Most patients with cystic fibrosis (CF) have a lipid abnormality involving both the n-6 and n-3 series of essential fatty acids (EFA)Reference Carlstedt-Duke, Bronnegard and Strandvik1–Reference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3. We recently reported that the fatty acid composition of serum phospholipids was significantly associated with bone mineral density (BMD) in children, but not in adults, with CFReference Gronowitz, Mellstrom and Strandvik4. We also previously reported a normal average annual accretion of bone massReference Gronowitz, Mellstrom and Strandvik5, which was in contrast to other studies reporting reduced bone accretion before peak bone mass and an accelerated bone loss in adultsReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6–Reference Bianchi, Romano, Saraifoger, Costantini, Limonta and Colombo8. High prevalence, 40–75 %, of osteopenia and osteoporosis with fractures has been reported in adult patients with CFReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6–Reference Buntain, Greer, Schluter, Wong, Batch, Potter, Lewingdon, Powell, Wainwright and Bell13. The low BMD has also been suggested to be linked to the cystic fibrosis transmembrane conductance regulator (CFTR) mutation, recently supported by reports of abnormal bone formation and resorption in CFTR − / − miceReference Dif, Marty, Baudoin, de Vernejoul and Levi14. The most common mutation in the CFTR gene, ΔF508, has been suggested as an independent risk factor for reduced BMDReference King, Topliss, Kotsimbos, Nyulasi, Bailey, Ebeling and Wilson15.
Physical activity has been shown to have a stimulatory effect on bone mass accretionReference Buntain, Greer, Schluter, Wong, Batch, Potter, Lewingdon, Powell, Wainwright and Bell13, Reference Frangolias, Pare, Kendler, Davidson, Wong, Raboud and Wilcox16–Reference Lorentzon, Mellstrom and Ohlsson18. Infection and inflammation, which start early in life in CF, have been reported to down modulate BMD by the effect of cytokinesReference Haworth, Selby, Webb, Martin, Elborn, Sharples and Adams19, Reference Conway20, also revealed by increased routine inflammatory markersReference Gronowitz, Mellstrom and Strandvik5. The influence of pancreatic insufficiency on fat absorption in CF patients might contribute to poor Ca absorption and low BMDReference Lark, Lester, Ontjes, Blackwood, Hollis, Hensler and Aris21. The relatively low serum concentrations of vitamin D despite regular supplementation might further contribute to low bone massReference Gronowitz, Mellstrom and Strandvik5, Reference Lark, Lester, Ontjes, Blackwood, Hollis, Hensler and Aris21–Reference Aris, Merkel and Bachrach23, although no correlation between the serum concentration of this vitamin and BMD has been reported in CF or healthy individualsReference Gronowitz, Mellstrom and Strandvik5, Reference Bianchi, Romano, Saraifoger, Costantini, Limonta and Colombo8, Reference Gronowitz, Garemo, Lindblad, Mellstrom and Strandvik22, Reference Guler, Sivas, Baskan, Gunesen, Alemdaroglu and Ozoran24, Reference Hogstrom, Nordstrom and Nordstrom25. Many CF patients also have a delayed onset of puberty; also, low levels of serum testosterone and abnormal menstruation have been reportedReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6, Reference Bhudhikanok, Wang, Marcus, Harkins, Moss and Bachrach7, Reference Bhudhikanok, Lim, Marcus, Harkins, Moss and Bachrach10–Reference Aris, Renner, Winders, Buell, Riggs, Lester and Ontjes12, Reference Leifke, Friemert, Heilmann, Puvogel, Smaczny, von zur Muhlen and Brabant26. The use of corticosteroids mandatory after lung transplantation is another identified risk factor for low BMDReference Bhudhikanok, Wang, Marcus, Harkins, Moss and Bachrach7–Reference Bhudhikanok, Lim, Marcus, Harkins, Moss and Bachrach10, Reference Aris, Renner, Winders, Buell, Riggs, Lester and Ontjes12.
EFA have been shown to be important for bone growth and density in animalsReference Watkins, Lippman, Le Bouteiller, Li and Seifert27, Reference Holman28. A recent published paper from Sweden studying a cohort of young males showed that n-3 fatty acids, especially DHA, were positively associated with bone mineral accrualReference Hogstrom, Nordstrom and Nordstrom29. Also in elderly patients and patients with renal disease, associations have been found between BMD, Ca metabolism and EFAReference Baggio, Budakovic, Ferraro, Checchetto, Priante, Musacchio, Manzato, Zaninotto and Maresca30, Reference Kruger, Coetzer, de Winter, Gericke and van Papendorp31. In a Norwegian study, dietary saturated fat showed a positive relationship with BMD in healthy childrenReference Gunnes and Lehmann32. EFA deficiency is common in CF patientsReference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3, and is more prevalent at prepubertyReference Lai, Kosorok, Laxova, Davis, FitzSimmon and Farrell33, a time usually considered to be a risk period for further decrease of BMD in CF patientsReference Aris, Merkel and Bachrach23.
