Since the 1960s, people in the Western world have been encouraged to increase their consumption of PUFA in order to decrease serum cholesterol concentrations1. Today, PUFA account for up to 12 % total energy intake (E%)Reference Hibbeln, Nieminen, Blasbalg, Riggs and Lands2, Reference Yam, Eliraz and Berry3 and n-6 PUFA, in particular linoleic acid (18: 2n-6), constitute up to 90–95 % of these dietary PUFAReference Kris-Etherton, Taylor, Yu-Poth, Huth, Moriarty, Fishell, Hargrove, Zhao and Etherton4. An increased intake of 18: 2n-6 has been shown to lower CVD end-pointsReference Sacks and Katan5, but Israelis, who traditionally have high intakes of 18: 2n-6, have been shown to have a high CVD mortality rateReference Yam, Eliraz and Berry3. Furthermore, the possible harmful effects of n-6 PUFA on inflammatory diseases and cancer risk have been disputedReference Sacks and Campos6, Reference Simopoulos7. High dietary and tissue levels of n-3 PUFA, especially n-3 long-chain PUFA (LCPUFA), have been associated with a protective effect on CVD and other chronic illnessesReference Hibbeln, Nieminen, Blasbalg, Riggs and Lands2, Reference Kremer8, Reference Bucher, Hengstler, Schindler and Meier9. The n-3 and n-6 PUFA compete metabolically with regard to tissue incorporation and conversion to active paracrine substances (e.g. eicosanoids)Reference Lauritzen, Hansen, Jorgensen and Michaelsen10. Therefore, it has been argued that the amount of 18: 2n-6 in the diet should be taken into account when designing studies and dietary recommendations on n-3 PUFAReference Hibbeln, Nieminen, Blasbalg, Riggs and Lands2.
Fish and fish oils (FO) are major dietary sources of n-3 LCPUFA, primarily DHA (22: 6n-3) and EPA (20: 5n-3). n-3 LCPUFA can be produced endogenously from α-linolenic acid (18: 3n-3), but the conversion is limited because of β-oxidation and competition for the rate-limiting enzyme, Δ6-desaturaseReference Lauritzen, Hansen, Jorgensen and Michaelsen10. This enzyme is involved in conversion of 18: 3n-3 to 20: 5n-3 and likely also in a second round of conversion to 22: 6n-3Reference Burdge and Calder11. It is also involved in metabolism of 18: 2n-6 to arachidonic acid (20: 4n-6)Reference Lauritzen, Hansen, Jorgensen and Michaelsen10. Accordingly, production of 22: 6n-3 from 18: 3n-3 is thought to depend on the amount of available substrate (18: 3n-3) and the amount of 18: 2n-6, but may also be limited by the total amount of 18: 2n-6+18: 3n-3 in the dietReference Blank, Neumann, Makrides and Gibson12.
