Se is an essential trace element for fish(Reference Khan, Zuberi and Fernandes1). Its deficiency has been demonstrated to exert various negative influences on fish, such as the suppression of growth performance and feed utilisation(Reference Lin and Shiau2), the increase of mortality(Reference Poston, Combs and Leibovitz3), oxidative stress(Reference Bell, Cowey and Adron4) and inflammation response(Reference Gao, Tang and Liang5), etc. In intensive aquaculture systems, fish primarily obtain sufficient Se from the proper feeds(Reference Khan, Zuberi and Fernandes1). Thus, the optimal dietary Se level is crucial for fish to maintain normal growth and physiological activities(Reference Khan, Zuberi and Fernandes1). Up to now, dietary Se requirements have been widely explored in a variety of fish species including grouper (Epinephelus malabaricus)(Reference Lin and Shiau2), rainbow trout (Oncorhynchus mykiss)(Reference Hilton, Hodson and Slinger6), channel catfish (Ictalurus punctatus)(Reference Gatlin and Wilson7), Nile tilapia (Oreochromis niloticus)(Reference Lee, Nambi and Won8), gibel carp (Carassius auratus gibelio var. CAS III)(Reference Zhu, Han and Zhu9) and blunt snout bream (Megalobrama amblycephala)(Reference Liu, Jiang and Lu10), etc. As a determinant for yield and economic efficiency in the aquaculture industry, fish growth performance has been extensively considered as a key index for the evaluation of dietary Se requirements. However, still little is known about the regulatory mechanism of dietary Se in fish growth.
Se being an integral part of selenoproteins plays an important role in the regulation of several biological pathways in fish body(Reference Lu and Holmgren11). Until now, a total of forty-one selenoproteins have been identified in teleost(Reference Mariotti, Ridge and Zhang12). Selenoproteins have a characteristic of hierarchy expression among tissues(Reference Sunde and Raines13). Previously, Wang et al.(Reference Wang, Zhang and Wu14) explored the differential expression pattern of a total of twenty-eight selenoprotein genes in different tissues of rainbow trout by feeding fish with gradually increased levels of dietary Se. Results showed that selenoprotein genes in trout muscle were much sensitive to dietary Se with more than 1/3 selenoprotein genes were up-regulated by the elevated dietary Se levels(Reference Wang, Zhang and Wu14). Furthermore, a remarkable phenomenon was observed that trout somatic growth was positively correlated with the expression of all the differentially expressed selenoprotein genes in the muscle(Reference Wang, Zhang and Wu14). These observations show the importance of dietary Se in the metabolic regulation of trout muscle growth, which might be an important way to control the somatic growth of rainbow trout. Muscle, which represents 40–60 % of fish body mass, is the largest tissue in fish, and its dry biomass is predominantly protein(Reference Valente, Moutou and Conceição15,Reference Seiliez, Dias and Cleveland16) . Nutrient modelling in rainbow trout suggests that protein deposition in muscle tissue is among the main determinants for fish somatic growth(Reference Bureau, Hua and Cho17). In view of this, the improvement of dietary Se for fish growth performance might be, at least partly, attributed to the accelerative protein deposition in fish muscle(Reference Khan, Zuberi and Fernandes1). And this inference could be supported by the elevated muscle protein content in rainbow trout(Reference Hunt, Berkoz and Ozkan18,Reference Wang, Wu and Liu19) and mahseer (Tor putitora)(Reference Khan, Zuberi and Nazir20) after feeding diet supplemented with exogenous Se.
Protein deposition in fish tissues depends on both rates of protein synthesis and protein degradation, with protein accumulation requiring the rate of protein synthesis to exceed that of degradation(Reference Johnston, Bower and Macqueen21). Recently, we have conducted an experiment on rainbow trout to explore the nutritional effects of Se on the basal protein synthesis and degradation in fish muscle and found that optimum dietary Se inhibited the basal protein degradation while exerted no influence on the basal protein synthesis(Reference Wang, Wu and Liu19). These results preliminarily reveal a regulatory effect of dietary Se on protein deposition in fish muscle by controlling the basal protein degradation. However, under normal farming conditions, cultured fish always receive multiple times of feeding per d. Ingestion of feeds has been reported to result in dynamic changes of protein synthesis and degradation in fish muscle after a meal(Reference Seiliez, Panserat and Skiba-Cassy22–Reference Seiliez, Gabillard and Skiba-Cassy25). From this consideration, our previous results focusing on the basal protein synthesis and degradation are still insufficient to explain the acceleration of dietary Se on protein deposition in fish muscle. Further investigation of the effect of dietary Se on postprandial dynamic changes of protein synthesis and degradation in fish muscle could help to better understand the nutritional effects of Se on fish muscle growth. In the present study, a postprandial kinetic-metabolic model was applied to juvenile rainbow trout, a commercially important fish species throughout the world, to comprehensively evaluate the nutritional effects of Se on protein deposition in its muscle tissue.
Materials and methods
The present experiment (HZAUFI-2018-017) was approved by The Scientific Ethic Committee of Huazhong Agricultural University, Wuhan, China.
Experimental diets
In this study, two experimental diets were prepared through supplementing a basal diet with or without 4 mg/kg Se from Se yeast and the analysed total Se contents were 0·75 and 4·68 mg/kg, respectively. Feed ingredients of the basal diet are presented in Table 1. The detailed procedure for diet preparation and storage was described in our previous study(Reference Wang, Zhang and Wu14). And the analysed proximate composition of the experimental diets is presented in Table 1.