In contrast to most other CF centres worldwide, we regularly compensate for severe EFA deficiency in our patientsReference Strandvik, Ghraf, Aggett, Lifschitz, Walker-Smith and Morán34, which in light of our recent finding of an associationReference Gronowitz, Mellstrom and Strandvik5 between EFA status and BMD would potentially have an impact on bone growth. We have previously found that bone mass in the adult animal is dependent on the EFA intake of the pregnant and lactating rat by programmingReference Korotkova, Ohlsson, Hanson and Strandvik35, Reference Korotkova, Ohlsson, Gabrielsson, Hanson and Strandvik36, and in a recent publication neonatal bone mineral content (BMC) in human healthy term-born infants was shown to be related to the perinatal maternal and infant EFA statusReference Weiler, Fitzpatrick-Wong, Schellenberg, McCloy, Veitch, Kovacs, Kohut and Kin Yuen37. Since several studies have shown that newborns with CF have a high prevalence of EFA deficiencyReference Lloyd-Still, Johnson and Holman38, Reference van Egmond, Kosorok, Koscik, Laxova and Farrell39 and further that EFA deficiency is associated with genotypeReference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3, the suggestion that the ΔF508 mutation is an independent risk factor for reduced BMD might be related to the fact that EFA deficiency seemed to be more common in patients with severe mutationsReference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3. Such a hypothesis would imply that it would be possible to influence BMC during growth and explain why we, contrary to others, obtained normal bone accrual in our patientsReference Gronowitz, Mellstrom and Strandvik5.
The aim of the present study was to investigate if our population of young adult men with CF, who might have reached or were close to reaching their final height, had normal bone mass, compared with healthy Swedish men of similar age. Dual-energy X-ray absorptiometry (DXA) was used to measure total BMC and BMD and focused on the lumbar spine (LS) and hip, since those sites contain more trabecular bone. In addition, cortical bone mass was specifically measured by peripheral quantitative computerised tomography (pQCT). The results were related to serum phospholipid fatty acid pattern. Anthropometry, bacterial colonisation, pulmonary function, and dairy intake as the major source of Ca, were also evaluated in relation to bone mass.
Subjects and methods
The study was conducted according to the Helsinki declaration and approved by the ethics committee at Gothenburg University (Sweden). Informed consent was obtained from patients and parents and from healthy participants.
Subjects
Fourteen men with CF, age 21·6 (sd 2·2) years, were included. All sixteen male patients in the age group of 18–25 years who regularly attended our CF centre were asked to participate in the study, but two declined. Thirteen patients were diagnosed in infancy or early childhood and only one after the age of 10 years. Sweat tests were pathological (>60 mmol/l) in all patients. Eleven patients had two ‘severe mutations’; ten were homozygous for the ΔF508 mutation. Three were compound heterozygotes for the same mutation and one of those had the severe mutation, 394delTT. The other mutations found were R117C and R75Q and in one patient one mutation could not be identified; these three latter patients were pancreatic sufficient. The pancreatic-insufficient patients were all treated with pancreatic enzyme supplementation. Multivitamin supplementation, including 10 μg vitamin D and extra vitamin E ( ≥ 0·1 g) were subscribed to all patients. All patients who, at the regular yearly check-up, disclosed low linoleic acid concentration in serum phospholipids were advised to add vegetable oil to their diets.
All patients had been physically active since diagnosis, as a part of our treatment policyReference Blomquist, Freyschuss, Wiman and Strandvik40, Reference Lannefors, Button and McIlwaine41. The Swedish programme of physiotherapy for patients with CF includes individualised physiotherapy and physical activities, such as daily trampoline exercise and different sports, including regular endurance and strength training several times per week. This was modified by continuous education and home visits for adjustment and optimising the programme as the patients grewReference Lannefors, Button and McIlwaine41. It also includes oral and inhalation therapy with mucolytics and bronchodilators to optimise airway clearance with devices according to the patients' preference in order to achieve optimal adherence to therapy. None of the patients were treated with steroids.
