Prostate diseases are a major health concern for the male population throughout the Western world. Benign prostatic hyperplasia (BHP) and chronic prostatitis (CP), two of the most common medical conditions affecting older men (aged over 40 years), are associated with lower urinary tract symptoms (LUTS) which can have a negative impact on the quality of life (QoL). LUTS are divided into irritative and obstructive symptoms. The former include frequency, urgency and nocturia. The latter consist of slow urine stream and incomplete bladder emptying. Recently, a significant association between the serum levels of C-reactive protein (CRP) and irritative LUTS in both men and women was found(Reference Kupelian, McVary and Barry1, Reference Nickel, Roehrborn and O'Leary2). On the other hand, CRP levels were not significantly associated with obstructive LUTS, or prostate-specific antigen (PSA) levels(Reference St Sauver, Sarma and Jacobson3). Untreated BHP and CP can lead to a number of medical complications, such as acute urinary retention, gross haematuria, repeated urinary tract infections, obstructive uropathy and cystolithiasis. The current standard of preventive care for men at risk of BHP and/or CP is treatment with α-adrenergic receptor blockers, 5-α-reductase inhibitors or antibiotics(Reference Chapple4). In recent years, there has been increasing interest in dietary supplements in the prevention of prostate diseases(Reference Klein5–Reference Wong, Lau and Leung8). The proposed active components of these preparations include Se, vitamin E, vitamin D, lycopene, plant oils, n-3 fatty acids, phytosterols, terpenoids, lectins, polysaccharides, flavonolignans, flavonols and isoflavones. Some important dietary supplements for prostate health are complex extracts from green tea leaf (Camellia sinensis), saw palmetto berry (Serenoa repens), milk thistle seed (Silybum marianum), pumpkin seed (Cucurbita pepo) and stinging nettle root (Urtica dioica). Cranberry (Vaccinium macrocarpon) is a source of organic and phenolic acids, flavonoids, flavonoid glycosides, anthocyanins, proanthocyanidins and triterpenoids of the ursane type with beneficial effects on the urinary tract(Reference Neto9, Reference Guay10). Cranberry preparations are used as natural treatments for urinary tract infections, may reduce the ability of Helicobacter pylori to cause gastrointestinal ulcers and display anti-plaque activity(Reference Howell11–Reference Yamanaka, Kimizuka and Kato13). The medicinal effectiveness and safety of intact cranberry fruits, juice and extracts have been critically evaluated recently(Reference Jepson and Craig14). Among recently reported effects of cranberry are its anti-inflammatory action through reduced cyclo-oxygenase-2 expression, suppression of IκBα degradation in human colon cancer cells(Reference Narayansingh and Hurta15) and inhibition of the growth and proliferation of several types of tumour cells including prostate(Reference Neto, Amoroso and Liberty16). However, to date there has been no published clinical study assessing whether cranberry reduces LUTS in men at risk of developing prostate diseases.
The aim of the present study was to evaluate the effect on urinary tract function of a 6-month daily consumption of 1500 mg cranberry fruit powder (CFP) in men with LUTS based on the International Prostate Symptom Score (IPSS), elevated PSA, BHP and histopathologically confirmed non-bacterial CP.
Materials and methods
Cranberry fruit powder characterisation
CFP (lot 070306-B/07-0659 supplied by Decas Botanical Synergies, LLC, Carver, MA, USA), containing 14·85 % (w/w) organic acids, 15·5 % sugars, 0·11 % anthocyanins, 1·95 % condensed tannins, 3·49 % total phenolics, was used for the clinical part of the study. One gelatine capsule contained 500 mg CFP.
Study subjects and data collection
The study was conducted according to the guidelines laid down in the Declaration of Helsinki, and all procedures involving human subjects/patients were approved by the Ethics Committee of the University Hospital and Faculty of Medicine and Dentistry, Palacky University in Olomouc, Czech Republic. Written informed consent was obtained from all participants. A 6-month randomised controlled trial was conducted from October 2008 to November 2009 at the Department of Urology of the University Hospital.