Many human trials studying the impact of dietary 18: 3n-3 and 18: 2n-6 on tissue n-3 LCPUFA and health have failed to show more than marginal increases in synthesis and incorporation of 22: 6n-3, as a result of decreasing the 18: 2n-6:18: 3n-3 ratio (see Reference Burdge and Calder11). However, most have focused on increasing the intake of 18: 3n-3Reference Hussein, Ah-Sing, Wilkinson, Leach, Griffin and Millward13–Reference Kelley, Nelson, Love, Branch, Taylor, Schmidt, Mackey and Iacono18, without considering the fact that as a substrate for the Δ6-desaturase, 18: 3n-3 may inhibit the conversion of docosapentaenoic acid (22: 5n-3) to 22: 6n-3. In an experiment where 18: 3n-3 was varied at a constant intake of 18: 2n-6, Blank et al. Reference Blank, Neumann, Makrides and Gibson12 found maximal incorporation of 22: 6n-3 in piglets when the dietary 18: 2n-6:18: 3n-3 ratio was 2:1 to 4:1, whereas both higher and lower ratios (the latter equivalent to both higher 18: 3n-3 and higher total C18 PUFA intake) gave lower incorporation. Therefore, both the dietary 18: 2n-6:18: 3n-3 ratio and the total consumption of C18 PUFA are likely to affect the endogenous 22: 6n-3 productionReference Blank, Neumann, Makrides and Gibson12. To our knowledge, only one randomized trialReference Liou, King, Zibrik and Innis19 has investigated n-3 LCPUFA incorporation in human subjects at dietary n-6:n-3 PUFA ratios below 7:1 in combination with a 18: 2n-6 intake of less than 4 E%, without strongly increasing dietary 18: 3n-3. Moreover, none of the studiesReference Hussein, Ah-Sing, Wilkinson, Leach, Griffin and Millward13–Reference Valsta, Salminen, Aro and Mutanen15, Reference Goyens, Spilker, Zock, Katan and Mensink17–Reference Liou, King, Zibrik and Innis19 investigated how tissue levels of n-3 LCPUFA are affected by a change in 18: 2n-6 intake in combination with FO-supplementation. This is interesting since it has been argued that a high intake of 18: 2n-6 impairs the health benefits of FO by decreasing the tissue incorporation of dietary n-3 LCPUFAReference Hibbeln, Nieminen, Blasbalg, Riggs and Lands2.
We supplemented sixty-four healthy men with FO or control (olive oil, OO) capsules in combination with different spreads and oils to provide a high or a low dietary 18: 2n-6 intake for 8 weeks. Diet and fatty acid composition of peripheral blood mononuclear cells (PBMC) were measured immediately before and after the intervention and after 8 weeks of wash-out, to assess whether any changes persisted after the participants had returned to their normal diet. PBMC was chosen as our model tissue since another purpose of the study was to investigate inflammatory effects, which is not reported in the present paper. We hypothesized that providing commercially available oils and spreads, which were high in 18: 2n-6, would increase the participants' 18: 2n-6 intake and the dietary ratio of n-6:n-3 PUFA and that this would result in a lower incorporation of 22: 6n-3 in PBMC. We also hypothesized that a high consumption of 18: 2n-6 would impair the incorporation of n-3 LCPUFA from FO-supplements. We tested this in a realistic setting in which the fats used by the participants on bread and for cooking were replaced with other commonly used types.
Methods
Study design and recruitment
The present study had a randomized, double-blinded 2 × 2 factorial design. Sixty-six healthy men aged 18–40 years were randomized to FO or OO (control) capsules (5 ml/d) and to either sunflower oil and Becel 60 margarine (S/B) or to rapeseed oil and a Danish rapeseed oil-enriched butter spread, Kaergaarden Light (R/K). The participants were instructed to use it for cooking, baking and on bread as substitutes for the oil and spreads that they normally used. Apart from being high and low in 18: 2n-6, respectively, the two fat combinations were also chosen because of their relatively high (>10:1) and low (approximately 2:1) ratio between n-6 and n-3 PUFA (Table 1). The oils were a kind gift from Aarhus United Denmark A/S (Aarhus, Denmark) and packed in identical, neutral 500 ml bottles. The spreads were kindly provided by Unilever Danmark A/S Foods (Brøndby, Denmark) and Arla Foods amba (Viby, Denmark) and packed in identical, white 250 g containers. The capsules were a kind gift from Pharma Nord ApS (Vejle, Denmark) and provided as 660 × 0·5 ml soft gelatine capsules with either FO (Bio-Marine, NEFA) or OO (TAG). Randomization was done separately for the fat and the capsule intervention by drawing notes from two envelopes. The capsule containers were numbered continuously to ensure that neither subjects nor investigators knew who were in the same capsule group. For logistic reasons, this could not be done with the fat intervention, so the fat groups were coded A and B. All allocations were blinded to the participants and investigators until the end of data analysis. However, when asking the participants after the intervention, 80 % correctly guessed which type of capsules they had received, due to the fishy taste of the FO. Less than 10 % correctly guessed what type of cooking oil they had been using, whereas about 45 % guessed that they had been using a butter spread or a margarine. Prior to the intervention period, the participants were provided with OO and butter for 2 weeks (run-in) to standardize their fat consumption and PBMC fatty acid composition. During the run-in and intervention period the participants were not allowed to eat fish except for five lean fish types (canned tuna in water, cod, coalfish, plaice, shrimps and shellfish), which were allowed once per week. There were no dietary restrictions during the wash-out period. Apart from these changes, the participants were told to maintain their dietary and lifestyle habits throughout the study.