Forced vital capacity and forced expiratory volume in 1 s (FEV1·0) were 103 (sd 22) and 94 (sd 24) % of predicted values, respectivelyReference Quanjer, Tammeling, Cotes, Pedersen, Peslin and Yernault42, Reference Solymar, Aronsson, Bake and Bjure43. Only one of the patients (age 23 years) had an FEV1·0 less than 60 %, i.e. 31 % of the predicted value. Seven patients were chronically colonised with Pseudomonas aeruginosa characterised by repeated growth of the bacteria in sputum for ≥ 6 months and increased serum titres to pseudomonal exotoxin AReference Hollsing, Granstrom, Vasil, Wretlind and Strandvik44. One patient was intermittently colonised without increased serum antibody titres. Two patients were chronically colonised with Staphylococcus aureus. Two patients had CF-related diabetes mellitus and two were treated with ursodeoxycholic acid for mild biochemical liver diseaseReference Lindblad, Glaumann and Strandvik45. All were in stable condition without signs of acute infection.
The two men with CF (age 20 and 23 years, respectively) who declined to participate in the study both had severe mutations, were pancreatic insufficient and chronically colonised with Ps. aeruginosa. Their FEV1·0 was 85 and 65 % of predicted values, respectively, and they had normal anthropometric data. Although a small sample, the studied CF group was thus representative for the male CF patients of the ages 18–25 years at our centre.
Forty-two healthy men from the same geographic area served as controls. The control subjects were a subgroup of the oldest individuals in a large study group, randomly identified using the national population register, contacted by telephone and asked to participateReference Lorentzon, Mellstrom and Ohlsson46. Their age was 19·5 (sd 0·2) years.
Questionnaire
A standardised questionnaire was used to collect information about weight-bearing physical activities (h/week) and dietary intake of dairy products, vegetables and vitamin supplementationReference Lorentzon, Mellstrom and Ohlsson46. Puberty onset was reported by a self-assessment enquiry (early, average or late compared with schoolmates).
Anthropometric measurements
Height was measured using a wall-mounted stadiometer, and weight was measured to the nearest 0·1 kg. The same trained staff carried out all the measurements. The CV were < 1 % for these measurements. BMI (kg/m2) was calculated.
Handgrip strength
Handgrip strength was measured with the JAMAR® hydraulic hand dynamometer (Sammons Preston Rolyan, Bolingbrook, IL, USA); each hand was measured three times and the best result was used.
Dual-energy X-ray absorptiometry
Bone area, BMC and BMD of the whole body, total femur, femoral neck and LS (L I–L IV) were assessed using Lunar Prodigy equipment (GE Lunar Corp., Madison, WI, USA). Lean body mass (LBM), fat mass and fat percentage of body weight were also measured. The CV of the BMD measurements ranged from 0·5 to 0·9 % and was based on measurements by repeated measurements on the same control. The measurement that was repeated included all steps of the measurement (everything from positioning the patient to image analysis) in order to cover every conceivable error of the measurement.
Peripheral quantitative computerised tomography
A pQCT device (XCT-2000; Stratec Medizintechnik GmbH, Pforzheim, Germany) was used to scan the non-dominant distal tibia and distal radius. The pQCT was calibrated every week, using a standard phantom, and once every 30 d using a cone phantom provided by the manufacturer. A 2 mm single tomographic slice was scanned with a voxel size of 0·50 mm. The cortical volumetric BMD (vBMD; mg/cm3), cortical BMC (mg/mm), cortical cross-sectional area (mm2) and cortical thickness (mm) were measured by scanning through the diaphysis at 25 % of the bone length in the proximal direction at the distal end of the bones. Trabecular vBMD was measured by scanning through the metaphysis at 4 % of the bone length in the proximal direction at the distal end of the bones. Tibia length was measured from the medial malleolus to the medial condyle, and forearm length was defined as the distance from the olecranon to the ulnar styloid process. The CV were less than 1 % for all pQCT measurements, estimated by repeated measurements on the same control as described earlier in the DXA section.
Fatty acids in serum phospholipids
Blood samples were obtained in relation to the bone measurements and frozen at − 70°C until analysis within 2 weeks. After lipid extraction, phospholipids were fractionated on a single SEP-PAK aminopropyl cartridge (Waters Corp., Beverly, MA, USA), transmethylated and separated by capillary GLC in a Hewlett-Packard 6890 gas chromatograph (Palo Alto, CA, USA) as previously describedReference Gronowitz, Mellstrom and Strandvik4.
Statistical analysis
Mean values and standard deviations are given if not otherwise indicated. Statistical analyses were performed by StatView® 5·0 (SAS Institute Inc., Cary, NC, USA), using Spearman rank test and multiple regression analysis for correlation tests. Student's t test was used for comparison between groups for normally distributed data, otherwise with non-parametric tests such as the Mann–Whitney U test. Statistically significant difference was set at P < 0·05.