Study subjects
We invited forty-two men, aged 45 to 70 years (mean age 63 (sd 5·5) years), to participate in the study. All subjects entering the study had LUTS, elevated PSA and/or BHP. Other inclusion criteria were histological findings of acute or chronic non-bacterial prostatitis, normal urinary sediment and negative bacterial cultivation of urine. The diagnosis was asymptomatic inflammatory prostatitis category IV according to the National Institute of Health classification system(Reference Krieger, Nyberg and Nickel17). Exclusion criteria were no supplements such as Se, vitamins E and D, lycopene or herbal products with possible effects on prostate health, a diet rich in isoflavones, antibiotics, anti-inflammatory drugs, α-blockers or 5α-reductase inhibitors, food allergies, chronic liver or kidney diseases, gastrointestinal or metabolic disorder or any other chronic health condition such as diabetes, all identified from interview. Participants were randomly divided into two groups: control (n 21; mean age 64·0 (sd 5·4) years) and cranberry (n 21; mean age 62·0 (sd 5·4) years). In the cranberry group, three capsules (1500 mg CFP per d) were taken at approximately equal intervals daily throughout the day for the 6-month period. The size of the daily dose was based on our double-blind study in young women(Reference Valentova, Stejskal and Bednar18). They were instructed not to consume food rich in phenolics, especially anthocyanin-containing fruits, and to make no other dietary or lifestyle changes during the study. The control group received the same instructions as the cranberry group but no cranberry supplementation.
Data collection
Each case report form included: (i) a detailed medical history; (ii) assessment of all concurrent medical drugs and therapies; (iii) digital rectal examination; (iv) dietary habits; (v) IPSS, QoL and the abridged five-item version of the International Index of Erectile Function (IIEF-5)(Reference Rosen, Cappelleri and Smith19); (vi) urinanalysis; (vii) uroflowmetry with post-voidal residual urine; (viii) kidney and bladder ultrasound; (ix) transrectal ultrasound prostate volume; (x) a complete blood laboratory analysis. The following data were also collected at baseline and at 3 and 6 months in all subjects: Se; testosterone; free PSA (PSAfree); total PSA (PSAtot); CRP; antioxidant status; urine ex vivo anti-adherence activity.
Lower urinary tract symptoms
All participants completed the IPSS including each of the seven areas (feeling of incomplete emptying, frequency, intermittency, urgency, weak stream, hesitancy and nocturia), QoL and five-item version of the International Index of Erectile Function (IIEF-5) questionnaires. Uroflowmetry data – maximal urinary flow rate (Qmax) and average urinary flow rate (Qave) – were measured using the FlowMic (Medkonsult, Olomouc, Czech Republic). Prostate bladder voiding volume (V) and post-void residual urine volume (RV) were assessed using the BK Medical Viking 2400 (BK Medical World Headquarters, Herlev, Denmark) with abdominal probe 3–7 MHz. V and RV were calculated using the formula for a prolate ellipsoid (width × length × height × 0·523). Histopathological examination of prostate tissue was done using ultrasound-guided prostate biopsy (BK Medical Viking 2400, transrectal probe 5–12 MHz; BK Medical World Headquarters) in all subjects.
Clinical biochemistry and haematology
Basic biochemical and haematological parameters were determined in all samples: Na, K, chlorides, total cholesterol, LDL, HDL, TAG, apoA, apoB, CRP, alkaline phosphatase, alanine aminotransferase, aspartate aminotransferase, γ-glutamyl transpeptidase, lactate dehydrogenase, urea, creatinine, and testosterone were quantified in serum using a Hitachi Modular Evo P Analyzer (Hitachi, Tokyo, Japan). PSA (PSAtot and PSAfree) in serum was determined using an Architect type LEIA Analyzer (Abbott Laboratories, Abbott Park, IL, USA). Analysis of selected parameters, i.e. total antioxidant capacity and total thiol (SH) groups in plasma, lipid peroxidation products such as malondialdehyde in plasma and erythrocytes, advanced oxidation protein products in serum, glutathione, glutathione peroxidase, catalase, glutathione reductase, glutathione transferase, and superoxide dismutase in erythrocytes was carried out as described by Psotova et al. (Reference Psotova, Vecera and Zdarilova20). Se in plasma was determined by atomic absorption spectrometry using the AA6300 instrument (Shimadzu, Kyoto, Japan). Hb, packed cell volume, erythrocytes, thrombocytes and leucocytes were measured in Na2EDTA blood.
