Starch is the main energy-yielding component of the daily diet for most mammals(Reference Knudsen, Lærke and Steenfeldt1). The major physiological properties of starch are characterised in the release of glucose as a source of energy for the body and the timeline of digestion in the small intestine(Reference Wiseman2). Generally, starch is thought to be a mixture of amylose and amylopectin, or resistant and digestible starch(Reference Annison and Topping3, Reference Robertson4). High resistant starch level is associated with a high level of amylose(Reference Yang, Shu and Zhang5). Our previous in vitro digestibility trial indicated that starch with an amylopectin:amylose ratio of 1:27·6 was digested only 45·83 % within 4 h, whereas those with ratios of 27·6:8·5 and 27·6:1 were digested up to 90·95 or 98·77 % in the same time period(Reference Yin, Zhang and Huang6). In vivo, the digestion rate of dietary starch will affect the circulating level of glucose, as well as other absorbed nutrients such as small peptides and free amino acids postprandially(Reference Gary7–Reference Regmi, Matte and van Kempen9), and a periodical variation in the circulating levels of glucose, insulin and other nutrients has previously been observed in response to the ‘two-time intake/d’(Reference Regmi, Matte and van Kempen9) or ‘three-time intake/d’ feeding procedures(Reference Bin10, Reference Dai11). Furthermore, the variation in the circulating levels of these metabolites was in accordance with the digestion rate of dietary starch well within postprandial 4 h, and the faster the starch digested, the higher the serum concentrations of these metabolites increased. Because the retention time of the digesta in the small intestine is limited (about 4 h from the proximal duodenum to the distal ileum)(Reference Wilfart, Montagne and Simmins12), the availability of dietary starch is also consequently affected by the retention time of the digesta in the small intestine. The unabsorbed glucose and undigested starch will flow into the large intestine, where they are fermented by microbes and absorbed into the portal blood stream in the form of SCFA(Reference Wiggins13, Reference Cummings and Macfarlane14), thus affecting lipid metabolism in the whole body(Reference Darcy-Vrillon, Cherbuy and Morel15). Previous in vivo and in vitro experiments strongly support the view that glucose and insulin are the potent signal molecules that up-regulate the levels of mRNA and the activities of lipogenic enzymes in rats(Reference Leturque, Postic and Ferre16, Reference Li, Yu and Pan17), as well as in pigs(Reference Louveau and Gondret18, Reference Dunshea19). In addition, an increased glucose metabolism is necessary for the expression of insulin effects on fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) mRNA accumulation in white adipose tissues(Reference Foufelle, Gouhot and Pégorier20, Reference Fukuda, Katsurada and Iritani21). The objectives of the present study were to investigate how and to what extent the digestion rate of dietary starch affects the systemic circulating levels of glucose, insulin and lipid profiles and lipid metabolism-related gene expression in different tissues when pigs consumed their meals under a ‘six-time intake/d’ feeding procedure.
Materials and methods
Animals, experimental design and diets
The protocol for the animal experiment was approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, the Chinese Academy of Sciences.
A total of twenty-four barrows, weaned at age 21 d with an average initial body weight of 6·25 (sem 0·82) kg, were allocated on the basis of weight and litter of origin to three dietary treatments in a randomised complete block design. The dietary treatment groups were as follows: the high-digestion rate starch (HDRS) group, the moderate-digestion rate starch (MDRS) group and the low-digestion rate starch (LDRS) group. The amylopectin:amylose ratios in the diets of each treatment were 27·6:1, 27·6:8·5 and 1:27·6, respectively. The dietary starches were commercially available from the Changsha food market (Changsha, Hunan, China). There were eight pigs in each group, with one pig per metabolism crate. Each pig was surgically fitted with a catheter in the jugular vein, according to previous protocols after a 3 d adaptation period to the new environment(Reference Huang, Yin and Wang22, Reference Li, Dai and Yin23). The preparation of catheters and the pre- and post-operative care were as described previously (Reference Li, Dai and Yin23). The pigs were returned to the metabolism crates immediately after surgery. Each crate was equipped with a suspended water line fitted with a low-pressure nipple and wire flooring. All pigs were trained to adapt to a new feeding procedure as described previously(Reference Yin, Zhang and Huang6). Briefly, all pigs were fed six times daily (04.00, 08.00, 12.00, 16.00, 20.00 and 24.00 hours) and trained to consume their meals within 10 min. The daily feed allowance to each pig was strictly limited and equal to 5 % of their body weight. Water was freely available. The temperature was kept at 26 ± 2 °C and relative humidity was maintained between 60 and 75 %. Following a 7 d recovery, all pigs were fed the experimental diets.