Table 1. Formulation and composition of the experimental diets (g/kg dry diet)
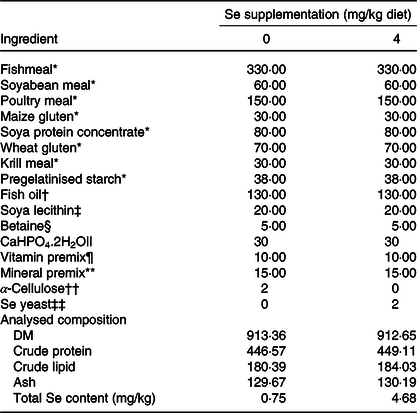
* Fishmeal (crude protein 678·2 g/kg, crude lipid 80·1 g/kg), soyabean meal (crude protein 485·2 g/kg, crude lipid 8·9 g/kg), poultry meal (crude protein 681·0 g/kg, crude lipid 198·2 g/kg), maize gluten (crude protein 637·4 g/kg, crude lipid 22·3 g/kg), soya protein concentrate(crude protein 857·2 g/kg, crude lipid 34·1 g/kg), wheat gluten (crude protein 838·8 g/kg, crude lipid 102·2 g/kg), krill meal (crude protein 507·4 g/kg, crude lipid 31·7 g/kg) and pregelatinised starch were purchased from Hubei Haida feed Co. Ltd.
† Dalian, China.
‡ Soya lecithin (≥ 70 %, Aladdin).
§ Betaine (98 %, Aladdin).
|| CaHPO4·2H2O (98 %, Aladdin).
¶ Vitamin premix (g/kg product): retinol acetate, 0·45, thiamine, 1·50; lactoflavine, 3·00; nicotinic acid, 17·50; d-calcium pantothenate, 5·00; pyridoxine-HCl, 1·50; d-biotin, 0·25; cyanocobalamin, 0·01; l-ascorbic acid, 500·00; cholecalciferol, 0·04; dl-a-tocopheryl acetate, 6; menadione, 0·50; folic acid, 50·00; inositol, 100·00; choline chloride, 200·00; α-cellulose, 114·26.
** Mineral premix (g/kg product): CaCO3, 143·33; Mg(OH)2, 199·87; FeSO4.7H2O, 13·33; KI, 0·03; ZnSO4.7H2O, 26·67; CuSO4.5H2O, 20·00; MnSO4.H2O, 20·00; CaHPO4.2H2O, 333·33; CoCl.6H2O, 0·13, KCl, 60·00; NaCl, 26·67; α-cellulose, 236·64.
†† α-Cellulose (Aladdin).
‡‡ Se yeast (selenium yeast, total Se content: 2 g/kg, Angel Yeast Co. Ltd).
Feeding trial and sampling
Sexually immature rainbow trout (O. mykiss) were obtained from Enshi Guoxi Fishery Development Co. Ltd and kept in experimental facilities at Huazhong Agriculture University, Wuhan, China. During the acclimatisation period, fish were fed the basal diet twice (09.00 and 16.00 hours) per d, for 2 weeks. The feeding trial was conducted in a flow-through system where each tank (1500 litres) was equipped with an inlet, outlet and continuous aeration. A flow rate of 1·5 litres/min was maintained throughout the feeding trial. Before the feeding trial, fish were fasted for 24 h and individually weighed. A total of 600 juvenile rainbow trout with an average body weight of 12·68 (sd 2·06) g and an average body length of 88·57 (sd 2·01) mm were randomly distributed in six tanks, with 100 fish in each tank. The tanks were randomly divided into two groups, and there were three tanks in each group. Fish were fed with the experimental diets twice (09.00 and 16.00 hours) per d, to visual satiation, for 6 weeks. During the feeding trial, the dissolved oxygen and the temperature of the water were 8·51 (sd 0·14) mg/l and 18·03 (sd 0·12) °C, respectively. A photoperiod of 12 h light–12 h dark was strictly maintained using an automatic time switch.
At the beginning of the feeding trial, six fish from the same population were randomly selected for the determination of the initial whole-body protein content. At the end of the feeding trial, fish were subjected to fasting. After 24 h of fasting, fish were individually weighed and counted. Furthermore, two fish were randomly sampled from each tank for the determination of muscle proximate composition and total Se content. Another two fish were randomly sampled from each tank for the determination of final whole-body protein content. After 48 h of fasting, fish reached the basal levels of metabolites in plasma and muscle(Reference Belghit, Panserat and Sadoul23,Reference Skiba-Cassy, Panserat and Larquier26) . Then, fish were re-fed once with their allocated diets until visible satiation to conduct the postprandial kinetic-metabolic study on protein synthesis and degradation in muscle tissues. Samples were collected before refeeding (0 h) and at 4, 8, 12 and 24 h after refeeding. At each interval, two fish from each tank were randomly taken and anaesthetised with tricaine methanesulphonate (MS-222, 100 mg/l water, Western Chemical, Inc.), subsequently killed by a sharp blow to the head. The blood was taken from the caudal vein and stored in heparinised tubes. After centrifugation at 5000 g for 15 min (4°C), the plasma was obtained and stored at –80°C for further analysis of the level of total free amino acids. Two dorsal muscle samples (about 100 mg) close to the vertebra and in the same location of each fish were sampled and stored at –80°C for further mRNA and protein analysis, respectively. The remaining dorsal muscles were mashed and stored at –20°C for the determination of muscle total free amino acid level and total Se content.
Determination of proximate composition and total selenium content
Proximate composition and total Se content of experimental diets, fish whole body and dorsal muscle were measured according to the methods described in our previous study(Reference Wang, Wu and Liu19). Briefly, DM was determined by drying in an oven at 105°C to constant weight. Crude protein content was determined with an automatic Kjeldahl analyzer (K9860; Jinan Hanon Instruments Co. Ltd), and the proportion of crude protein was calculated as total N × 6·25. Crude lipid content was extracted after acid hydrolysis according to Soxhlet method. Ash was determined by incineration at 550°C to constant weight. Total Se content was measured by inductively coupled plasma MS (Agilent 7500c, Yokogawa Analytical Systems) after digestion with concentrated HNO3 and H2O2.