Urinanalysis
Urine samples were collected from a midstream clean catch and analysed by the IQ200 Automated Urinanalysis System (IRIS International, Inc., Chatsworth, CA, USA).
Anti-adherence activity of urine
Four biofilm-positive micro-organisms were used: Escherichia coli FB42, Enterococcus faecalis FB16 and Candida parapsilosis BC 12 (clinical strain; Collection of the Department of Microbiology, Faculty of Medicine, Masaryk University, Brno, Czech Republic) and Staphylococcus epidermidis CCM 7221 from the Czech Collection of Micro-organisms (CCM; Faculty of Sciences, Masaryk University, Brno, Czech Republic). The bacteria were stored in cryotubes at − 76°C. The biofilm formation was detected using a modification of the adherence assay(Reference Christensen, Simpson and Younger21). The experiment was repeated three times.
Statistical analysis
The data were analysed using the non-parametric Wilcoxon two-tailed tests (paired-samples and independent-samples) to test the statistical significance of differences in all parameters on days 0, 90 and 180. The level of significance was 5 %. Values are presented as 1st quartile, median and 3rd quartile or mean value and standard deviation. Box and empirical cumulative distribution plots were used as graphic illustration of significant differences in progression over 3 and 6 months between the groups.
Results
At baseline both groups had similar clinical and demographic characteristics except for significant differences in BMI and Qmax values (Table 1). The daily dose of CFP contained 223 mg organic acids, 1·65 mg anthocyanins, 29·5 mg condensed tannins and 52 mg total phenols. Patients who received cranberry for 6 months had a statistically significant lower IPSS and QoL score than controls. A lower IPSS score reflected improvement in the irritative and obstructive symptoms (Table 2). All parameters of urination (Qmax, average urinary flow rate (Qave), prostate bladder voiding and post-void residual urine volumes) were significantly improved in at least 70 % of participants of the cranberry group (Fig. 1); in the control group, the tested parameters did not change with the exception of post-void residual urine volume where a statistically significant deterioration was found (Table 3). Haematology values were unchanged with the exception of a significant increase in erythrocytes in the cranberry group, which, however, was within physiological limits (Table 4). PSAtot decreased in approximately 80 % of patients in the cranberry group while, in contrast, the PSAfree:PSAtot ratio mostly increased (Table 4; Fig. 2). Although changes in the values of several ‘safety’ markers were statistically significantly different, after 6 months for both groups, the fluctuation was within normal physiological limits. From this point of view, the cranberry group compared with the control group stabilised and this might be true for oxidative stress markers as well (Table 5). Differences in urine adherence ex vivo in both groups were not significantly different. No adverse events were recorded.
Table 1 Baseline demographics and clinical characteristics
(Mean values and standard deviations)
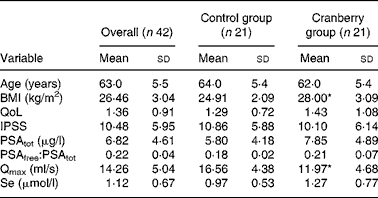
QoL, quality of life questionnaire; IPSS, International Prostate Symptom Score; PSAtot, total prostate-specific antigen; PSAfree, free prostate-specific antigen; Qmax, maximal urinary flow rate.
* Mean value was significantly different from that of the control group (P < 0·05).
Table 2 International Prostate Symptom Score (IPSS), quality of life (QoL) score and International Index of Erectile Function (IIEF-5) in control and cranberry groups
(Mean values and standard deviations)
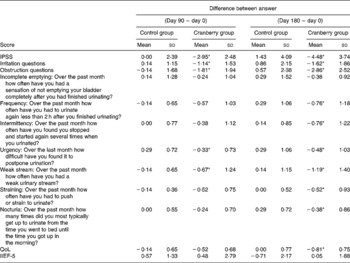
* Mean value was significantly different from that of the control group (P < 0·05).