Dietary crude protein, nutritional indispensable amino acids, vitamins and minerals were supplemented to meet or exceed the National Research Council nutritional requirements for swine(24), with a body weight of 5–10 kg. The ingredients and nutrient levels of the diets are summarised in Table 1.
Table 1 Ingredient and chemical composition of the experimental diets
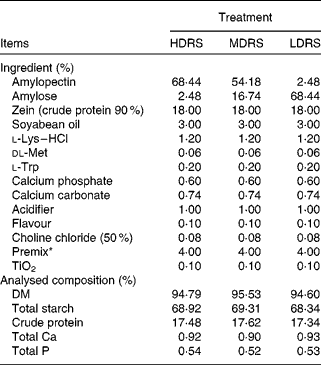
HDRS, high digestion rate starch; LDRS, low digestion rate starch; MDRS, moderate digestion rate starch.
* Supplied/kg of diet: vitamin A, 6 mg; vitamin D3, 8 mg; vitamin E, 30 mg; vitamin K, 3 mg; vitamin B2, 27 mg; vitamin B6, 2 mg; vitamin B12, 30 μg; biotin, 80 μg; folic acid, 8 mg; nicotinic acid, 24 mg; Na (NaCl), 3 g; Zn (ZnSO4), 165 mg; Fe (FeSO4), 165 mg; Mn (MnSO4), 33 mg; Cu (CuSO4), 165 mg; I (CaI2), 297 μg; Se (Na2SeO3), 297 μg.
Sample preparation
Venous blood samples were taken from each pig via the catheter into 5 ml heparin-free vacutainer tubes (Becton Dickinson Vacutainer Systems, Franklin Lakes, NJ, USA) once per hour, from 08.30 to 15·30 hours on day 7 after the experiment started. All samples were centrifuged at 750 g (Biofuge 22R centrifuge; Heraeus Instruments, Hanau, Germany) for 10 min at 4 °C, the supernatant (serum) was immediately collected and placed into test-tubes and stored at − 20 °C for later analysis. The pigs still consumed their meals six times daily, according to the feeding procedure during the sample collection period. On day 8, after the experiment started, all pigs consumed their meals at 08.00 hours and were euthanised at 10.00 hours. About 5 g of the myocardium, liver, abdominal adipose and interscapular brown adipose tissues were collected and immediately frozen in liquid N2, and stored at − 80 °C for the determination of the activities of lipogenic enzymes, respectively. The tissue samples (2 g) were cut into approximately 5 × 5 × 1–2 mm pieces and placed immediately in RNAlater (Applied Biosystems, Austin, TX, USA), and stored at room temperature for a few hours, respectively, then frozen at − 20 °C until further processing for RNA extraction and complementary DNA synthesis.
Serum analyses
The serum concentrations of glucose, TAG, total cholesterol, LDL-cholesterol, HDL-cholesterol and SCFA (including acetate, propionate and butyrate) were determined using the Automatic Biochemical Analyser (Beckman, Miami, FL, USA) with corresponding kits (commercially available from Leadman Biochemistry Technology Company, Beijing, China). The serum insulin concentration was analysed using a commercially available 125I Radio Immunoassay Analyser kit (Beijing North Institute of Biological Technology, Beijing, China) with the γ-calculating instrument GC-300 (Zhongjia Company, Beijing, China), according to the manufacturers' instructions.
