In Egypt, pregnant women who attend antenatal care (ANC) services are routinely given Fe and folic acid supplements but not Zn supplement. The International Zinc Nutrition Consultative Group Steering Committee estimated that 9 % of the population in Egypt was at a risk of inadequate Zn intake( Reference Brown, Rivera and Bhutta 1 ). Meanwhile, the Egyptian Demographic and Health Survey 2008 indicated that 29 % of children under the age of 5 years were stunted( Reference El-Zanaty and Way 2 ). Based on the aforementioned two proxy indicators of Zn deficiency, Egypt had been classified to be in the medium risk category of Zn deficiency( Reference Wessells and Brown 3 ).
Adequate Zn nutrition is necessary for normal pregnancy outcome and child growth, immune function and neurobehavioural development( Reference Brown, Rivera and Bhutta 4 ). Most observational studies have noted that low serum Zn levels are linked to prolonged labour and post-partum haemorrhage, pregnancy-induced hypertension, preterm birth and poor perinatal outcome( Reference Shah and Sachdev 5 – Reference Deshpande, Joshi and Giri 7 ), while some have shown association with low birth weight (LBW)( Reference Karimi, Bagheri and Nematy 8 , Reference Angelova, Nedkova and Nicolov 9 ). However, supplementation trials have reported inconsistent findings of the effects of Zn supplementation. Meta-analyses( Reference Mori, Ota and Middleton 10 , Reference Gebreselassie and Gashe 11 ) and systematic reviews( Reference Chaffee and King 12 , Reference Hess and King 13 ) did not reveal significant association between birth weight and Zn supplementation. In contrast, a systematic review (2012) of twenty independent trials( Reference Chaffee and King 12 ) has revealed that Zn supplementation lowers the risk of preterm birth. Darnton-Hill( 14 ) claimed that the effect of Zn supplementation on preterm birth may be due to a reduction in the incidence or severity of maternal infections, which is a known risk factor for preterm birth.
Zn deficiency commonly coexists with other micronutrient deficiencies. Similar to other developing countries, many women in Egypt suffer from multiple micronutrient (MM) deficiencies, not just Fe deficiency( 15 , 16 ). Considerable evidence suggests a role for micronutrients in improving some pregnancy outcomes including birth weight, gestational age at birth, and stillbirths and neonatal morbidity and mortality( Reference Fall, Fisher and Osmond 17 ). However, nutrition intervention studies( Reference Haider and Bhutta 18 – Reference Bhutta and Haider 21 ) have not provided unequivocal evidence of an association between micronutrient intakes and pregnancy outcomes. This was attributed to considerable methodological variation across these studies. Small sample size, study population not representing women at high risk for low micronutrient intakes, and use of non-randomised study design have probably limited the chances of these studies to demonstrate a positive association( Reference Ramakrishnan, Grant and Goldenberg 22 ).
A meta-analysis (Kawai et al.)( Reference Kawai, Spiegelman and Shankar 23 ) and systematic reviews( Reference Ramakrishnan, Grant and Goldenberg 22 , Reference Zerfu and Ayele 24 ) have shown that MM supplementation, compared with control supplementation that was usually Fe plus folic acid in most studies, is effective at reducing the risk of LBW (relative risk (RR) 0·86, 95 % CI 0·79, 0·93) and small size for gestational age (RR 0·85, 95 % CI 0·78, 0·93), but no overall effect on preterm birth, stillbirth and maternal or neonatal mortality.
The doses used by supplementation trials vary widely. Most studies have assessed the effect of Zn against a background of other micronutrient supplements where the dose of Zn ranged from 5 to 44 mg/d( Reference Mori, Ota and Middleton 10 ). In contrast, the trials that used the international multiple micronutrient preparation (UNIMMAP)( Reference Bhutta and Haider 21 ) used a dose of 15 mg Zn, which is equivalent to one RDA.
Till now, no attempts were made to deliver Zn and/or multivitamin supplements in ongoing large-scale programmes in Egypt. However, planning to introduce these micronutrients based on the WHO recommendation to use the UNIMMAP to high-risk groups may take place in the near future. Since the typical Egyptian diet is largely based on plants with high content of Zn inhibitors, mainly phytic acid( 25 ), higher doses of Zn may be required to cause a detectable effect on pregnancy outcomes. The present study was designed to determine whether doubling the dose of Zn in a MM supplement (i.e. to be equivalent to two RDA) and using different doses of B1, B6, E, D3 and vitamin C will result in pregnancy outcomes different from those reported by studies using the UNIMMAP preparation (i.e. which contains a dose of Zn equivalent to one RDA). Meanwhile, we used a three-arm trial design with sufficient power to test in one trial the effects of supplementing Zn alone compared with combining it with MM on the outcomes of pregnancy. Results would provide useful information to policy makers in making decisions about which type of supplement to be introduced in large-scale programmes.