Determination of total free amino acid level in plasma and dorsal muscle
Dorsal muscle samples were put in 2 ml sterile centrifugal tubes and homogenised with nine volumes (w/v) of ice-cold normal saline for 3 min at 4°C and centrifuged for 15 min at 4000 g . The supernatants were collected for the determination of total amino acid levels. Plasma was directly subjected to further analysis after being thawed on ice. Total amino acid level was detected using the corresponding detection kits purchased from Nanjing Jiancheng Bioengineering Institute (Jiangsu, China) according to the manufacturer’s recommendations.
Protein extraction and Western blot analysis
Muscle samples (about 100 mg) were homogenised on ice using 1 ml of radio immunoprecipitation assay buffer (P0013B, Beyotime Biotechnology) with protease inhibitor (P1010, Beyotime Biotechnology) and phosphatase inhibitor (P1081, Beyotime Biotechnology). Lysates were centrifuged at 12 000 g (4°C) for 10 min. Protein concentrations were determined using a BCA Protein Assay Kit (P0012S, Beyotime Biotechnology) with bovine serum albumin as standard. Lysates (10 μg of total protein) were subjected to SDS-PAGE and transferred to 0·45 μm nitrocellulose membrane (Millipore Co.) for Western blot analysis. Primary antibodies against phospho-S6 (Ser235/Ser236, catalogue no. 4856), carboxyl terminal S6 (catalogue no. 2217), phospho-4E-BP1 (Thr37/46, catalogue no. 9459), 4E-BP1 (catalogue no. 9452), phospho-eIF2α (Ser51, catalogue no. 9721), carboxyl terminal eIF2α (catalogue no. 9722) and LC3B (catalogue no. 2775) were purchased from Cell Signaling Technology (CST) Inc. and have been previously validated in rainbow trout(Reference Seiliez, Gabillard and Riflade27,Reference Belghit, Skiba-Cassy and Geurden28) . For primary antibodies against phospho-eEF2 (Thr56, catalogue no. 2331, CST), eEF2 (catalogue no. 2332, CST), ubiquitin (P4D1, catalogue no. 3936, CST) and β-tubulin (catalogue no. AC008, ABclonal), the amino acid sequences were monitored in the SIGENAE database (http://www.sigenae.org) to check for a good conservation of the antigen sequence. Nitrocellulose membranes were washed and incubated with an IRDye Infrared secondary antibody (LI-COR Inc. Biotechnology). Bands were visualised by infrared fluorescence using the Odyssey imaging system (LI-COR Inc. Biotechnology) and were quantified by Odyssey Infrared imaging system software (version 5.2; LI-COR Inc. Biotechnology).
Extraction of total RNA and DNA
Total RNA and DNA were extracted from 100 mg muscle samples using TRIzol reagent (Invitrogen) according to the manufacturer’s instructions. After being extracted, RNA and DNA pellets were resuspended in 40 μl nuclease-free water. The concentrations of RNA and DNA were determined by NanoDrop ND-1000 (NanoDrop Technologies®). RNA samples were then run on a 1·5 % agarose gel and visualised on a Gel Doc EQ imaging system (BIO-RAD Laboratories). Densitometric measurements of the 28S and 18S ribosomal RNA were performed with ImageJ software (version 1.8.0).
Gene expression analysis
Complementary DNA synthesis was performed with a PrimeScript™ RT Reagent kit with gDNA Eraser (Takara) following the manufacturer’s instructions. The mRNA abundances of target genes were determined by quantitative real-time PCR using specific primers (Table 2). PCR was performed on a QuantStudio 6 Flex Real-Time PCR System (Applied Biosystems) using SYBR® Premix Ex Taq ™ (Tli RNaseH Plus) (Takara) as previously described(Reference Wang, Zhang and Wu14). The relative quantification of the target gene was performed using the mathematical model described by Pfaffl(Reference Pfaffl29) and normalised to the geometric mean of the best combination of eukaryotic translation elongation factor 1α (EF1α) and β-actin (Reference Vandesompele, De Preter and Pattyn30).
Table 2. Oligonucleotide primers used in the quantitative real-time PCR assays

CAPN1, catalytic subunits of μ-calpain; CAPN2, catalytic subunits of m-calpain; CAST-L, calpastatin long isoform; CAST-S, calpastatin short isoform; Atg4b, autophagy-related 4b; Atg12l, autophagy-related 12-like; LC3B, microtubule-associated light chain 3B; Gabarapl1, γ-aminobutyric acid type A receptor-associated protein-like 1; Cts, cathepsin; MuRF, muscle RING finger; Fbx, F-box protein; EF1α, eukaryotic translation elongation factor 1α.
Statistical analysis
Data obtained from each tank at each sampling time were pooled (n 3 tanks per diet) and statistically analysed using SPSS 19.0 (SPSS Inc.). Shapiro–Wilks test and Levene’s test were performed to test the normal distribution and homogeneity of the variances, respectively. Data with non-normal distribution were subjected to logarithmic (Log10) or square root (SQRT) transformations. Data on growth performance, feed utilisation and muscle proximate composition were analysed by two-sided Student’s t test. The postprandial dynamic changes (over time, T) of total free amino acid levels in tissues (plasma and muscle) and factors related to protein synthesis and degradation in muscle tissue of rainbow trout fed diets with different levels of Se (Se) were subjected to a two-way (T × Se) ANOVA followed by a Bonferroni–Dunn multiple comparison. The level of significance was set at P < 0·05.