S/B, sunflower oil/Becel margarine; R/K, rapeseed oil/Kaergaarden spread.
* For details of subjects and procedures, see Methods.
† Based on the National Danish Food Composition Tables20, information from the manufacturers and our own laboratory analyses.
‡ The fish oil (FO) was provided as NEFA and the olive oil (OO) as TAG. Ten capsules contained 5 ml oil. The fish oil capsules also contained 0·67 g/l α-tocopherol.
Participants were recruited by notes at universities throughout Copenhagen. Written information was sent out by email and the inclusion criteria were checked by telephone screening. Eligible subjects were invited to an information meeting where verbal information on the study was given. The study protocol was approved by the Scientific Ethical Committee of Frederiksberg and Copenhagen (J.no. KF 01267804) and registered in the US National Institutes of Health clinical trial database (ClinicalTrials.gov, no. NCT00266292). Informed written consent was obtained from all participants.
Eligible persons were apparently healthy 18–40 year old males with no chronic diseases, no regular medication and no strong allergies, who were smoking less than five cigarettes/week, exercising < 7 h/week, eating home-made meals ≥ 5 d/week and who had a daily consumption of butter, margarine and/or oil. Blood donation or dietary supplements such as vitamins were not allowed 2 months before or during the study. Most of the participants were students. Two participants abandoned the study during the intervention period, one due to personal problems and one because of trouble swallowing the capsules. Accordingly, sixty-four participants completed the intervention period, after which one person in the OO+R/K group withdrew without explanation.
Visits and measurements
The present study included four examination visits to the Department of Human Nutrition: 1) before the start of the 2-week run-in-period; 2) at baseline before the start of the intervention; 3) after 8 weeks of intervention; 4) after 8 weeks of wash-out. Dietary intake was recorded three times by 4-d weighed dietary records on three workdays and a Saturday, just before the start of the run-in period and before visits 3 and 4. Nutritional calculations were done with Dankost 3000® software (Dansk Catering Center, Herlev, Denmark) based on the National Danish Food Composition Tables20 but with the specific fatty acid composition of the intervention spreads and oils entered into the database. At every visit the participants filled out questionnaires on illnesses, use of medication, dietary habits and use of fats and oils. The median length of the intervention and wash-out periods were 8 (range 7–9) weeks and 8 (range 7–8) weeks, respectively, and did not differ between the groups (P>0·17, Kruskall-Wallis test).
At the first visit, height and weight were measured by standard procedures. Instructions on food recording were given and diet registration forms and a kitchen scale were provided. The participants were asked to hand in the completed dietary records and the scale to a dietitian a couple of weeks later and to pick up new forms and a scale before the second and third food recording. Finally, 500 ml bottles of OO (Oleificio R. M. s.p.a., Lucca, Italy) and 500 g packs of butter (a kind gift from Arla Foods amba) were provided to the participants. They were instructed to use it after finishing the first dietary recording and to discard all other types of oil, butter and margarine in the household. On the second visit, participants received their allocated capsules and fats. As in the run-in period, they were told to use the fats instead of their usual butter, margarine and oils and to pick up more if they ran out. Since no fixed amounts were set, they were allowed to share it with other members of their household. They were also told to consume the capsules in combination with a meal, to return the remaining capsules at the end of the intervention period and to bring back remaining oils and spreads to ensure their use only during the intervention. No fats or oils were provided in the wash-out period, where the participants were asked to eat their habitual diet. Counting of the returned capsules showed that the median FO and OO consumption during the intervention was 4·4 (range 2·0–5·6) ml/d in both groups. Based on the dietary records, the median consumption of the provided intervention fats was 3·4 g/d spread (0·0–32·5 g/d) and 10·3 g/d oil (0·0–52·5 g/d) with no significant differences between the groups (P>0·78, Kruskal–Wallis test). There were 12 % and 31 % of the participants in the OO and FO groups, respectively, who had episodes of nausea, slight headache or felt bloated during the supplementation period, likely due to the gelatine in the capsules or the fishy taste of the FO capsules.