Subjects and methods
Study design
A double-blind, placebo-controlled, parallel group, randomised trial was conducted in Alexandria, Egypt, from February 2007 to September 2009. Women with low serum Zn level were eligible for enrolment to the study. Zn-alone supplementation in pregnancy is not feasible since Fe–folic acid supplement is given routinely to women who attend ANC services in Egypt. Accordingly, the effects of Zn supplementation are in fact compared with those of control supplementation with Fe plus folic acid.
Eligible participants (n 675) were randomly assigned to one of the three parallel groups in 1:1:1 ratio. The control group received placebo, the Zn group received a daily supplement of 30 mg of ZnSO4 and the combined Zn plus multivitamin group received 30 mg of ZnSO4 added to multivitamins. We defined ‘multivitamins supplement’ as a ‘single tablet containing more than three micronutrients other than zinc’( Reference Zerfu and Ayele 24 ). The MM supplement used in the present study contains Zn along with vitamins B1, B6, D3, E and C.
Study participants and study settings
The study population was pregnant women who presented themselves for ANC in two clinics (Smouha and Moharam Bec Maternal and Child Health centres) serving low- and middle-income population in Alexandria, Egypt. Of the 1055 apparently healthy pregnant women aged 20–45 years assessed for the eligibility of low serum Zn level, 675 had serum Zn level below the estimated median for the gestational age. Serum Zn level was measured by using flame atomic absorption spectrophotometry at enrolment and a second measurement was performed between 28 and 32 weeks of gestation.
Eligibility criteria for participants
The inclusion criteria for the recruitment were gestational age < 16 weeks assessed by recall of last menstrual period and confirmed by ultrasonography, age range between 20 and 45 years, BMI between 18 and 26 kg/m2, normal course of pregnancy and Zn level at the time of enrolment below the estimated median for gestational age (7·5 μg)( Reference El-Kassas 26 ).
Women were excluded if they were identified through interviews to be on any other form of Zn supplements at any dosage, were older than 35 years at first pregnancy, had an established risk of having reduced or excessive birth weight of infants (e.g. diabetes, hypertension, and renal and heart disease) and cases that developed complications or had a twin pregnancy during the follow-up period.
Ethical considerations
The present study was conducted according to the guidelines laid down in the Declaration of Helsinki. Informed consent forms were obtained before enrolment and eligible women were given copies of the signed forms. Each woman was given a unique serial number that was recorded on her antenatal care card. Confidentiality was maintained throughout the experiment. The protocol of the study was approved by the ethics committee of Ministry of Health and the ethics committee of High Institute of Public Health. Permission to carry out the study in the ANC clinics in Alexandria was obtained from the local health authority. The trial was registered in the WHO Pan African Trial Registry after both recruitment and data analysis (PACTR201303000453309).
The intervention
The supplements were supplied as a package (box of thirty capsules) every month during the regular ANC visits. Checking the regularity of intake was done every visit. The supplements were taken every day from enrolment till delivery. Capsules were all of the same shape, smell, taste and colour. Boxes of the supplement were labelled by the specific number of the woman. The eligible women receive one of the following regimens: (1) placebo capsule (270 mg lactose); (2) ZnSO4 capsule (30 mg Zn2+ and 270 mg lactose); (3) multivitamin capsule with Zn (30 mg Zn2, vitamin B1 (0·13 mg), vitamin B6 (1·94 mg), vitamin E (5·73 mg), vitamin D3 (3·1 mg (approximately equal to 309 IU)), vitamin C (66·5 mg) and 270 mg lactose).
All recruited women were screened for eligibility. They were subjected to initial assessment that includes the following:
-
(1) Collection of sociodemographic and obstetric data: the trial physician used a predesigned questionnaire to collect the following:
-
(a) Personal and sociodemographic data such as age, residence and educational level (including the following categories: read and write or illiterate, primary, preparatory, secondary and university level or higher); working status (unskilled, skilled, clerical, professional and not working); family size (two persons, three, four, five and six persons, or more); and income (always in debt, sometimes in debt, just sufficient and saving).
-
(b) Obstetric data such as gravidity, parity, abortions and obstetric complications in previous pregnancies or deliveries.
-
-
(2) Anthropometric measurements: weight was measured to the nearest 0·1 kg on an electronic bathroom weighing scale and height was measured to the nearest 0·1 cm with a height stick. BMI was calculated as follows: BMI = weight in kg/height in m2.