Results
Growth performance and feed utilisation
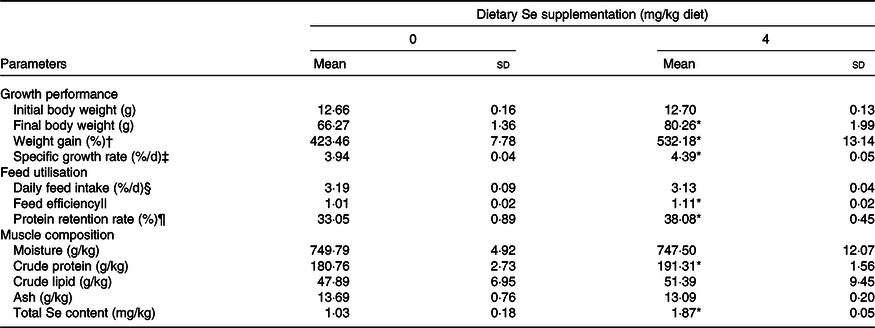
Juvenile rainbow trout accepted the experimental diets well, and no death was observed throughout the feeding trial. Fish fed diet supplemented with 4 mg/kg Se from Se yeast exhibited the significantly larger body size (weight and length), higher growth rate (weight gain and specific growth rate) and higher feed utilisation (feed efficiency and protein retention rate) than those fed the basal diet after 6 weeks of feeding (Table 3, P < 0·05). However, fish daily feed intake exhibited no significant difference between the treatments (Table 3).
Table 3. Growth performance, feed utilisation and muscle proximate composition of juvenile rainbow trout (Oncorhynchus mykiss) fed a basal diet supplemented with or without 4 mg/kg selenium (as selenium yeast) for 6 weeks
(Mean values and standard deviations, n 3)
* Mean values in fish fed diet supplemented with 4 mg/kg Se significantly differ from those fed the basal diet (P < 0·05, two-sided Student’s t test).
† Weight gain = 100 × (final body weight – initial body weight)/initial body weight.
‡ Specific growth rate (%/d) = 100 × (ln final body weight – ln initial body weight)/d.
§ Daily feed intake (%/d) = 100 × total feed consumption/(42 d × (initial body weight + final body weight)/2).
|| Feed efficiency = wet weight gain (g)/total feed consumed (g).
¶ Protein retention rate (%) = (100 × (final body weight × final whole-body protein content) – (initial body weight × initial whole-body protein content)/total feed consumption × protein content of diet.
Proximate composition and total selenium content in dorsal muscle
As shown in Table 3, dietary Se significantly increased the crude protein content and total Se content in the dorsal muscle of juvenile rainbow trout (P < 0·05) after the 6-week feeding trial while exerted no influences on the content of moisture, crude lipid and ash.
Postprandial total free amino acid levels in plasma and dorsal muscle
After refeeding, the total free amino acid levels in both plasma and dorsal muscle significantly increased from 4 to 12 h and reached the maximum levels at 8 h (Fig. 1, P < 0·05). Subsequently, they decreased to similar levels as those at 0 h (Fig. 1). No significantly different total free amino acid levels in plasma and dorsal muscle were observed between the treatments at each time point before and after refeeding (Fig. 1).
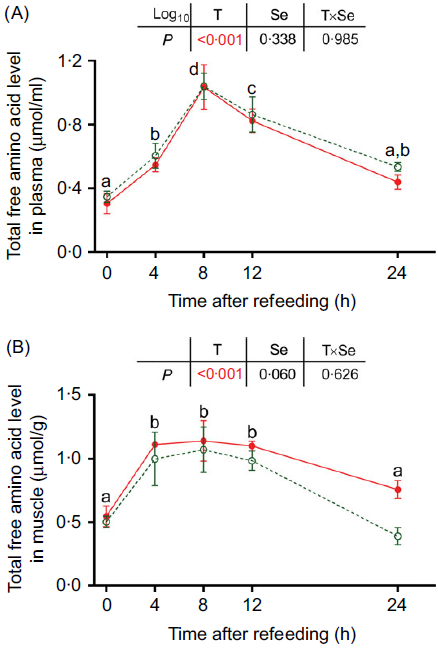
Fig. 1. Effects of dietary selenium on postprandial total free amino acid levels in (A) plasma and (B) dorsal muscle of juvenile rainbow trout. Values are means (n 3) and standard deviations and analysed by two-way ANOVA followed by Bonferroni–Dunn multiple comparison. a,b,c,d Mean values among time points with unlike letters are significantly different (P < 0·05). ![]() , 0 mg/kg selenium;
, 0 mg/kg selenium; ![]() , 4 mg/kg selenium.
, 4 mg/kg selenium.
Postprandial protein synthesis in trout dorsal muscle
Postprandial changes of protein synthesis-related factors in terms of the ratio of RNA:DNA, ribosomal RNA and the phosphorylation levels of ribosomal protein S6 (S6) on Ser235/236, eukaryotic translation initiation factor 4E binding protein 1 (4E-BP1) on Thr37/46, eukaryotic translation initiation factor 2 (eIF2) α subunit on Ser51 and eukaryotic translation elongation factor 2 (eEF2) on Thr56 are presented in Fig. 2. These factors were significantly influenced by the refeeding (P < 0·05). The ratio of RNA:DNA (Fig. 2(A)) and the phosphorylation level of S6 (Fig. 2(C)) significantly increased from 8 h to 12 h after refeeding and declined to the basal level (as 0 h) at 24 h (P < 0·05). The ribosomal RNA (Fig. 2(B)) significantly increased from 4 to 24 h after refeeding (P < 0·05). The phosphorylation level of 4E-BP1 (Fig. 2(D)) in fish fed the basal diet significantly increased from 4 to 12 h after refeeding and declined to the basal level at 24 h (P < 0·05), while in fish fed diet with Se supplementation, it significantly increased from 4 to 24 h after refeeding (P < 0·05). eIF2α exhibited a significantly lower phosphorylation level at 8 h after refeeding and a significantly higher phosphorylation level at 12 h after refeeding (Fig. 2(E), P < 0·05). Furthermore, the phosphorylation level of eEF2 significantly declined from 4 to 12 h after refeeding and increased to the basal level at 24 h (Fig. 2(F), P < 0·05).
