq/kg(Reference Borucki Castro, Phillip and Girard49)) and that of portal venous blood (25–400 mEq/kg(Reference Haydon, West and McCarter39)) and arterial and portal blood ( − 100 to 200 mEq/kg(Reference Dersjant-Li, Verstegen and Jansman50)) in pigs. In the present study, the observed postprandial blood pH values (7·38–7·59) were lower than those reported previously for fish (7·8–8·1)(Reference Cooper and Wilson11, Reference Bucking, Fitzpatrick and Nadella12), which is most probably caused by the difference in blood sampling methods; for instance, anaesthetising fish has been found to reduce blood pH(Reference Iwama, McGeer and Pawluk51).
Importantly, the high blood pH in the heart of tilapia fed the 800 dEB diet reduced substantially between 3 and 7 h –post-feeding to such an extent that the heart blood pH at 7 h turned out to be inversely related to dEB. This indicates the prolonged effect of dEB on systemic acid–base homeostasis in tilapia. Unlike the heart blood pH, the caudal vein blood pH did not differ significantly at 7 h post-feeding, suggesting an active adjustment of pH imbalance in the gills. The impact of dEB at 3 h postprandially on the caudal vein blood pH might suggest that at the high dEB level (800 mEq/kg), the gills could not fully compensate for the dietary challenge of acid–base homeostasis. The direct transepithelial transfer of acid–base homeostasis-relevant molecules between the gills of the fish and the water has been suggested as the primary compensation mechanism of pH regulation(Reference Claiborne, Edwards and Morrison Shetlar6, Reference Cameron52). In freshwater rainbow trout, gills have been suggested to contribute more than kidney to the relieving of metabolic alkalosis(Reference Bucking, Landman and Wood53), which might include electrogenic H+-ATPase and Na+–K+-ATPase ion transporters(Reference Claiborne, Edwards and Morrison Shetlar6). Accordingly, the excess inclusion of salt (NaCl) in the diet of rainbow trout in freshwater resulted in the upregulation of seawater-specific ion transporter in gills(Reference Perry, Rivero-Lopez and McNeill54). The present results suggest that dEB disrupts acid–base homeostasis as indicated by blood pH in Nile tilapia and was actively adjusted over time with compensatory mechanisms, which might be the underlying reason for the increase in MEm.
In summary, our data show that dEB affects acid–base homeostasis (chyme and blood pH) in tilapia, which imposes increased energy expenditure to maintain acid–base homeostasis. The increased energy expenditure towards acid–base regulation caused by dEB reduces the amount of energy available for retention (growth) despite the enhanced nutrient digestibility. For Nile tilapia, the dEB of the 200 mEq/kg diet was found to be optimal, which showed minimal influence on acid–base homeostasis and MEm. Finally, as dEB affects MEm, further studies are needed to explore the impact of dEB on feed intake in ad libitum-fed fish.
Acknowledgements
We thank Menno ter Veld and Ep Eding for their technical support in conducting the experiment and Ronald Booms and Tino Leffering for their assistance during the sampling and laboratory works. The present study was funded by Wageningen University and performed in the framework of the collaborative INRA-WUR Platform for Sustainable Aquaculture. S. S., I. G., S. J. K., J. A. J. V. and J. W. S. contributed to the concept and design of the present study. Z. G. A. O. and J. W. S. executed the experiment and performed the laboratory analysis and data collection. S. S., I. G. and J. W. S were involved in the data analysis. S. S. wrote the first draft of the manuscript. All the authors contributed to the interpretation of the results and writing and agreed with the content of the final manuscript. There are no conflicts of interest.