-
(3) Determination of gestational age: gestational age was determined using the last menstrual period. Ultrasound was used for the verification of gestational age for all cases. The dates for the assessment of gestational age at recruitment and at delivery were all recorded in the data collection form. The length of gestation was subsequently calculated in completed weeks from the recorded data.
-
(4) Routine examination: it included blood pressure measurement, chest and heart examination, fundal level examination and checking for lower limb oedema.
-
(5) Routine laboratory investigations: it included urine analysis, random blood glucose level and measurement of Hb concentration using the cyanmethaemoglobin method.
After this initial assessment, women (n 1055) were tested for eligibility in terms of Zn deficiency.
-
(1) Determination of serum zinc: non-fasting venous blood was collected during morning hours for the determination of serum Zn level by using plastic syringes, stainless steel needles and trace mineral-free plastic tubes. Serum was separated at a maximum of 6 h after collection and stored at − 20°C until analysed. Zn concentration was measured by using flame atomic absorption spectrophotometry. The reference median serum Zn level at each gestational age was based on the values obtained from a study on pregnant women attending maternal and child health centres in Alexandria( Reference El-Kassas 26 ).
-
(2) Assessment of dietary intake: dietary intake was measured by using 24 h recall method and a FFQ, focussing on the intake of foods that might enhance or inhibit Zn absorption. This assessment was done on a subsample of 100 women in the three studied groups. Enhancers of Zn absorption were enquired about their protein intake and low Ca intake represented in low consumption of dairy products. Inhibitors of Zn absorption were represented in fibres, phytates and Fe. The nutritive value of the daily diet was computed using the Egyptian food composition tables( 27 ). The mean daily intake was compared with that of the recommended dietary intake level of the Food and Nutrition Board, Institute of Medicine( Reference Mahan, Escott-Stump, Raymond and Krause 28 ), to get the per cent adequacy of intake from the specified nutrient.
Those having Zn deficiency (n 675) were randomised into the three trial groups. During the monthly follow-up, the trial physician carried out the following:
-
(1) Routine antenatal examination.
-
(2) The second measurement of serum Zn: a second blood sample was taken between 28 and 32 weeks of gestation.
-
(3) Anthropometry of women: weight gain was measured monthly. Total weight gain defined as: weight at last visit before delivery − weight at enrolment was measured and compared with the recommended total weight gain in pregnant women by pre-pregnancy BMI (in kg/m2)( 29 ). Ultrasound examination to all women was performed at 24–34th week of gestation and as indicated to assess the gestational age and the anthropometric measurements of the fetus.
-
(4) Post-partum data: data on delivery, postnatal complications, birth weight and neonatal complications were obtained by a trained nurse/midwife during the post-partum home visits and also during the routine first visit of the newborn to the health facility for immunisation and thyroid blood sample testing (first week after delivery).
Outcome measures
Primary outcome measures
-
(1) Birth weight was measured within 72 h of birth to the nearest 10 g.
-
(2) Delivery data included mode of delivery, second- and third-stage complications, stillbirth and preterm delivery.
-
(3) Neonatal data included head, chest and arm circumferences to the nearest 0·1 cm, clinically apparent congenital malformation and early neonatal morbidity, mainly infections. Neonatal infection was ascertained on the basis of either maternal report of a specific severe respiratory sign such as distress or report of respiratory disease-related hospitalisations.
Secondary outcome measures
These included serum Zn after supplementation and total weight gain.
Sample size
The sample size required to enable detection of a mean difference of 150 g in birth weight with 80 % power, 0·5 sd for each group, 5 % level of significance was estimated to be 534 (n 178 each group). The study included up to 675 cases to compensate for the potential exclusion after randomisation and loss to follow-up. So, after randomisation of participants (n 675) to the different groups, there were 223 cases in the placebo group, 225 cases in the Zn group and 227 cases in the combined Zn and multivitamin group.
Randomisation
Participants were randomly assigned to one of the three study groups. An independent statistician generated the allocation sequence using computer-generated random numbers, using Excel software. After obtaining informed consent for enrolment, the investigators randomly assigned participants till the required sample size was met. There was no stratification during the randomisation.
Concealment of random allocation sequence
The assignment of participants to study conditions was carried out at the study centres. A co-worker wrote the treatment allocations (A, B and C) on sequentially numbered opaque plastic boxes. One box containing thirty capsules of a given random number was prepared for monthly use. Thus, five boxes were totally numbered for each participant. Additional packs were available for non-compliant or replaced cases. The assigned box number was transferred to a folder prepared for each woman where data such as any complications/withdrawal/non-compliance were recorded. Two copies of randomisation lists were prepared and kept by two independent staff not involved in the study. They kept them until collection and analysis of data were completed. Deciphering of group labels took place after completing the analysis and commenting on the results.