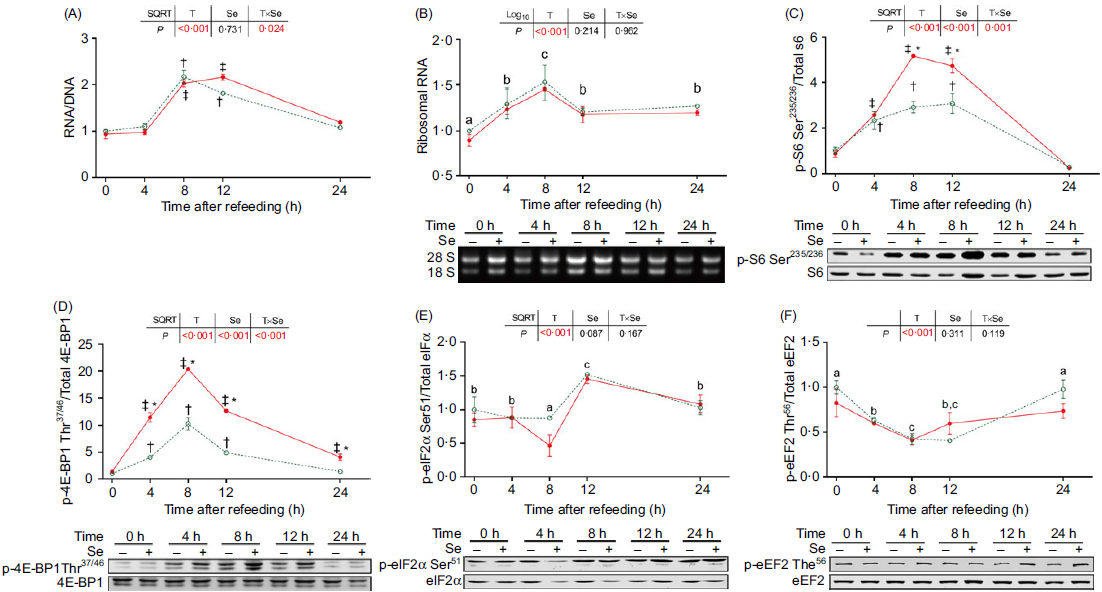
Fig. 2. Effects of dietary selenium on postprandial changes of factors related to protein synthesis in dorsal muscle of juvenile rainbow trout. Data were normalised to those in fish fed the basal diet at 0 h. Values are means (n 3) with their standard errors and analysed by two-way ANOVA followed by Bonferroni–Dunn multiple comparison. a,b,c Mean values among time points with unlike letters are significantly different (P < 0·05). * Mean values in fish fed diet supplemented with 4 mg/kg selenium significantly differ from those fed the basal diet at the same time point (P < 0·05). † Mean values after refeeding (4–24 h) significantly differ from those before refeeding (0 h) in fish fed the basal diet (P < 0·05). ‡ Mean values after refeeding (4–24 h) significantly differ from those before refeeding (0 h) in fish fed diet supplemented with 4 mg/kg selenium (P < 0·05). S6, ribosomal protein S6; 4E-BP1, eukaryotic translation initiation factor 4E binding protein 1; eIF2α, eukaryotic translation initiation factor 2α; eEF2, eukaryotic translation elongation factor 2. ![]() , 0 mg/kg selenium;
, 0 mg/kg selenium; ![]() , 4 mg/kg selenium.
, 4 mg/kg selenium.
Dietary Se supplementation exerted no influences on the ratio of RNA:DNA, ribosomal RNA and the phosphorylation levels of eIF2α and eEF2. However, it significantly increased the phosphorylation of S6 (Fig. 2(C)) from 8 to 12 h and the phosphorylation of 4E-BP1 (Fig. 2(D)) from 4 to 24 h after refeeding (P < 0·05).
Postprandial protein degradation in trout dorsal muscle
Protein degradation in fish muscle is under the control of the calpain system, autophagy–lysosome system and ubiquitin–proteasome system. We analysed the expression of numerous genes related to these three proteolysis systems first. In the calpain system, all the genes exhibited no significant response to both refeeding and dietary Se supplementation (Fig. 3(A)–(D)). In the autophagy–lysosome system, the expression of all the genes was significantly affected by both refeeding and dietary Se supplementation (Fig. 3(E)–(K)). Before refeeding (0 h), all the autophagy-related genes presented relatively lower expression in the fish fed diet with Se supplementation compared with those fed the basal diet. After refeeding, most of these genes exhibited gradually decreasing expression along with the time course, except for γ-aminobutyric acid type A receptor-associated protein-like 1 (Gabarapl1) and cathepsin B (CtsB), which exhibited significantly elevated expression at 12 and 12–24 h, respectively (Fig. 3(E)–(K), P < 0·05). Two-way ANOVA showed that dietary Se supplementation exerted a significant inhibition on the expression of autophagy-related genes throughout the postprandial period (P < 0·05). In the ubiquitin–proteasome system (Fig. 3(L)–(P)), the expression of most genes was significantly influenced by both refeeding and dietary Se supplementation (P < 0·05) except for muscle RING finger 2 (MuRF2). Before refeeding (0 h), all the differently expressed genes presented relatively lower expression in the fish fed diet with Se supplementation compared with those fed the basal diet before refeeding (0 h). After refeeding, their expression was significantly declined from 4 to 8 or 12 h (P < 0·05). In particularly, the expression of F-box 32 (Fbx32) was even significantly declined from 4 to 24 h after refeeding (P < 0·05). Two-way ANOVA showed that dietary Se supplementation exerted a significant inhibition on the expression of all the differently expressed ubiquitin–proteasome-related genes throughout the postprandial period (P < 0·05).