Fig. 1 Effect of cranberry (Vaccinium macrocarpon) on uroflowmetry parameters of maximal urinary flow rate (Qmax; a), average urinary flow rate (Qave; b), prostate bladder voiding volume (V; c) and post-void residual urine volume (RV; d) during 6 months of treatment. (![]() ), Cranberry group; (□), control group. The box-and-whisker graphs show the median as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. ○, Outside values. * Median was significantly different from that of the control group (P < 0·05).
), Cranberry group; (□), control group. The box-and-whisker graphs show the median as the middle line. The box extends from the 25th to the 75th percentile and the whiskers extend from the lowest value to the highest. ○, Outside values. * Median was significantly different from that of the control group (P < 0·05).
Table 3 Values of uroflowmetry in control and cranberry groups
(First quartiles, medians and third quartiles)
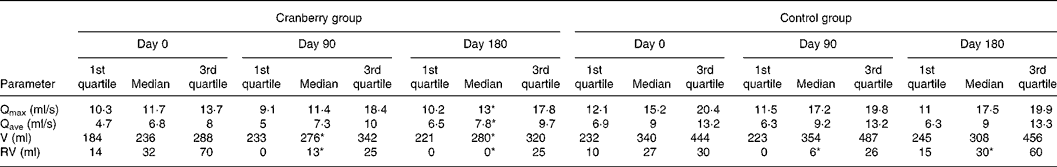
Qmax, maximal urinary flow rate; Qave, average urinary flow rate; V, prostate bladder voiding volume; RV, post-void residual urine volume.
* Median was significantly different from that at day 0 (P < 0·05).
Table 4 Markers of haematology and clinical chemistry in control and cranberry groups
(First quartiles, medians and third quartiles)
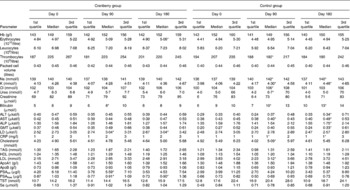
ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, alkaline phosphatase; GMT, γ-glutamyl transpeptidase; LD, lactate dehydrogenase; CRP, C-reactive protein; PSAtot, total prostate-specific antigen; PSAfree, free prostate-specific antigen; TST, testosterone.
* Median was significantly different from that at day 0 (P < 0·05).

Fig. 2 Effect of cranberry (Vaccinium macrocarpon) on free prostate-specific antigen:total prostate-specific antigen ratio after 90 and 180 d of consumption. The values are expressed as difference values based on day 90 and day 0 (a) and day 180 and day 0 (b) of study. (—), Cranberry group; ![]() , control group. * P < 0·05 v. control. The numbers near to the lines correspond with the number of each participant.
, control group. * P < 0·05 v. control. The numbers near to the lines correspond with the number of each participant.
Table 5 Markers of oxidative stress in control and cranberry groups
(First quartiles, medians and third quartiles)
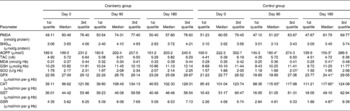
PMDA, plasma malondialdehyde; SHGtot, total thiol groups; AOPP, advanced oxidation protein products; TAC, total antioxidant capacity; MDA, malondialdehyde; GSH, glutathione; SOD, superoxide dismutase, GPX, glutathione peroxidase; CAT, catalase; GST, glutathione transferase; GSR, glutathione reductase.
* Median was significantly different from that at day 0 (P < 0·05).