Lipogenic enzyme activity analyses
The protocol for lipid metabolism-related enzyme activity analyses in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues was as described previously(Reference Swierczynski, Goyke and Wach25). Briefly, approximately 1 g of the tissue sample was rinsed, blotted dry, weighed and placed in 8 ml ice-cold 20 mm-riffs HCl buffer (pH 7·8) containing 0·2 % Triton X-100. The tissue was minced finely with scissors, homogenised manually with a Teflon-pestle homogeniser and centrifuged at 30 000 g for 20 min. After removal of the fat cake, the resulting supernatant was decanted, and the pellet was re-suspended in 5 ml isolation medium, re-homogenised and centrifuged as before. The supernatant was combined with that obtained after the first centrifugation step and used for enzyme assay. The activities of FAS (EC 2.3.1.85) and ATP-citrate lyase (EC 4.1.3.8) were measured as described previously(Reference Zelewski and Swierczynski26). All assays were performed in duplicate at 37°C using a Biochrom ultrosprc 3100 spectrophotometer (Biochrom, Science Park, Cambridge, UK). The absorbance change both against time and enzyme concentration was linear. ACC (EC 6.4.1.2) activity was measured by the H14CO3-fixation assay according to the method of Salati & Clarke(Reference Salati and Clarke27). All assays were conducted in the range of linearity with respect to the amount of enzyme and time. Soluble protein in the tissue supernatants was measured according to the method as described previously(Reference Bradford28), using bovine serum albumin as the standard. All reagents were obtained from Sigma (St Louis, MO, USA).
Gene expression profiles of lipogenic enzymes
The gene expression profile of lipid metabolism-related enzymes was determined according to the following protocol. Briefly, approximately 100 mg of the tissue sample was homogenised using a JT-B homogeniser (Luohe Jintian Institute of Test Equipment, Luohe, Henan, China). Total RNA was isolated from each homogenised tissue by using TRIZOL reagent (Invitrogen, Inc., Carlsbad, CA, USA) and then treated with DNase I (Invitrogen) as described by the manufacturer. The RNA quality was checked by 1 % agarose gel electrophoresis, stained with ethidium bromide (10 μg/ml). The RNA with an optical density 260/280 ratio between 1·8 and 2·0 was performed with Oligo (dT) 20 and Superscript II reverse transcriptase (Invitrogen)(Reference Wang, Gu and Tang29). The quantitative PCR assays were performed using the Brilliant SYBR Green quantitative PCR Master Mix (Stratagene, La Jolla, CA, USA) and a Stratagene MX4000 Thermal Cycler (Stratagene). Briefly, a 10 μl volume reaction system contained 0·4 μl of the complementary DNA template, 5 μl of the SYBR Green Mix and 0·3 μl of each of the forward and reverse primers and 4 μl of double distilled water. The amplification programme started at 95°C for 30 s followed by forty cycles of 95°C for 5 s, the gene-specific annealing temperature for 30 s and extension at 72°C for 30 s. Fluorescence measurements were collected after each annealing during the cycles. PCR primers targeting FAS and ACC were as described previously(Reference Zhao, Wang and Song30), while those targeting ATP-CL and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were designed with the Primer Premier (Premier Biosoft International, Palo Alto, CA, USA) based on the available porcine GenBank sequences (Table 2). GAPDH was used as an internal reference gene to normalise target gene transcript levels. The identity of each product was confirmed by the dideoxy-mediated chain termination sequencing at Sangon Biotechnology, Inc. (Shanghai, China). The relative expression ratio (R) of mRNA was calculated by R = 2(CT(GAPDH) − CT(test)) (Reference Livak and Schmittgen31). The amplification efficiency (E) of the quantitative PCR was acquired by the amplification of a dilution series of complementary DNA according to the equation E = 10( − 1/slope) and was consistent between the target mRNA and GAPDH rRNA. Negative controls were performed in which complementary DNA was replaced with water(Reference Wang, Shi and Zhang32).
Table 2 Quantitative PCR primers
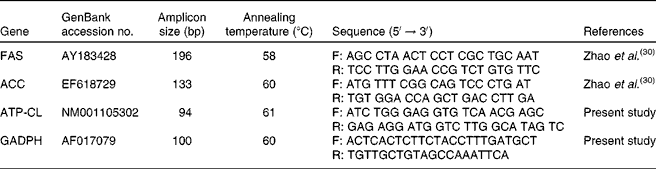
FAS, fatty acid synthase; F, forward; R, reverse; ACC, acetyl CoA carboxylase; ATP-CL, ATP-citrate lyase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analyses
All physico-chemical analyses were performed at least in duplicate. The data on the serum biochemical parameters were analysed as a split-plot design for repeated measures using the generalised linear model procedure of SAS 9.13 (SAS Institute, Inc., Cary, NC, USA). The statistical model included the effect of treatment as the main plot (tested by the animal within treatment variance) and the effects of sampling time and the treatment × sampling time interaction as the subplot. The comparisons among treatments within sampling time were made when a significant F test (P < 0·05) for the treatment × sampling time interaction was observed. The data on the variation in the activities and mRNA expression of FAS, ACC and ATP-CL were also analysed as a split-plot design for repeated measures. The statistical model included the effect of treatment as the main plot and the effects of tissues and the treatment × tissue interaction as the subplot. The comparisons among treatments within tissues were made when a significant F test (P < 0·05) for the treatment × tissue interaction was observed. The Duncan's multiple comparison test was used to determine the differences among the means of treatment groups. A value of P < 0·05 was considered to be statistically significant.