Results
Table 1 summarises the clinical characteristics of CF patients and controls. The CF patients were slightly, but significantly, older than the control group. The anthropometric data were similar. There was no significant difference between reported duration of physical activity per week or handgrip strength between the two groups. The CF patients consumed more vitamin supplements (P < 0·0001) and more dairy products (P < 0·05) than the controls. The intake of vegetables was similar (data not shown). DXA did not reveal differences in bone parameters or LBM between CF patients and controls. Percentage of fat mass was lower in the patients (Table 2), but the difference disappeared when the one most severely ill patient (FEV1·0 31 %) was excluded, indicating that body composition was similar to controls in thirteen of the fourteen patients. Of the CF patients and the controls, 21 and 10 % respectively considered themselves to have a late puberty; the difference was not significant, probably due to the low number of patients (Table 1). The BMD at all sites measured by DXA and the endosteal circumference measured by pQCT in relation to self-assessed puberty stage showed a similar trend in controls and CF patients. Fig. 1 illustrates this for BMDLS and the endosteal circumference of radius.
Table 1 Clinical data in patients with cystic fibrosis (CF) and healthy controls
(Mean values and standard deviations)

Value was significantly different from that of the control group: *P < 0·05, ***P < 0·001.
† One control missing.
Table 2 Body composition and bone mass evaluated by dual-energy X-ray absorptiometry in patients with cystic fibrosis (CF) and healthy controls
(Mean values and standard deviations)
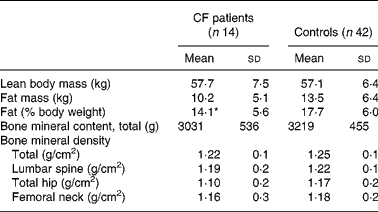
* Mean value was significantly different from that of the control group (P < 0·05).
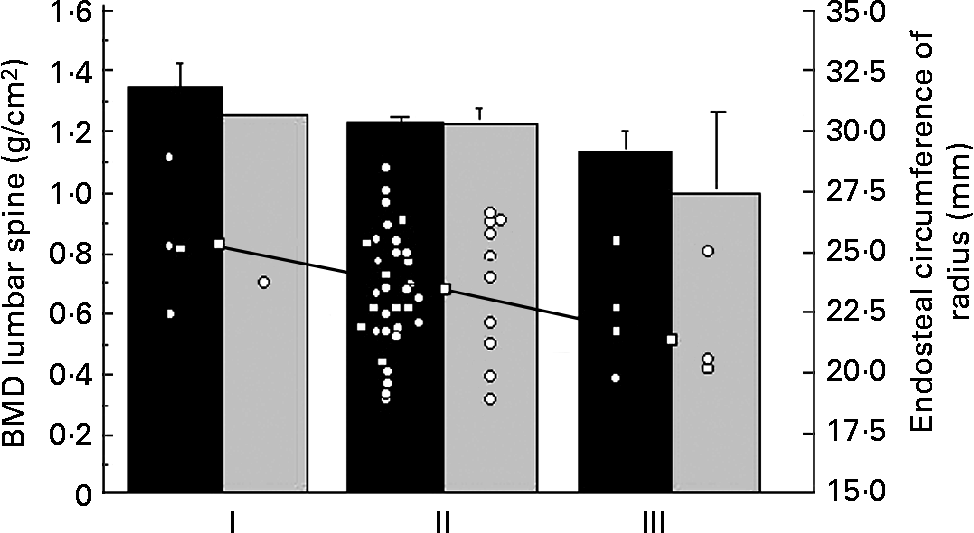
Fig. 1 Bone mineral density (BMD) of the lumbar spine measured by dual-energy X-ray absorptiometry in fourteen patients with cystic fibrosis (![]() ) and forty-two healthy controls (■) in relation to self-assessed onset of puberty (early (I), average (II) or late (III)), as reported in the questionnaire. Values are means, with their standard errors represented by vertical bars. Endosteal circumference of radius (○) measured by peripheral quantitative computerised tomography. Median (□) of endosteal circumference for the different puberty stages showed a significant trend (r − 0·27; P < 0·05).
) and forty-two healthy controls (■) in relation to self-assessed onset of puberty (early (I), average (II) or late (III)), as reported in the questionnaire. Values are means, with their standard errors represented by vertical bars. Endosteal circumference of radius (○) measured by peripheral quantitative computerised tomography. Median (□) of endosteal circumference for the different puberty stages showed a significant trend (r − 0·27; P < 0·05).