Discussion
Plant extracts for urinary tract disorders have long been used in traditional medicine. Today, botanical diuretics, antimicrobials and anti-adherence agents, renal protectives and herbs for patients with LUTS or BHP are requested by patients, even though accepted only with reservation by urologists(Reference Yarnell22, Reference Dedhia and McVary23). Cranberry fruit and juice are noted for their ability to inhibit the binding of pathogenic E. coli strains and other microbes to the bladder epithelium. This effect has been attributed to proanthocyanidins (condensed tannins), even if a more simple explanation might be the direct antibacterial action of hippuric acid(Reference Jepson and Craig14). Cranberry prophylaxis is also recommended to women with recurrent urinary tract infection. In a recent publication, for example, the antibiotic Trimethoprim was shown to have minimal advantage over cranberry extract in the prevention of recurrent urinary tract infections in women and had side effects(Reference McMurdo, Argo and Phillips24). LUTS refer to a complex of irritative and obstructive voiding symptoms that are common in both ageing women and men. Prostate enlargement and BPH affect primarily older men. The incidence of LUTS associated with BPH increases dramatically with advancing age(Reference Nickel and Roehrborn25). Unfortunately, no trials have yet been published assessing the effect of cranberry components on men with indicated LUTS and/or increased PSA levels. Recently published results have demonstrated that CP might be linked to a higher prostate cancer risk(Reference Vasto, Carruba and Candore26). The present study was focused on men with diagnosed LUTS, increased PSA level, and histologically confirmed non-bacterial prostatitis. We selected cranberry whole fruit powder in preference to cranberry extract for the present study. Our previous work had shown equivalent efficacy between CFP and two different cranberry extracts(Reference Palikova, Vostalova and Zdarilova27). The daily dose of 1500 mg dried cranberries was based on our double-blind study in young women(Reference Valentova, Stejskal and Bednar18). This dose elicited urine anti-adherence activity but had no adverse effects. In participants taking cranberry for 6 months there was, in addition to a marked improvement in all urodynamic parameters (Fig. 1), a statistically significant decrease in the IPSS score, and an increase in the quality of life evaluated by the QoL questionnaire (Table 2). Taking cranberry affected the value of PSA (Table 4). In the cranberry group, both a decrease in the PSA value and an increase in the PSAfree:PSAtot ratio (Fig. 2) without affecting CRP or testosterone levels were recorded. The use of selective 5-α-reductase inhibitors has often been linked to hormone changes associated with unpleasant sexual side effects, in particular, erectile dysfunction and decreased libido(Reference Tindall and Rittmaster28, Reference Giuliano29). The treatment approach in patients with elevated PSA and histologically confirmed prostatitis is rather complicated and may involve long-term antibiotics with the expectation of lowering the PSA level. The decrease in PSA in the cranberry group demonstrates that prophylaxis by cranberry may be as effective as antibiotic treatment but without the risk of antimicrobial resistance and a minimum of adverse effects. The diuretic effects of cranberries may also have contributed to the reduction in LUTS in the cranberry group(Reference Duke, Bogenschutz-Godwin and DuCellier30). Cranberries contain several structurally different groups of compounds that modulate various cellular pathways in man including the urinary tract and the prostate. However, phenolics, as phenolic acids, anthocyanins and proanthocyanidins, that are metabolised mainly to hippuric acid, are assumed to be the active components. The synergistic effects of cranberry constituents may improve their bioactivity. Use of the whole berries may be more beneficial than single components and with minimal adverse effects.
Conclusions
Our trial is the first to evaluate cranberry in the treatment of LUTS specifically in men with BHP, elevated PSA levels and non-bacterial prostatitis. The present results show that dried cranberries improve prostate health very effectively both in men with elevated PSA in histologically proven prostatitis and for improving voiding dysfunction. In the cranberry group, no associations were found between dried powdered berry consumption and CRP levels. Unlike currently used medication for prostatitis and LUTS, cranberry has no adverse effects. Our findings may assist men suffering from LUTS, and also their clinicians, to decide on a treatment that is both inexpensive and natural, like cranberry. The limitations of the present study include the relatively small number of men. Given the probability that some responses on the IPSS and QoL questionnaires in the cranberry group may have been secondary to a placebo effect, there is a need to control for this in future clinical trials.
Acknowledgements
The present study was supported by the Ministry of Education, Youth and Sport of the Czech Republic (grant no. MSM 6198959216).
The original authors and their contributions were as follows: A. V. was involved in the development of the protocol; V. Student and D. S. participated in the clinical observation of the subjects; J. Vrbkova carried out the statistical analysis; F. R. performed the microbial anti-adherent assay; R. R. performed the cranberry analysis; V. Simanek. and J. U. analysed the clinical chemistry data; J. Vostalova was responsible for the management of the study, and was the principal investigator and guarantor.
There is no conflict of interest.