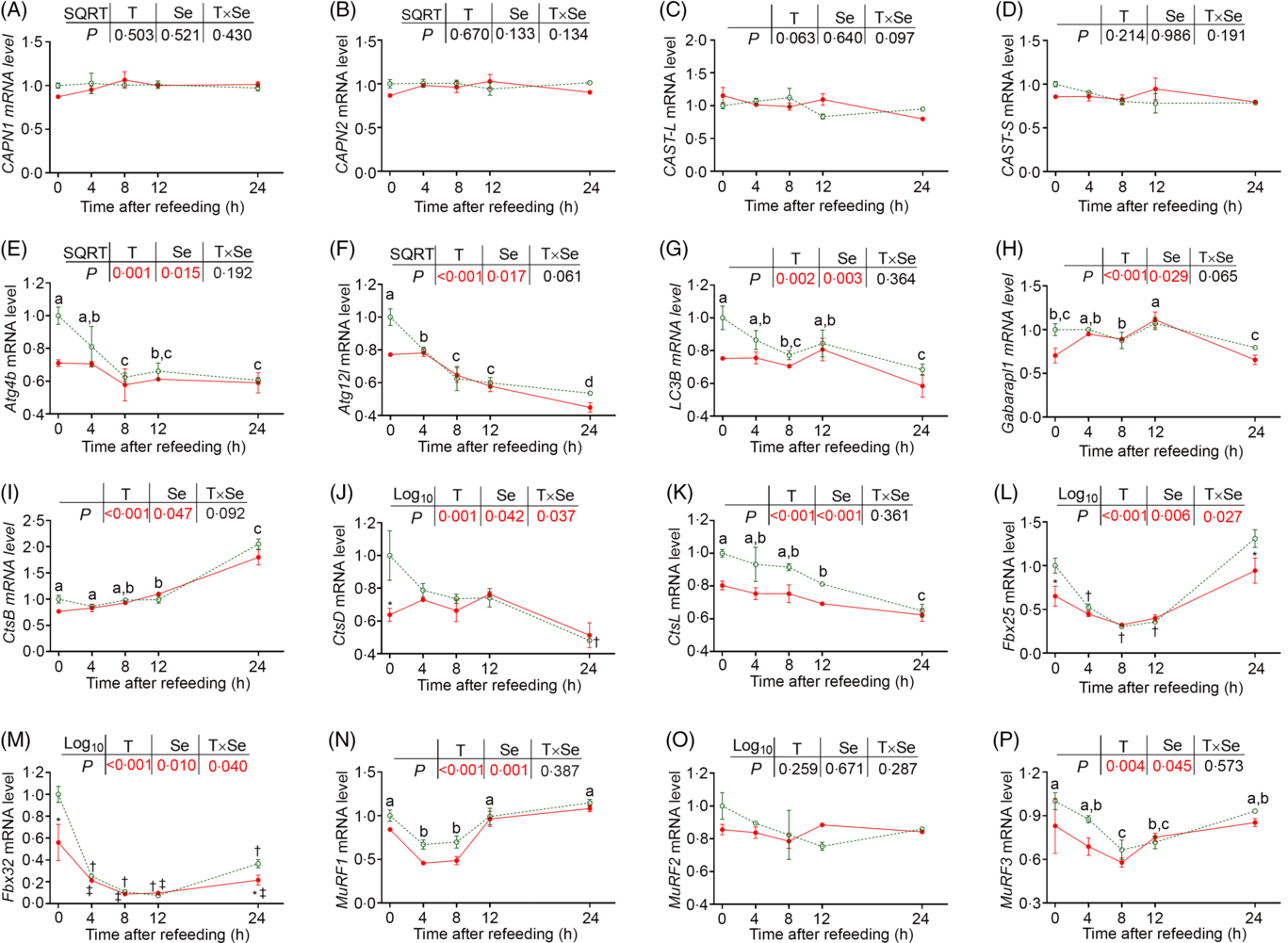
Fig. 3. Effects of dietary selenium on postprandial expression of genes related to protein degradation in dorsal muscle of juvenile rainbow trout. Data were normalised to those in fish fed the basal diet at 0 h. Values are means (n 3) with their standard errors and analysed by two-way ANOVA followed by Bonferroni–Dunn multiple comparison. a,b,c Mean values among time points with unlike letters are significantly different (P < 0·05). * Mean values in fish fed diet supplemented with 4 mg/kg selenium significantly differ from those fed the basal diet at the same time point (P < 0·05). † Mean values after refeeding (4–24 h) significantly differ from those before refeeding (0 h) in fish fed the basal diet (P < 0·05). ‡ Mean values after refeeding (4–24 h) significantly differ from those before refeeding (0 h) in fish fed diet supplemented with 4 mg/kg selenium (P < 0·05). Atg4b, autophagy-related 4b; Atg12l, autophagy-related 12-like; LC3B, microtubule-associated light chain 3B; Gabarapl1, γ-aminobutyric acid type A receptor-associated protein-like 1; Cts, cathepsin. CAPN1, catalytic subunits of μ-calpain; CAPN2, catalytic subunits of m-calpain; CAST-L, calpastatin long isoform; CAST-S, calpastatin short isoform; Fbx, F-box protein; MuRF, muscle RING finger. ![]() , 0 mg/kg selenium;
, 0 mg/kg selenium; ![]() , 4 mg/kg selenium.
, 4 mg/kg selenium.
Based on the results of gene expression, we further analysed the amount of ubiquitinated proteins and the ratio of microtubule-associated light chain 3B (LC3)-II:LC3-I, biomarkers for the activities of ubiquitination and autophagy, respectively (Fig. 4(A)). Before refeeding (0 h), the amount of ubiquitinated proteins and the LC3-II:LC3-I ratio presented no significant difference in fish fed diet with Se supplementation compared with those fed the basal diet. After refeeding, the amount of ubiquitinated proteins significantly declined from 4 to 24 h (Fig. 4(B), P < 0·05), while the LC3-II:LC3-I ratio presented no change (Fig. 4(C)). Two-way ANOVA showed that dietary Se supplementation posed no significant influence on the amount of ubiquitinated proteins and LC3-II:LC3-I ratio throughout the postprandial period (Fig. 4).