Cortical BMC, vBMD and periosteal and endosteal circumferences of tibia and radius did not differ between CF patients and controls. The trabecular vBMD in the tibia differed between patients and controls (P = 0·04; Table 3), but not after adjustment for age and weight (data not shown). There were no differences in bone parameters between patients with pancreatic insufficiency and those with pancreatic sufficiency or controls. Chronic colonisation with Ps. aeruginosa was associated with significantly lower total BMC and BMD measured by DXA, both compared with controls and with CF patients without pseudomonal colonisation (P = 0·01 for each). BMDLS differed between controls and patients chronically colonised with Ps. aeruginosa (P = 0·04). With pQCT, the chronically colonised patients showed lower cortical BMC and bone area in both tibia and radius than patients who were not colonised (P = 0·02 for each). The colonised patients also differed from controls in cortical BMC, cortical thickness and cross-sectional area and trabecular BMD of the tibia (P = 0·01 for each). No differences were found in corresponding bone parameters of radius. Physical activity was similar in both chronically colonised and non-colonised patients (data not shown).
Table 3 Bone parameters measured by peripheral quantitative computerised tomography in patients with cystic fibrosis (CF) and healthy controls
(Mean values and standard deviations)
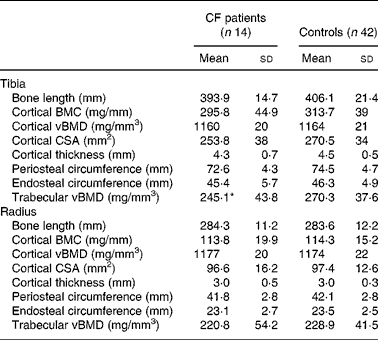
BMC, bone mineral content; vBMD, volumetric bone mineral density; CSA, cross-sectional area.
* Mean value was significantly different from that of the control group (P < 0·05).
The serum concentration of fatty acids in phospholipids differed significantly in CF patients from those in controls (Table 4). The total SFA was decreased and MUFA increased. The oleic acid:stearic acid ratio was higher in CF patients than in controls, indicating an increased Δ9-desaturase activity (P < 0·001). The molar concentration of eicosatrienoic acid and the eicosatrienoic acid:arachidonic acid ratio were increased, suggesting EFA deficiency in the patients with CF. This was supported by the significantly lower concentration of linoleic acid in the patients. The dihomo-γ-linolenic acid:linoleic acid ratio indicated a higher Δ6-desaturase activity in the patients than controls (P < 0·001), but a lower Δ5-desaturase activity, as represented by the arachidonic acid:dihomo-γ-linolenic acid ratio (P = 0·02; Table 4). No correlation was found between the desaturase activities and bone parameters (data not shown). In the patients with CF, the endosteal circumference of the radius was inversely correlated to the n-6:n-3 fatty acid ratio (r − 0·73; P < 0·01; Fig. 2 (A)), but with a positive trend to cortical thickness (r 0·38; P = 0·18; Fig. 2 (B)). This was related to the n-3 fatty acids, since the endosteal circumference was strongly correlated both to total n-3 and to the long-chain product, DHA (r 0·79; P < 0·001 for both; Fig. 2 (C)). As expected then, the corresponding association with cortical thickness was negative (r − 0·57; P = 0·035; Fig. 2 (D)). The same trend of correlations was seen in the tibia although not significant (DHA, r 0·38, P = 0·18; n-6:n-3 fatty acids, r − 0·28, P = 0·31). A positive correlation was also found between arachidonic acid:DHA and cortical thickness, (r 0·57; P < 0·05), suggesting that the balance between n-6:n-3 fatty acids was of importance for skeletal size in the CF patients. This was further indicated by bone length being negatively associated with arachidonic acid both in the radius and tibia in the healthy controls, being r − 0·44 (P < 0·01) and r − 0·32 (P < 0·05), respectively.
Table 4 Molar percentage of major fatty acids in serum phospholipids in patients with cystic fibrosis (CF) and healthy controls
(Mean values and standard deviations)

OA, oleic acid; ETA, eicosatrienoic acid; DGLA, dihomo-γ-linolenic acid; LA, linoleic acid; AA, arachidonic acid.
Mean value was significantly different from that of the control group: *P ≤ 0·05, **P ≤ 0·01, ***P ≤ 0·001.