Blinding
The supplements were supplied by a local pharmaceutical company (Pharo Pharma). The capsules were of the same shape, colour and taste. All study personnel – except the statistician who generated the sequence – and the participants were blinded to the allocation.
Administration of supplements and monitoring of compliance
Each participant was supplied with enough supplements to last for 1 month at a time. Participant was instructed to take one capsule daily after meals and to bring the boxes of the supplement during each follow-up visit to check the regularity of intake. Compliance was monitored by pill counting. Compliance was considered adequate when it exceeds 90 % of total intake during the trial period.
Data collection and data management
A pilot study was conducted on thirty women. The questionnaire was completed in about 20–30 min. Some dietary data were added and the questionnaire was put in its final form.
Statistical analysis
Data was analysed using the Predictive Analytics Software (PASW Statistics 18). Intention-to-treat strategy was used for primary analysis, and involved all participants who were randomly assigned. Association between categorical variables was tested using χ 2 test. Yate's exact correction was applied when more than 20 % of the cells have expected count less than 5. Quantitative data were described using means and standard deviations. When there were ≥ 30 observations per group, parametric statistics were used for comparing means in the study with disregard to the state of normality of the data as the conclusions drawn by both the t and F distributions will not be seriously affected. Independent one-way ANOVA was used to compare quantitative variables among more than two groups. Paired t test was used for comparing the quantitative variables before and after the intervention. The RR and a 95 % CI were calculated to compare the proportions of cases having a negative outcome in the different groups relative to the control group. The effect size was determined using the RR, absolute risk reduction and numbers needed to treat.
Results
Fig. 1 shows the flow chart of participants: of the 1450 healthy pregnant women aged 20–45 years assessed for eligibility, 395 cases were excluded. Of the remaining 1055 women, 675 had low serum Zn level and were enrolled. During the follow-up period, thirteen participants developed complications such as diabetes and hypertension and were excluded at different times. Also, sixty-four women lost to follow up (9·48 %) due to different reasons such as lack of tolerance to the supplement (some women disliked the smell of the supplement, others disliked the capsule size and some women could not adhere to daily intake), development of side effects such as nausea or vomiting and desire of some women to deliver at their home villages. Finally, 597 women completed the study (199 cases in the placebo group, 198 in the Zn group and 200 in the Zn plus multivitamin group).

Fig. 1 Flow chart of participants of the randomised controlled trial. MM, multiple micronutrients.
The number of women who completed the study in each group was higher than the minimum sample size required to detect a 150 g mean birth-weight difference at 80 % power. There were no differences in the compliance levels between the three groups. Women in all groups consumed >90 % of the supplements provided, and few reported adverse effects such as vomiting, diarrhoea and abdominal pain (see online Supplementary Table S1). Also, characteristics of the women lost to follow-up were not different from participants who continued the trial.
Table 1 presents the baseline demographic and clinical characteristics for each group. The mean age of the study participants at recruitment was 26·54 (sd 4·42), 26·56 (sd 4·45) and 27·90 (sd 4·76) years for placebo, Zn and Zn plus multivitamin groups, respectively. The mean gestational age at recruitment was 15·10 (sd 1·41), 15·22 (sd 1·37) and 15·44 (sd 1·09) weeks for the three groups, respectively. The study groups did not differ significantly in terms of their baseline characteristics, except for gestational age where the combined Zn and multivitamin group had later age at enrolment (P= 0·017) and the serum Zn level as the Zn group entered the trial with a significantly lower level than the other groups (P= 0·025).
Table 1 Baseline sociodemographic and clinical characteristics of the three groups of the trial, Alexandria, Egypt (Mean values and standard deviations, numbers of participants and percentages)
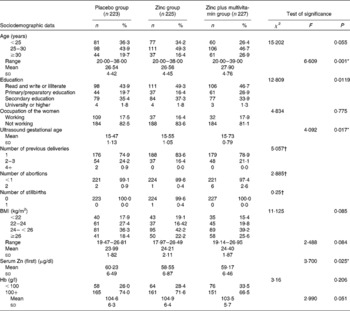
* P< 0·05.
† Yates' χ2.