Fig. 4. Effects of dietary selenium on postprandial changes of (B) ubiquitinated proteins and (C) LC3-II:LC3-I ratio in dorsal muscle of juvenile rainbow trout. Data were normalised to those in fish fed the basal diet at 0 h. Values are means (n 3) with their standard errors and analysed by two-way ANOVA followed by Bonferroni–Dunn multiple comparison. a,b,c Mean values among time points with unlike letters are significantly different (P < 0·05). LC3, microtubule-associated light chain 3B. (B,C) ![]() , 0 mg/kg selenium;
, 0 mg/kg selenium; ![]() , 4 mg/kg selenium.
, 4 mg/kg selenium.
Discussion
In the present study, juvenile rainbow trout exhibited an excellent growth performance with a high specific growth rate of 3·94–4·39 %/d throughout the 6-week feeding trial, which was comparable with previous observations (2·21–2·61 %/d) from the same species(Reference Vilhelmsson, Martin and Médale31,Reference Rider, Davies and Jha32) . An optimal level of dietary Se is crucial for fish growth(Reference Khan, Zuberi and Fernandes1). Our previous study reported that rainbow trout reached the maximum growth rate when fed diet supplemented with 4 mg/kg Se as Se yeast. Based on this result, rainbow trout in this study were fed a basal diet unsupplemented or supplemented with 4 mg/kg Se as Se yeast, respectively. As expected, dietary Se supplementation led to an enhancement of rainbow trout growth.
Protein deposition in muscle tissue has been demonstrated as an important determinant for fish somatic growth(Reference Bureau, Hua and Cho17). In this study, we observed that dietary Se supplementation led to an increase of protein content in trout muscle, which is consistent with previous reports that diets supplemented with 3–4 mg/kg Se as Se yeast increased the protein content in rainbow trout muscle(Reference Hunt, Berkoz and Ozkan18,Reference Wang, Wu and Liu19) . In addition to the increase of muscle protein content, dietary Se supplementation also led to an elevated protein retention rate in rainbow trout. The elevated muscle protein content and protein retention rate suggest a promoting effect of nutritional level of dietary Se on protein deposition in trout muscle. It is reported that protein deposition in fish tissues depends on both rates of protein synthesis and protein degradation(Reference Johnston, Bower and Macqueen21), both of which are sensitive to the feeding status and exhibit dynamic changes after a meal(Reference Seiliez, Panserat and Skiba-Cassy22–Reference Seiliez, Gabillard and Skiba-Cassy25). Thus, this study chose a postprandial kinetic-metabolic model to further investigate the effects of nutritional level of dietary Se on the postprandial (within 24 h after a meal) protein synthesis and degradation in rainbow trout muscle.
Previous studies reported that ingestion of feeds led to an increased level of total free amino acids in plasma of rainbow trout with a peak level at postprandial 6–12 h(Reference Belghit, Panserat and Sadoul23,Reference Seiliez, Gabillard and Skiba-Cassy25,Reference Karlsson, Eliason and Mydland33,Reference Seiliez, Panserat and Lansard34) . In agreement, in the present study, the level of total free amino acids in the plasma of rainbow trout significantly increased from 4 to 24 h after refeeding and peaked at 8 h. As the increase of free amino acids in plasma, the amino acid pool in trout muscle has been replenished from 4 to 12 h after refeeding. The repletion of the amino acid pool provided enough substrates for the subsequent protein synthesis(Reference Fruhbeck35).
Protein synthesis is a multistep, multifactorial process of mRNA translation on the polyribosome(Reference Sivan and Elroy-Stein36). This process can be divided into initiation, elongation and termination phases, etc.(Reference Pestova and Hellen37) It is reported that the rate of cellular protein synthesis is influenced by both the translation capacity (ribosome biomass) and the translation activity (activation of translation initiation and elongation)(Reference Sivan and Elroy-Stein36,Reference Figueiredo and McCarthy38) .
The ribosomal RNA and RNA:DNA ratio have been widely regarded as indicators for translational capacity and used to evaluate protein synthesis in muscle tissues of both mammal(Reference Goodman, Mabrey and Frey39,Reference West, Baehr and Marcotte40) and fish(Reference McNamara, Caldarone and Buckley41–Reference Bennett, O’Donohue and Gundry43). Our results showed that both ribosomal RNA and RNA:DNA ratio significantly increased after refeeding, which was consistence with the observations in turtle(Reference Ji, Liu and Song44), squid(Reference Vidal, DiMarco and Lee45) and rats(Reference Millward, Nnanyelugo and James46). However, they showed no significant differences between the fish fed diet with Se supplementation and those fed the basal diet, suggesting no influence of dietary Se on postprandial translational capacity in rainbow trout muscle.
In eukaryotic cells, translation initiation is primarily mediated by the target of rapamycin complex 1 (TORC1) and the eIF2B-eIF2 pathways(Reference Sivan and Elroy-Stein36). Postprandial activation of TORC1 pathway is a primary driving force for protein synthesis in fish tissues(Reference Xu, He and Mai47) and has been extensively observed in the muscle of rainbow trout(Reference Seiliez, Gabillard and Skiba-Cassy25,Reference Seiliez, Panserat and Lansard34,Reference Mennigen, Panserat and Larquier48) . Consistent with previous findings, an increase of the postprandial phosphorylation levels of S6 and 4E-BP1, two downstream molecules of TORC1(Reference Ma and Blenis49), in trout muscle has been observed in the present study. Elevated phosphorylation level of S6 and 4E-BP1 enhances the translation initiation efficiency and drives the 5′-cap-dependent translation, respectively(Reference Goodman, Mayhew and Hornberger50). A previous study reported that dietary Se had a promoting effect on the activation of TORC1 pathway in the skeletal muscle of pigs(Reference Zhao, Barcus and Kim51). In the present study, trout fed diet supplemented with 4 mg/kg Se exhibited persistently higher phosphorylation levels of S6 (postprandial 8–12 h) and 4E-BP1 (postprandial 4–24 h) than those fed the basal diet, suggesting a persistent promotion of dietary Se on postprandial activation of TORC1 pathway in trout muscle. Unlike the TORC1 pathway, the eIF2B-eIF2 pathway controls a global translation initiation in a GTP-dependent mode(Reference Goodman, Mayhew and Hornberger50,Reference Hinnebusch52) . eIF2B acts a vital role in this pathway by catalysing the conversion of eIF2-GDP to eIF2-GTP, which is necessary for the new round of translation initiation(Reference Hinnebusch52). However, the catalytic activity of eIF2B will be inhibited by eIF2 when a phosphorylation occurs on the α subunit of eIF2(Reference Hinnebusch52). The phosphorylation level of eIF2α in fish muscle is sensitive to amino acids availability and has been reported to be declined after a meal(Reference Xu, He and Mai47), which was in line with our observation that the phosphorylation level eIF2α in trout muscle exhibited a significant decline after 8 h of refeeding. However, no difference was found in postprandial phosphorylation level of eIF2α in trout muscle between the fish fed diet with Se supplementation and the basal diet.