Results
Serum circulating glucose and insulin
The variation in postprandial systemic circulating glucose and insulin is summarised in Figs. 1 and 2, respectively. Both glucose and insulin were affected (P < 0·05) by the sampling time as well as by the treatment × sampling time interaction, and changed periodically in response to the present feeding procedure. Furthermore, the serum level of glucose in pigs of the HDRS group was increased sharply to the peak point (at postprandial 1·5 h), while that in the MDRS and LDRS groups were increased slowly to their peak points at postprandial 2·5 h, respectively. The peak level of glucose in the HDRS group was higher (P < 0·05) than that in the MDRS group, and that in the MDRS group was also higher (P < 0·05) than in the LDRS group. The serum insulin levels in the three dietary treatment groups were increased quickly to the peak point, and then decreased gradually within each feeding cycle; however, the variation in insulin level did not respond simultaneously to the postprandial blood glucose levels. The serum insulin concentration in the HDRS group was higher (P < 0·05) than that in the MDRS group, and that in the MDRS group was also higher (P < 0·05) than in the LDRS group at postprandial 0·5, 1·5 and 2·5 h, respectively. During the second feeding cycle, from 12.30 to 15.30 hours, the variation in postprandial systemic circulating glucose and insulin was similar to that observed during the first feeding cycle, from 08.30 to 11.30 hours.

Fig. 1 Variation in postprandial serum systemic circulating glucose in two feeding cycles. ![]() , High-digestion rate starch group;
, High-digestion rate starch group; ![]() , moderate-digestion rate starch group;
, moderate-digestion rate starch group; ![]() , low-digestion rate starch group. a,b,c Mean values within the same sampling time with unlike letters were significantly different (n 8, P < 0·05).
, low-digestion rate starch group. a,b,c Mean values within the same sampling time with unlike letters were significantly different (n 8, P < 0·05).

Fig. 2 Variation in postprandial serum systemic circulating insulin concentration in two feeding cycles. ![]() , High-digestion rate starch group;
, High-digestion rate starch group; ![]() , moderate-digestion rate starch group;
, moderate-digestion rate starch group; ![]() , low-digestion rate starch group. a,b,c Mean values within the same sampling time with unlike letters were significantly different (n 8, P < 0·05). 1 mIU = 6·945 μmol.
, low-digestion rate starch group. a,b,c Mean values within the same sampling time with unlike letters were significantly different (n 8, P < 0·05). 1 mIU = 6·945 μmol.