Table 2 presents the change in total mean weight gain and the serum Zn level in the three studied groups. The total weight gain of the combined Zn plus multivitamin group was significantly higher than that of the placebo and Zn groups (P< 0·001). In the second testing, the mean Zn level of the Zn group was significantly higher than that of the placebo group and the Zn plus multivitamin group (P< 0·001). For all the three groups, the second serum Zn level was significantly higher than the first one (F within= 864·64; P< 0·001). It increased, yet differently, among the three groups (F interaction= 222·21; P< 0·001). The change was highest among the Zn group (mean difference 16·52 μg/dl) and of less magnitude in the combined Zn plus multivitamin group (mean difference 11·18 μg/dl) compared with the mean of 60·68 (sd 9·48) μg/dl among the placebo group.
Table 2 Comparison between the mean total weight gain and serum zinc level among pregnant women of the three groups using mixed ANOVA test* (Mean values and standard deviations)
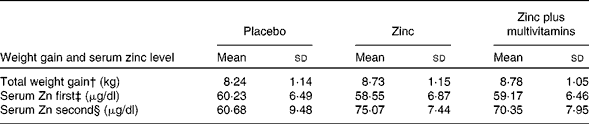
* Post hoc significant at (P< 0·05).
† ANOVA for total weight gain = 14·315 (P< 0·001).
‡ ANOVA for first serum zinc = 3·700 (P= 0·025).
§ ANOVA for second serum zinc = 154·405, (P< 0·001).
Table 3 presents the occurrence of delivery complications, stillbirth and preterm births among the three groups. Differences in the mode of delivery among the three groups were not statistically significant (P= 0·58). The group supplemented by both Zn and multivitamins was less likely to be delivered by caesarean section (RR 0·779, 95 % CI 0·562, 1·081) than the Zn supplemented one (RR 0·889, 95 % CI 0·649, 1·214). Prolonged labour was the most apparent complication. The risk of prolonged labour was lower among the Zn group (RR 0·434, 95 % CI 0·313, 0·601) than among the combined group of Zn plus multivitamins (RR 0·543 95 % CI 0·405, 0·727).
Table 3 Delivery data of women of the three groups regarding mode of delivery, second- and third-stage complications, stillbirth and preterm delivery (Numbers of participants and percentages, relative risk (RR) and 95 % confidence intervals)

ARR, absolute risk ratio; NNT, numbers needed to treat.
* P< 0·05.
† RR was calculated as normal v. abnormal (preterm and stillbirth).
Preterm delivery was significantly higher among the placebo group than among the Zn and combined groups (21 (10·6 %), compared with 2 (1 %) and 4 (2 %), respectively). Cases of Zn group were less likely to experience preterm labour and stillbirth by about 88 % (RR 0·012, 95 % CI 0·036, 0·377) and cases of Zn plus multivitamins by 73 % (RR 0·268, 95 % CI 0·119, 0·603) compared with placebo group (Table 3).
The anthropometric measurements and early morbidity of the neonates are presented in Table 4. The birth weight ranged between 2150 and 4000 g with a mean of 2929·12 (sd 330·28), 2922·22 (sd 324·05) and 2938·48 (sd 317·39) g for the placebo, Zn and Zn plus multivitamin groups, respectively. Although the mean weight of babies of the combined group was higher than that of the other groups, the difference was not statistically significant (P= 0·88; Table 4). There were also no statistically significant differences between the groups regarding head circumference (P= 0·28), chest circumference (P= 0·51) and arm circumference (P= 0·58).
Table 4 Anthropometric measurements and early morbidity of the neonates of the three groups of the trial† (Mean values, standard deviations, numbers of participants, percentages, relative risk (RR) and 95 % confidence intervals)

ARR, absolute risk ratio; NNT, numbers needed to treat.
* P< 0·05.
† Three neonates died in the placebo group.
Difference between the study groups regarding early neonatal morbidity was statistically significant (P= 0·001). Participants of the Zn-supplemented group were less prone to the occurrence of early neonatal morbidity by 0·225 times (95 % CI 0·146, 0·346) than those of the placebo group, and participants of the multivitamin-supplemented group were less prone to the occurrence of early neonatal morbidity by about 0·246 times (95 % CI 0·163, 0·372) than those of the placebo group.
Discussion
The present randomised controlled trial (RCT) examined the effects of maternal supplementation with two regimens of Zn, namely Zn alone against combined Zn and multivitamins, on pregnancy outcome. The present study was adequately powered to examine a birth-weight difference of 150 g between the supplemented and the placebo groups. The findings are consistent with recent systematic reviews in showing that maternal micronutrient supplementation can reduce the risk of pregnancy complications, preterm birth and neonatal infection but not birth weight( Reference Ramakrishnan, Grant and Goldenberg 22 , Reference Kawai, Spiegelman and Shankar 23 ).