Translation elongation in eukaryotic cells is under the control of various eukaryotic translation elongation factors (eEF)(Reference Goodman, Mayhew and Hornberger50). Among the eEF, eEF2 mediates the translocation of the ribosome to the next codon during translation(Reference Sivan and Elroy-Stein36). Phosphorylation of eEF2 by its sole kinases dissociates it from ribosomes and thus inhibits its activity(Reference Sivan and Elroy-Stein36). Previous studies in rats reported that the postprandial phosphorylation level of eEF2 was significantly declined(Reference Wilson, Moulton and Garlick53,Reference Wilson, Layman and Moulton54) . Similarly, a significant decrease of phosphorylation level of eEF2 was observed in rainbow trout muscle from 4 to 12 h after refeeding. However, no significant difference existed in the fish fed diet with Se supplementation compared with the basal diet.
To sum up the postprandial dynamics of factors related to protein synthesis in trout muscle, dietary Se led to a persistently high activity of TORC1 pathway, which involves in the regulation of translation initiation. Given that translation initiation is the rate-limiting step of universal protein synthesis(Reference Simonetti, Marzi and Myasnikov55). The present observation provides evidence for the promoting effect of dietary Se on postprandial protein synthesis in rainbow trout muscle.
Protein degradation is a highly complex, temporally controlled and tightly regulated process(Reference Hershko, Ciechanover and Varshavsky56). In vertebrate, muscle protein degradation relies on three major proteolytic systems: the calpain system, the ubiquitin–proteasome system and the autophagy–lysosome system(Reference Johnston, Bower and Macqueen21,Reference Sandri57,Reference Nemova, Lysenko and Kantserova58) . Until now, these proteolytic systems have been widely proved to involve in the regulation of muscle protein degradation in fish by both in vivo and in vitro researches(Reference Seiliez, Dias and Cleveland16,Reference Seiliez, Gabillard and Riflade27,Reference Seiliez, Gutierrez and Salmerón59–Reference Salem, Nath and Rexroad61) . It is demonstrated that proteins in the fish body are subject to continuous breakdown and replacement(Reference Johnston, Bower and Macqueen21). And ingestion of feeds led to a declined rate of protein degradation in fish muscle(Reference Seiliez, Panserat and Skiba-Cassy22,Reference Belghit, Skiba-Cassy and Geurden28,Reference Mennigen, Panserat and Larquier48) . In line with the previous reports, our results showed that the expression of numerous genes related to the autophagy–lysosome pathway and ubiquitin–proteasome pathway in trout muscle was down-regulated after refeeding. We previously observed that diet supplemented with 2 and 4 mg/kg Se from Se yeast exerted a negative influence on the expression of autophagy–lysosome system- and ubiquitin–proteasome system-related genes in the muscle of rainbow trout(Reference Wang, Wu and Liu19). Similarly, in the present study, dietary Se supplementation down-regulated the expression of most of the autophagy–lysosome system- and ubiquitin–proteasome system-related genes both at the basal metabolic level (0 h) and throughout the whole postprandial period (0–24 h). However, the postprandial LC3-II:LC3-I ratio and ubiquitinated proteins, markers for the activities of autophagy and ubiquitination, respectively(Reference Seiliez, Panserat and Skiba-Cassy22,Reference Wang, Li and Zhao62) , in trout muscle showed no significant difference between the fish fed different experimental diets, suggesting no influence of dietary Se on postprandial activities of the autophagy–lysosome system and ubiquitin–proteasome system in rainbow trout muscle.
In conclusion, this study systematically analysed the nutritional effects of Se on protein deposition in the muscle of rainbow trout using a postprandial kinetic-metabolic model. The results of the present study demonstrated a promoting effect of nutritional level of dietary Se on postprandial protein synthesis in trout muscle by persistently promoting the activation of the TORC1 pathway. Furthermore, the nutritional level of dietary Se exerted no influences on the postprandial activities of the three major proteolytic systems: the calpain system, the ubiquitin–proteasome system and the autophagy–lysosome system. Our findings reveal an involvement of nutritional level of dietary Se in mechanisms regulating the postprandial dynamics of protein deposition in fish muscle, which could help to better understand the regulatory role of dietary Se in fish growth.
Acknowledgements
The authors thank Enshi Guoxi Fishery Development Co. Ltd. for the provision of rainbow trout.
This work was supported by the Fundamental Research Funds for the Central Universities (X. Z., grant numbers 2662019FW013 and 2662015PY024) and the Da Bei Nong Group Promoted Project for Young Scholar of HZAU (X. Z., grant number 2017DBN018). These funders had no role in the design, analysis and writing of this article.
Li Wang and X. Z. designed the study; Li Wang, Long Wang, D. Z., S. L. and J. Y. performed data acquisition and data analysis; Li Wang, Z. X. and X. Z. wrote the manuscript; X. Z. had primary responsibility for the final content. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.