Serum circulating lipid profiles and SCFA
The postprandial serum concentrations of lipid and SCFA were also affected (P < 0·05) by both the sampling time and the treatment × sampling time interaction (Tables 3 and 4), and changed periodically in response to the present feeding procedure. The serum levels of TAG, total cholesterol, LDL-cholesterol and HDL-cholesterol in the HDRS group were increased to the peak point at postprandial 2·5, 2·5, 1·5 and 1·5 h, those in the MDRS group were at postprandial 3·5, 3·5, 3·5 and 3·5 h and those in the LDRS group were at postprandial 3·5, 3·5, 1·5 and 3·5 h, respectively. Furthermore, the serum TAG level in the HDRS group was higher (P < 0·05) than that in the MDRS group at postprandial 1·5 and 2·5 h, and was even higher (P < 0·05) than that in the LDRS group at different time points postprandially, and that in the MDRS group was higher (P < 0·05) than that in the LDRS group at postprandial 3·5 h in the first feeding cycle. The serum TAG level in the MDRS group was lower (P < 0·05) than that in the MDRS group at postprandial 3·5 h, and was higher (P < 0·05) than that in the LDRS group at postprandial 1·5, 2·5 and 3·5 h; the serum TAG level in the MDRS group was higher (P < 0·05) than that in the LDRS group at different time points postprandially in the second feeding cycle, respectively. The serum levels of total cholesterol and HDL-cholesterol in the HDRS group were higher (P < 0·05) than those in the MDRS group at postprandial 0·5, 1·5 and 2·5 h, and those in the MDRS group were higher (P < 0·05) than those in the LDRS group at different time points postprandially. The serum LDL-cholesterol level in the HDRS group was lower (P < 0·05) than that in the MDRS group at postprandial 0·5, 2·5 and 3·5 h, and was higher (P < 0·05) than that in the LDRS group at postprandial 1·5 h, and that in the MDRS group was higher (P < 0·05) than that in the LDRS group at different time points postprandially in the first feeding cycle; the serum LDL-cholesterol level in the MDRS group was lower (P < 0·05) than that in the MDRS group at postprandial 2·5 and 3·5 h, and was higher (P < 0·05) than that in the LDRS group at postprandial 1·5 h, and that in the MDRS group was higher (P < 0·05) than that in the LDRS group at postprandial 0·5, 2·5 and 3·5 h, respectively. The serum acetate, propionate and butyrate levels in pigs of the HDRS and MDRS groups increased to the peak point sharply, at postprandial 1·5 h; those in the LDRS group increased to the peak points at postprandial 2·5 h, respectively. Similar results were observed during the second feeding cycle, from 12.30 to 15.30 hours.
Table 3 Serum lipid profiles and SCFA concentration after the first feeding
(Mean values with their pooled standard errors, n 8)

HDRS, high digestion rate starch; MDRS, moderate digestion rate starch; LDRS, low digestion rate starch; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Table 4 Serum lipid profiles and SCFA concentration after the second feeding (continued from Table 3)
(Mean values with their pooled standard errors, n 8)

HDRS, high digestion rate starch; MDRS, moderate digestion rate starch; LDRS, low digestion rate starch; TC, total cholesterol; LDL-C, LDL-cholesterol; HDL-C, HDL-cholesterol.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Activities of lipogenic enzymes
The activities of the lipogenic enzymes were affected (P < 0·05) by the tissue and the treatment × tissue interaction (Table 5). The activity of FAS in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of pigs of the HDRS and MDRS groups was higher (P < 0·05) than that of the LDRS group; that in the liver and abdominal adipose tissues of the HDRS group was also higher (P < 0·05) than that of the MDRS group. The activity of ACC in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of pigs of the HDRS group and that in the myocardium, liver and abdominal adipose tissues of the MDRS group were higher (P < 0·05) than that of the LDRS group; the activity of ACC in the myocardial adipose and interscapular brown adipose tissues of the HDRS group was higher (P < 0·05) than that of the MDRS group. The activity of ATP-CL in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of pigs of the HDRS group and that in the myocardium and liver of the MDRS group were higher (P < 0·05) than that of the LDRS group; the activity of ATP-CL in the liver and interscapular brown adipose of the HDRS group was higher (P < 0·05) than that of the MDRS group.
Table 5 Activity of the lipogenic enzymes after pigs consumed the experimental diets (nmol/min per mg protein)
(Mean values with their pooled standard errors, n 8)
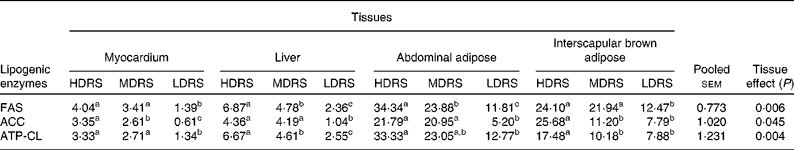
HDRS, high digestion rate starch; MDRS, moderate digestion rate starch; LDRS, low digestion rate starch; FAS, fatty acid synthase; ACC, acetyl CoA carboxylase; ATP-CL, ATP-citrate lyase.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
mRNA levels of lipid metabolism-related genes
The mRNA levels of the lipid metabolism-related genes were affected (P < 0·05) by the tissue and the treatment × tissue interaction (Table 6). The mRNA level of FAS in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of pigs of the HDRS group and that in the myocardium, abdominal adipose and interscapular brown adipose tissues of the MDRS group were higher (P < 0·05) than that of the LDRS group; the mRNA level in the myocardium, liver and interscapular brown adipose tissues of the HDRS group was also higher (P < 0·05) than that of the MDRS group. The mRNA level of ACC in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of pigs of the HDRS and MDRS group was higher (P < 0·05) than that of the LDRS group; that in the myocardium and abdominal adipose tissue of the HDRS group was higher (P < 0·05) than that of the MDRS group. The mRNA level of ATP-CL in the myocardium, liver, abdominal adipose and interscapular brown adipose tissues of the HDRS and MDRS group was higher (P < 0·05) than that of the LDRS group; that in the liver, abdominal adipose and interscapular brown adipose tissues was higher (P < 0·05) than that of the MDRS group.