Women in the present study suffered not only from Zn deficiency but also from multiple micro- and macronutrient deficiencies( Reference Naem, El-Sayed and Nossier 30 ). This could explain the better outcome parameters in the group that received both Zn and multivitamins than the group receiving Zn only. For example, the mean weight gain was relatively higher and the birth weight was higher in the combined multivitamins than the other study groups, although no significant differences in birth weight between the three groups was revealed. This group was also more likely to have normal delivery than the Zn-alone group (RR 0·779, 95 % CI 0·562, 1·081 v. RR 0·889, 95 % CI 0·649, 1·214).
Increased Zn concentrations after supplementation was reported in some but not in all of the supplementation trials( Reference Moran, Skinner and Medina 31 ). The present study provides further evidence for the effectiveness of Zn supplementation in improving maternal Zn status. At follow-up, serum plasma Zn levels were significantly higher in the supplemented groups than in the placebo group (75·07 (sd 7·44) and 70·35 (sd 7·95) v. 60·68 (sd 9·48) mg, respectively). Also, within each supplemented group, the difference between the first and the second measurement of serum Zn concentration was significant. We believe that this may be attributed to many factors: compliance with taking the supplements was good; the supplements were consumed between meals to avoid potential competition for absorption; doubling the dose of Zn substantially increased the daily dietary Zn intake. The Zn-alone group had lower level at enrolment (58·55 (sd 6·87)) but highest level after supplementation (75·07 (sd 7·44)), which supports the notion that the more deficient cases benefit more from supplementation.
In spite of the significant improvement of Zn concentration in the supplemented groups in this trial, both supplement modalities had no significant effect either on birth weight or on the other fetal anthropometric measurements. These findings are consistent with the results of recent reviews, meta-analyses and RCT( Reference Mori, Ota and Middleton 10 , Reference Chaffee and King 12 , Reference Ramakrishnan, Grant and Goldenberg 22 , Reference Norrozi, Borna and Hanachi 32 ). The bioavailability of Zn supplement was the main reason suggested to explain the insignificant effect on birth weight reported in supplementation trials. Results of a recently published meta-analysis (Ramakrishnan et al. ( Reference Ramakrishnan, Grant and Goldenberg 22 )) have supported this claim as although a higher dose (25–62 mg/d) of Zn was used in five studies out of twelve in this meta-analysis, the effect of Zn on birth weight was insignificant even after stratification. Absorption of Zn supplement can be inhibited by Fe and phytate intake, as dietary Zn. Hence, Zn level adequate to promote birth weight may not be achieved after the supplementation of Zn( Reference Hunt, Murphy and Cleaver 33 ). In the present study, the intake of protein, which is a potent enhancer of Zn absorption, was low( Reference Kawai, Spiegelman and Shankar 23 ) and the weight gain was subnormal. These may partly explain the lack of association between Zn status and birth weight.
Unlike Zn supplementation, the evidence shows that supplementation with MM has been associated with increase in birth weight and larger micronutrient doses seem to produce greater impact. A meta-analysis of twelve RCT( Reference Bhutta and Haider 21 ) comparing MM supplementation with Fe–folic acid has reported a small significant increase in the mean birth weight and a reduction in the prevalence of LBW after maternal supplementation with MM in low-income countries. We found a small positive effect of joint supplementation of Zn and MM on birth weight of the neonates (+9·4 g), yet this effect was not significant (Table 4). Also, there was no significant effect on the other anthropometric measurements of the neonates in the three groups. The small effect detected by the present trial may be partly explained by the difference in the dose of the MM. Most of the trials in the meta-analysis( Reference Bhutta and Haider 21 ) used the UNIMMAP, which contains the RDA of fifteen vitamins and minerals, while our supplement contains the RDA of five micronutrients only.
Gestational weight gain has been positively associated with fetal growth, and observational studies of food supplementation in pregnancy have reported increases in gestational weight gain and fetal growth( Reference Frederick, Williams and Sales 34 , Reference Abrams, Altman and Pickett 35 ). Results of supplementation trials, however, had been inconsistent( Reference Ota, Tobe-Gai and Mori 36 ). The present results indicated that although the total weight gain was significantly higher in supplemented groups, yet there was no significant association between weight gain and birth weight in the three groups. Most of our cases (97·6 %) had subnormal weight gain (below 11·5 kg according to Institute of Medicine classification), which may explain the absence of an effect of supplementation on birth weight. Weight gains outside the Institute of Medicine's recommended ranges( Reference Mahan, Escott-Stump, Raymond and Krause 28 ) had been associated with twice as many poor pregnancy outcomes than are weight gains within the ranges. In addition, deviations in maternal weight gain can act as useful markers of biological and social factors that relate to poor pregnancy outcome( Reference Deshpande, Joshi and Giri 7 , Reference Ota, Tobe-Gai and Mori 36 ). The present results were in consistent with these studies where poor women of the placebo group attained the least amount of weight gain and had the poorest outcome.