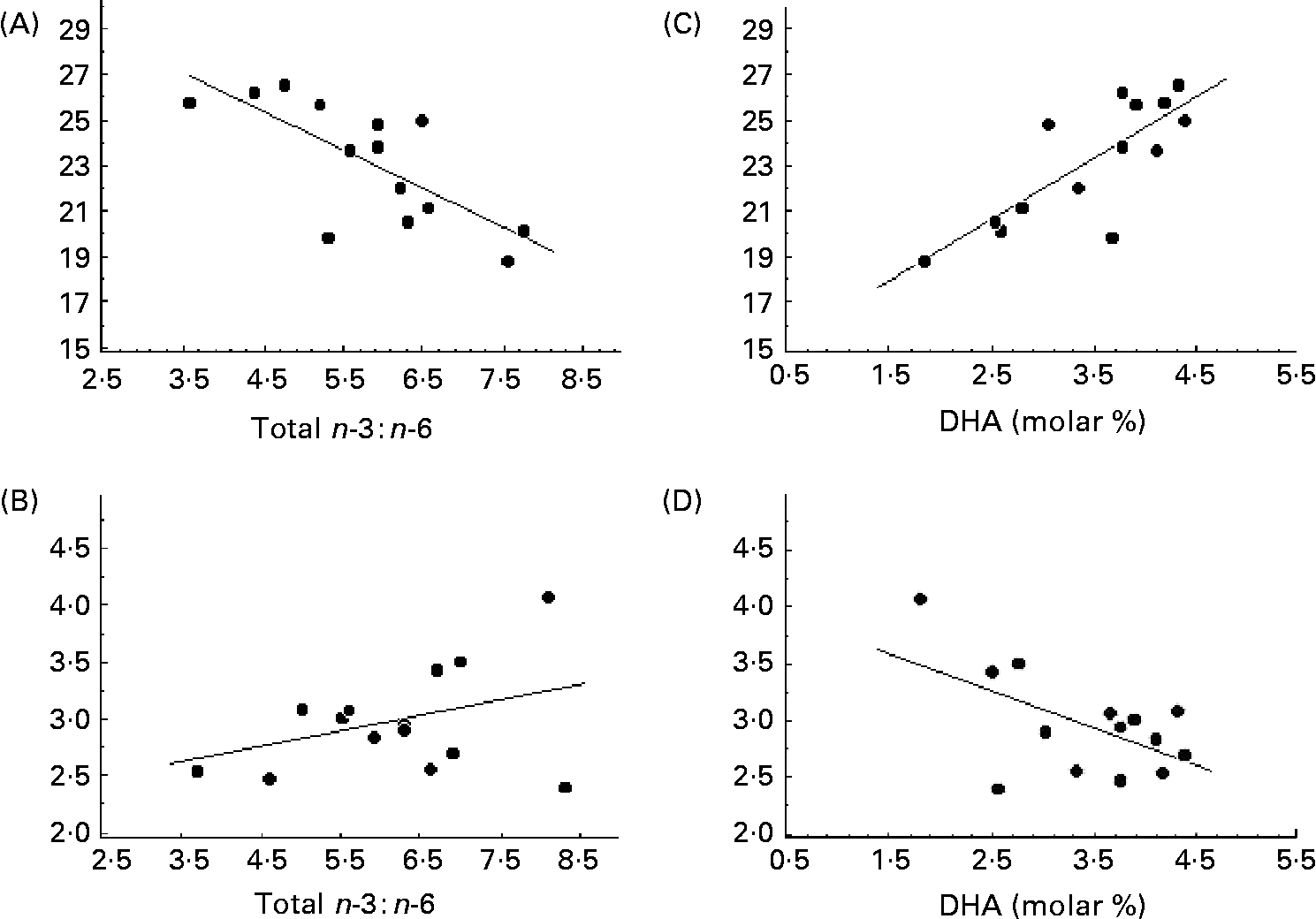
Fig. 2 (A) Total n-6 : n-3 fatty acids ratio in relation to the endosteal circumference of radius in fourteen patients with cystic fibrosis (r − 0·73; P = 0·03); (B) n-6 : n-3 fatty acids ratio in relation to the cortical thickness of radius (r 0·38; P = 0·18); (C) molar concentration of DHA in serum phospholipids in relation to the endosteal circumference of radius (r 0·79; P = 0·0008); (D) molar concentration of DHA in serum phospholipids in relation to the cortical thickness of radius (r − 0·57; P = 0·035).
Correlations between bone parameters and BMI, LBM, fat mass and FEV1·0 in patients with CF and controls are shown in Table 5. Multiple regression analyses showed that LBM was stronger correlated to BMC, analysed with both DXA and pQCT, than was BMI in both CF patients and controls (data not shown). Mainly similar correlations were found in CF subjects and controls, supporting similar associations between body composition and bone mass. In patients with CF, multiple regression analyses showed FEV1·0 to be the strongest predictor for cortical BMC, eliminating the influence of BMI and LBM (P = 0·02).