Table 6 Relative key lipogenic enzyme gene expression profiles after pigs consumed the experimental diets (arbitrary units)
(Mean values with their pooled standard errors, n 8)
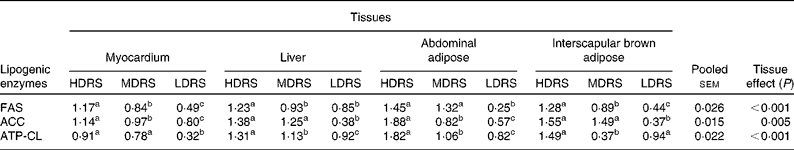
HDRS, high digestion rate starch; MDRS, moderate digestion rate starch; LDRS, low digestion rate starch; FAS, fatty acid synthase; ACC, acetyl CoA carboxylase; ATP-CL, ATP-citrate lyase.
a,b,c Mean values within a row with unlike superscript letters were significantly different (P < 0·05).
Discussion
Animal nutrition researchers have been trying to develop novel strategies to get maximal deposition of carbon, nitrogen as well as other nutrients in food animals, of which elevating the postprandial circulating levels of insulin, glucose and amino acids has been proved to be practical in mammals(Reference Jeyapalan, Orellana and Suryawan33, Reference Tremblay and Marette34). An important observation from the present study was that the postprandial circulating levels of glucose and insulin were changed periodically in response to the ‘six-time intake/d’ feeding procedure. Besides, about 81·90, 47·17 and 30·14 % of dietary starch were digested in the anterior jejunum of pigs in the HDRS, MDRS and LDRS groups, respectively(Reference Yin, Zhang and Huang6). The higher starch digestibility in the anterior small intestine results in higher and rapid postprandial circulating glucose and insulin responses(Reference Deng, Wu and Bin35). Since insulin is secreted primarily in response to the elevated blood glucose concentration, stimulated by the feeding frequency and activity(Reference Solomon, Chambers and Jeukendrup36, Reference Armentano, Mills and de Boer37), and even controlled by the central nervous system through some signal pathways(Reference Shankar, Zhu and Ladd38), the difference in the postprandial serum insulin levels in the present study should be a combined result of both the feeding activity and the efficiency of glucose absorption. In other words, there is a clear correlation between the starch digestibility and the insulin response for the three diets.
Lipogenesis is a process by which simple sugars such as glucose are converted to fatty acids(Reference Kersten39), which is regulated by a wide array of interdependent factors, including nutrients, hormones, nuclear transcription factors and lipogenic enzymes, of which glucose and insulin are two important regulating molecules(Reference Yue, Yin and Li40, Reference Zhang, Huang and Dúvel41). In this regard, the postprandial circulating lipid profile may reflect how blood glucose and insulin affected the lipid metabolism. An important finding from the present study is that the postprandial circulating levels of lipids and SCFA were also changed periodically. Interestingly, the higher the starch digestion rate was, the faster the postprandial circulating levels of TAG, total cholesterol and HDL-cholesterol responded, and the lower the circulating levels of SCFA responded. Insulin promotes the synthesis of fatty acids in the liver and inhibits the breakdown of fat in the adipose tissue by inhibiting the intracellular lipase that hydrolyses TAG to release fatty acids(Reference Marshall42), and high glucose induces adipogenic differentiation in porcine(Reference Yue, Yin and Li40), as well as in human tissues(Reference Wang, Zhang and Zheng43). Therefore, the higher level of postprandial glucose and insulin stimulated the process of adipogenesis and elevated the circulating lipid profiles. SCFA are the major end products of bacterial metabolism in the large intestine of animals(Reference Macfarlane and Macfarlane44, Reference Cummings, Pomare and Branch45), which stimulate adipogenesis via the G protein-coupled receptor 43 pathway(Reference Hong, Nishimura and Hishikawa46). In the present study, lower levels of postprandial circulating lipid profiles were observed in pigs of the LDRS group with higher levels of fatty acids at the same time. The lower availability of glucose would result in higher oxidation of other nutrients, such as protein and lipids, for energy requirement(Reference Higgins, Higbee and Donahoo47, Reference Jéquier48), which may explain the lower concentration of serum lipids in pigs of the LDRS group, and a similar phenomenon was observed previously in rats(Reference Kabir, Rizkalla and Champ49), as well as in mice(Reference So, Yu and Kuo50).