Although most studies have found no significant overall impact of Zn supplementation on preterm delivery, some studies( Reference Christian, Khatry and Katz 37 , Reference Simmer, Lort-Phillips and James 38 ) have found a beneficial impact in selected subgroups of women. A systematic review of twenty RCT including over 15 000 women and their babies( Reference Mori, Ota and Middleton 10 ) has found a small but significant reduction in preterm birth and no similar reduction in numbers of LBW. The authors concluded that the evidence for a 14 % relative reduction in preterm birth was primarily represented by trials involving women of low income and living in areas of high perinatal mortality.
Conversely, other systematic reviews and meta-analyses have shown significant benefit of MM supplementation in reducing LBW and small-for-gestational age births compared with the Fe–folate supplements, but no significant effects on the duration of gestation or incidence of preterm birth( Reference Mori, Ota and Middleton 10 , Reference Fall, Fisher and Osmond 17 – Reference Kawai, Spiegelman and Shankar 23 ).
In the present study, there were significantly fewer preterm and stillbirth infants in the supplemented groups compared with the control group (1·5 and 3·5 % v. 13·1 %, respectively). The reduced risk of preterm and stillbirth among the supplemented groups (RR 0·12, 95 % CI 0·036, 0·377 and RR 0·27, 95 % CI 0·12, 0·60) is higher than the reduced risk reported by systematic reviews( Reference Mori, Ota and Middleton 10 , Reference Chaffee and King 12 ). Poor maternal health and infection are closely related to preterm birth. Participants in this trial had poor nutritional status at enrolment( Reference Naem, El-Sayed and Nossier 30 ), and they were more likely to benefit from supplementation. Zn alone was more effective than Zn combined with MM in averting preterm and stillbirth cases. The effect size achieved by supplementation with Zn alone was higher than that of joint supplementation with MM. For every nine pregnant women receiving Zn supplement, one stillbirth/preterm birth would be averted compared with ten cases when combined with MM (Table 3). Prolongation of the time spent in utero was suggested to be the main effect of Zn in the few studies, which observed increases in average birth weight with Zn supplementation and not the improvement of fetal growth rate( Reference Caulfield, Zavaleta and Shankar 39 ).
Second- and third-stage complications – mainly prolonged labour and haemorrhage – were lower in the two supplemented groups than the control group, and the effect was higher in the Zn-alone group (RR 0·43, 95 % CI 0·31, 0·60 for Zn group v. RR 0·54, 95 % CI 0·40, 0·73 for combined Zn and MM group). Results of supplementation trials were variable and even contradictory in some cases. Some trials have reported positive effects( Reference Mahomed, James and Golding 40 , Reference Jonsson, Hauge and Larsen 41 ), others no effect( Reference Mahomed, James and Golding 40 , Reference Jonsson, Hauge and Larsen 41 ), and in contrast, Dijkhuizen and Wieringa( Reference Dijkhuizen and Wieringa 42 ) found significantly more deliveries with complications in the group receiving Zn (30 mg/d) plus Fe and folic acid than in the groups receiving Fe and folic acid alone, Fe and folic acid plus β-carotene, or Fe and folic acid plus β-carotene and Zn.
The differences among these studies may be related to the dose of Zn, the initial Zn status of the mothers, the co-intake of other micronutrient supplements and obstetric practices( Reference Caulfield, Zavaleta and Shankar 39 ). It is worth mentioning that in the present trial, data on the occurrence of complications was obtained in post-partum visits and based on women's reports, which may be influenced by subjective judgement and recall bias. It is also worth mentioning that the number needed to treat to avoid one complication was lower (n 4) when using Zn alone than when using the combination of Zn and multivitamin capsules (n 5; Table 3). This has an implication on the decision to introduce a new nutrient to the routine ANC programmes as the cost is a key element.
Zn deficiency during pregnancy affects not only the mother but it also has immunological consequences for the fetus. Concurrent deficiencies of MM, many of which function as cofactors or regulatory molecules in immune or inflammatory cascades (e.g. vitamins C, D and Se), increase the adverse effects of Zn deficiency. Therefore, MM supplements may theoretically augment the effect of Zn on infectious disease resistance( Reference Maggini, Wintergerst and Beveridge 43 ). However, Roth et al. ( Reference Roth, Caulfield and Ezzati 44 ) in their review found that the evidence for the contribution of MM supplementation to perinatal mortality and neonatal morbidity is limited and the evidence base for individual micronutrient effects on neonatal mortality and morbidity is patchy.