Table 5 Correlations between clinical parameters and bone parameters in fourteen patients with cystic fibrosis (CF) and forty-two healthy controls, measured by dual energy X-ray absorptiometry (DXA) and peripheral quantitative computerised tomography (pQCT)

LBM, lean body mass; FEV1·0, forced expiratory volume in 1 s; BMC, bone mineral content; BMD, bone mineral density.
Regression coefficient was significant: *P < 0·05, **P < 0·01, ***P < 0·001.
Discussion
In this small group, but for our centre representative and non-selected male young adult patients with CF, we found that cortical and trabecular BMC were not different from the healthy controls. The only difference found was in trabecular vBMD of the tibia. It might be speculated that this difference between CF patients and controls would be stronger if the group of CF patients had been larger. Many studies have shown that trabecular bone density is more affected in CF and associated with inflammatory markersReference Gronowitz, Mellstrom and Strandvik5–Reference Baroncelli, De Luca, Magazzu, Arrigo, Sferlazzas, Catena, Bertelloni and Saggese11, Reference Buntain, Greer, Schluter, Wong, Batch, Potter, Lewingdon, Powell, Wainwright and Bell13, Reference Haworth, Selby, Webb, Martin, Elborn, Sharples and Adams19, Reference Conway20, Reference Gronowitz, Garemo, Lindblad, Mellstrom and Strandvik22. Since the tibia and radius are the last bones to attain peak bone massReference Lorentzon, Mellstrom and Ohlsson46, the finding might be complementary to the results that endosteal contraction in the radius was not finished in the CF patients. Further studies are indicated to determine if measurement of the tibia might be a more sensitive marker of low BMD than LS and femoral neck.
The present results are in line with our previous finding in a prospective 2-year study, where the average annual increase of BMDLS and BMDfemoral neck were normal in children and the levels constant in the adultsReference Gronowitz, Mellstrom and Strandvik5. These results differ markedly from most other reportsReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6–Reference Buntain, Greer, Schluter, Wong, Batch, Potter, Lewingdon, Powell, Wainwright and Bell13 and might be associated with many factors related to treatment. Our patient population does not differ genetically from most other studiesReference Strandvik, Björck, Fallström, Gronowitz, Thountzouris, Lindblad, Markiewicz, Wahlström, Tsui and Zielenski47, but generally has a better growth, pulmonary function and BMDReference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3–Reference Gronowitz, Mellstrom and Strandvik5, Reference Gronowitz, Garemo, Lindblad, Mellstrom and Strandvik22.
The present results first of all show that normal bone parameters are possible to obtain in a non-selected group of young men with classical CF and in whom 79 % carried severe CFTR mutations. A few studies have shown that normal bone mass can be achieved in patients with CF compared with other patients of similar height, i.e. normal but stunted patients, both children and adultsReference Salamoni, Roulet, Gudinchet, Pilet, Thiebaud and Burckhardt48, Reference Hardin, Arumugam, Seilheimer, LeBlanc and Ellis49. Our patients were compared with controls representative to the general population of similar age. It is interesting to note that despite the good condition of the patients, the influence of bacterial colonisation and lung function on bone mass was significant, implicating that the pulmonary treatment might be the strongest predictor of bone mass in CF in physically active patientsReference Gronowitz, Mellstrom and Strandvik5, Reference Aris, Merkel and Bachrach23. It also implies that in a larger CF population there might have been a small, but significant, difference from controls.
In a recent study of adult patients with much more severe pulmonary impairment and a high percentage of steroid treatment, an association was found with the ΔF508 mutation independent of lung function and malnutritionReference Haworth, Selby, Webb, Martin, Elborn, Sharples and Adams19. Others and we have not found such a relationship and it cannot be excluded that the relationship with ΔF508 might reflect a common delineator such as EFA deficiency, which has been shown to be related to genotypeReference Strandvik, Gronowitz, Enlund, Martinsson and Wahlstrom3. In our centre the EFA deficiency in the patients is balanced by oral supplementation of vegetable oils or in severe cases by lipid emulsions intravenouslyReference Strandvik, Ghraf, Aggett, Lifschitz, Walker-Smith and Morán34. The extent of EFA deficiency is therefore probably lower than in many other centres.