Lipids are considered to be crucial in the metabolic process of mammals(Reference Wisneski, Gertz and Neese51, Reference Martinez-Puig, Mourot and Ferchaud-Roucher52). There are two sources of lipids for metabolism, exogenously derived (dietary) lipids and endogenously synthesised lipids(Reference Ortega, Mayas and Moreno-Navarrete53). The biosynthesis of the latter (lipogenesis) depends on well-known enzyme-regulated processes, of which FAS, ACC and ATP-CL are the primary lipogenic enzymes, and were considered as rate-limiting enzymes of lipogenesis in pigs(Reference Scott, Cornelius and Mersmann54, Reference Huang, Xu and Han55). A novel finding of the present study was that the activities of FAS, ACC and ATP-CL in the myocardium, liver, abdominal adipose and interscapular brown adipose in pigs of the HDRS group were higher than those in the MDRS and LDRS groups, and these results are, at least partially, in accordance with the gene expression profiles of these enzymes. Metabolic regulation in mammals relies partly on transcriptional control of the expression level of key enzymes(Reference Desvergne, Michalik and Wahli56), and gene expression analysis is useful for inferring transcriptional activity. Glucose is known to directly affect lipogenic gene expression by altering the transcriptional repressor level via the lipid synthesis pathway in a range of tissues(Reference Hasegawa, Osatomi and Wu57), while insulin regulates the lipogenic gene expression indirectly, either by increasing glucose transportation and metabolism through the translocation of the insulin-responsive GLUT-4 to the plasma membrane in adipocytes or by speeding up glucose metabolism in the liver through the activated glucokinase pathway(Reference Weickert and Pfeiffer58). In the present study, the mRNA levels of FAS, ACC and ATP-CL in the myocardium, liver, abdominal adipose and interscapular brown adipose in pigs of the HDRS group were higher than those of the LDRS group, corresponding with the postprandial circulating glucose and insulin responses in these groups. However, an interesting observation was that not only the mRNA levels but also the activities of these rate-limiting lipogenic enzymes in the same tissue were significantly different. Furthermore, the mRNA level and the activity of the same lipogenic enzyme gene in different tissues were also different. These significant differences may be due to the different functions of these lipogenic enzymes, different responses of these enzymes to blood glucose and insulin and different metabolic patterns of lipids in these tissues(Reference Nguyen, Leray and Diez59–Reference Gaikwad, Long and Stringer62).
In summary, the present study shows that dietary starch with a higher digestion rate significantly elevated the postprandial blood glucose and insulin response, and also the profiles of circulating lipids but not of SCFA. Glucose and insulin have a priority in regulating lipogenesis in different tissues over that of SCFA. The activity of lipogenic enzymes and their gene expression were affected by the postprandial circulating glucose and insulin in weaned pigs.
Acknowledgements
The present study was jointly supported by grants from the National 863 project (2008AA10Z316), the National Natural Science Foundation of China (30901040, 30928018), the Cooperative Projects of Guangdong Province and Chinese Academy of Science (2009B091300043, 2009B091300079, 2009B091300089). The authors are grateful to all staff in the Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, the Chinese Academy of Sciences for their assistance in the present study. Y. Y. was in charge of the whole trial. F. Y. and Z. Z. conducted the animal experiment and wrote the manuscript. J. H., R. H. and T. L. assisted with the animal trial and laboratory analyses. The authors have no conflicts of interest.