In the present trial, early neonatal morbidity – in terms of infections and respiratory distress – was significantly lower in the two supplemented groups compared with the placebo group (11·1 % and 11·7 v. 47·4 %, respectively). The risk reduction resulting from the use of Zn alone did not greatly differ from that resulting from addition of MM to Zn supplementation (77 % (RR 0·23, 95 % CI 0·15, 0·35)) for the Zn group, and 75 % for the combined group (RR 0·25, 95 % CI 0·16, 0·37).
For programme managers, the number needed to treat is important before deciding to introduce a new supplement. Of all effects investigated in the present trial, the highest effect size was noticed in aversion of early neonatal infection. For every three pregnant women receiving Zn or Zn plus multivitamin supplement, one early neonatal morbidity would be averted.
Limitations of the trial
Although we controlled many of the factors previously claimed to be responsible for lack of evidence on association between Zn supplementation and pregnancy outcome; a double-blind design was used, an adequate sample size was recruited to ensure the detection of the smallest worthwhile differences, eligible women were Zn deficient at enrolment, and full participants were only those with high compliance; yet, the present study has a number of limitations that warrant mention. The dosage of Zn used in the Zn-alone supplements may be considered low, especially because women's intake of enhancers of Zn absorption, protein in particular, was very low( Reference Kawai, Spiegelman and Shankar 23 ). Some studies that used high doses have revealed positive effect( Reference Mori, Ota and Middleton 10 , Reference Hunt, Murphy and Cleaver 33 , Reference Abrams, Altman and Pickett 35 , Reference Maggini, Wintergerst and Beveridge 43 ). In addition, verifying the occurrence of complications during delivery and postnatal period was based on self-report and not validated clinically, because it was difficult to follow up many deliveries that took place in different hospitals and even in different governorates. Although outcome ascertainment based on maternal recall alone may nullify true effects, we have no particular reason to doubt the validity of these self-reports, because research shows that recalls of obstetric events are highly valid. An additional limitation is that the follow-up extended only for 6 weeks after delivery, which is too short to observe differences in weight gain and morbidity of the newborns.
Conclusion
Zn supplementation proved to be significantly effective in lowering the risk of second- and third-stage complications, stillbirth and preterm births, and early neonatal morbidity (respiratory tract infection). Supplementation had also positive effects on secondary outcomes such as total weight gain and mean Zn level. The effect was slightly higher among Zn-supplemented group rather than the combined Zn and multivitamin group in most outcomes. In contrast, there was no detectable difference in the mean birth weight between the study groups.
Recommendations
We recommend to include Zn in the prenatal supplementation programmes for women at risk of Zn deficiency in Egypt. The data on the effect size of the two supplements tested in this RCT should be used when carrying out cost-effective intervention studies for introducing new supplements to the routine ANC programme in Egypt. Nutritional health education should be used as a preventive approach to allow the large sector of the low-income population in our society to maximise the use of the limited resources in the best means. Providing nutritional advice, improving access to micronutrient-rich local foods with reasonable price such as beans (kidney bean), whole-grain cereals (wheat and maize), seeds (sesame), eggs and dairy products, and balanced energy and protein supplements to women during pregnancy may be beneficial. Further studies using various combination and doses of micronutrient supplements in different system settings are recommended.
Supplementary material
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S000711451500166X
Acknowledgements
The authors specially thank the managers and personnel of Smouha and Moharam Bec Maternal and Child Health centres. The guidance provided by Dr Emad Darwish, Professor of Gynecology and Obstetrics, Faculty of medicine, Alexandria University, in the supervision of the clinical aspects of the research is highly appreciated. They also specially thank the Pharo Pharma Company for the preparation of the supplements. The present trial was carried out entirely by the authors and was not financially supported by grants from any organisations.
The authors' contributions are as follows: Samia A. Nossier conceived the idea for the study, supervised the data collection, revised and interpreted the results, and wrote the final manuscript; Noha E. Naeim prepared the study tool, conducted the experiment, searched the references, communicate with the statistician during data analysis and wrote a preliminary draft of the manuscript; Nawal A. El-sayed supervised the collection, managed the nutrition data, prepared the study tools, revised the nutrition-related results and its discussion and critically revised the manuscript; Azza A. Abu-zeid facilitated day-to-day collection of data, supervised the laboratory work and facilitated communication with authorities for permissions and withdrawal of blood samples. All authors read the final draft of the manuscript and approved it.
The authors declare that they have no conflicts of interest.