Late puberty and low testosterone concentrations have been shown to be common in CF patients, and might probably contribute to a later achievement of peak bone massReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6, Reference Bhudhikanok, Wang, Marcus, Harkins, Moss and Bachrach7, Reference Conway, Morton, Oldroyd, Truscott, White, Smith and Haigh9–Reference Baroncelli, De Luca, Magazzu, Arrigo, Sferlazzas, Catena, Bertelloni and Saggese11, Reference Leifke, Friemert, Heilmann, Puvogel, Smaczny, von zur Muhlen and Brabant26. It has been shown that free testosterone is a positive predictor of cortical bone size in young menReference Lorentzon, Swanson, Andersson, Mellstrom and Ohlsson50. Although we had no possibility to measure testosterone in the present study, the self-assessment suggested that more CF patients than controls considered themselves to have a late puberty (Table 1). This might indicate that a significant difference of BMD might be found in a larger population of patients, as indicated by the significantly lower trabecular vBMD of tibia in the CF patients, which disappeared when adjusted for weight and height. Late puberty will also include a later peak bone mass, which might explain why an association between fatty acids and bone mass was only found in the CF patients but not in the controls, corroborating our previous results in CFReference Gronowitz, Mellstrom and Strandvik4. During growth the endosteal circumference increases in the long bones, but after cessation of linear growth the endosteal circumference restrains, when the cortex grows thickerReference Lorentzon, Mellstrom and Ohlsson46. The long bones are the last ones, where this can be measured. Self-assessment of puberty and peak height velocity, which occurs at the time of puberty, have been found strongly correlatedReference Kindblom, Lorentzon, Norjavaara, Hellqvist, Nilsson, Mellström and Ohlsson51, the correlation coefficient being r 0·50 (P < 0·01) in the controls.
The trend between endosteal circumference and self-assessed puberty supports an earlier report of a large healthy populationReference Lorentzon, Mellstrom and Ohlsson46 (Fig. 1). Since it has previously been shown a relationship between fatty acids and bone mass in children but not in adults with CFReference Gronowitz, Mellstrom and Strandvik4 and a relationship between n-3 fatty acids in healthy menReference Hogstrom, Nordstrom and Nordstrom29, the association in the present study between EFA and endosteal circumference and thickness only in the CF patients suggests that the patients were later in puberty than the controls. It also implies that EFA, especially the n-6:n-3 ratio, are of importance for the final bone modelling of the long bones (Fig. 2). High concentrations of DHA were associated with thinner cortical thickness and a similar association was found between maternal blood erythrocyte DHA concentration and bone mineralisation of both femur and LS in healthy newbornsReference Weiler, Fitzpatrick-Wong, Schellenberg, McCloy, Veitch, Kovacs, Kohut and Kin Yuen37. That a balance between the n-6 and n-3 fatty acids is important was also found in the animal studies, where a high-n-3 diet perinatally resulted in lower bone mass in the adult animalReference Korotkova, Ohlsson, Hanson and Strandvik35. This implies that a selective supplementation of DHA to CF patients as well as to other categories of patients might have other non-desirable effects.
Conclusion
Contrary to all previous reports of adult CF patients with high prevalence of osteoporosisReference Haworth, Selby, Horrocks, Mawer, Adams and Webb6–Reference Buntain, Greer, Schluter, Wong, Batch, Potter, Lewingdon, Powell, Wainwright and Bell13, the present study showed that normal BMD can be obtained in a small but non-selected group of young CF men with classical severe CF. Several factors in our treatment policy might be responsible:
social insurance did not limit the use of expensive antibiotics in the treatment of mild exacerbations of pulmonary diseaseReference Strandvik52;
physical activity has been a part of the regular treatment regimen from an early age onReference Blomquist, Freyschuss, Wiman and Strandvik40, Reference Lannefors, Button and McIlwaine41;
there is a restricted use of steroids;
fat is supplemented on a more-or-less regular basis, contributing to normal anthropometric data, although a total normalisation of the phospholipid fatty acid pattern was not obtainedReference Strandvik, Ghraf, Aggett, Lifschitz, Walker-Smith and Morán34.
We suggest that one common denominator for the previously reported association of low BMD and the ΔF508 mutation might be the lipid abnormalityReference Carlstedt-Duke, Bronnegard and Strandvik1, Reference Miele, Cordella-Miele, Xing, Frizzell and Mukherjee2, Reference Watkins, Lippman, Le Bouteiller, Li and Seifert27. Such association can be explored by early nutritional support including EFA as suggested by the Wisconsin CF Neonatal Screening GroupReference Shoff, Ahn, Davis and Lai53, as well as by other prospective intervention investigations.
Acknowledgements
The authors are grateful to Sofia Heigis, Sarah McGovern, Emelie Svensson, Martin Svensson and Veronika Sjöstrand for performing the DXA and pQCT scans. The present study was supported by grants from the Faculty of Medicine, Gothenburg University and the Swedish Association of Cystic Fibrosis.