At the second, third, and fourth visit a number of cardiovascular measurements were performed and blood was sampled. Participants were instructed to be 12 h fasted (except for 0·5 litres water), not take any medication or drink alcohol 24 h prior to the blood sample and to avoid exercise the last 36 h. They were also told not to smoke the last week, to have the same meal every night before the blood sample and to reschedule their visit if they were ill. These criteria were checked at every visit. Finally, the participants received a breakfast meal, kindly sponsored by Arla Foods amba and Lantmännen Schulstad A/S (Hvidovre, Denmark).
Laboratory analyses
For isolation of PBMC, blood was sampled with Na-heparin and diluted 1:1 in sterile RPMI-1640 culture medium (# BE12-167F; Lonza, Basel, Switzerland) with 1 % antibiotics (10 000 units penicillin/10 000 μg streptomycin, # P4333; Sigma-Aldrich, Mannheim, Germany) within 30 min, layered on Ficoll-Paque Plus (# 17144003; Amersham Biosciences, Piscataway, NJ, USA) and centrifuged for 30 min at 950 g, 20°C. PBMC were isolated from the interface and washed twice in ice-cold RRMI medium by centrifugation at 700 g, 20°C, 10 min. PBMC from 20 ml blood (about 2–4 × 107 cells) were resuspended in 1·5 ml methanol with 0·01 % butylated hydroxytoluene, sealed with N2 and frozen at − 80°C within 6 h after sampling. Lipids were extracted by the Folch procedureReference Folch, Lees and Sloane Stanley21 and transmethylated with boron trifluoride in methanolic NaOH. The resulting fatty acid methyl esters were separated by GLC on a HP-6890 Series II chromatograph (Hewlett-Packard Inc., Waldbronn, Germany) with an injection volume of 5 μl, split-ratio 10:1 and injector and detector temperatures of 250°C and 300°C, as described elsewhereReference Lauritzen, Jorgensen, Mikkelsen, Skovgaard, Straarup, Olsen, Hoy and Michaelsen22. About 98 % of the fatty acids in the chromatograms were identified, corresponding to a mean of 222 (range 93–408) μg fatty acids in PBMC in one sample. The intra- and inter-assay variations were quantified by analysis of a reference sample. If the reference sample in a given assay deviated more than 3 sd from its usual value, the whole assay was re-analysed. Intra- and inter-assay CV were 1·1 and 2·4 % for SFA, 0·8 and 1·8 % for MUFA, 1·8 and 2·3 % for n-6 PUFA and 4·8 and 6·1 % for n-3 PUFA, respectively. The relative content of specific fatty acids is expressed as an area percentage relative to all fatty acids in a chromatogram, comparable to g/100 g. The fatty acid composition of the spreads, oils and oil capsules were also analysed by the afore-mentioned procedures.
Statistical analyses
Data were checked for normal distribution with the Shapiro-Wilk normality test and visual inspection of histograms. Dietary 18: 3n-3 and n-6: n-3 PUFA and 20: 5n-3 in PBMC were logarithmically transformed before analysis. Comparisons between the four intervention groups at baseline were done in one-way ANOVA with Tukey's post hoc test. Comparisons after the intervention and after the wash-out period were done in analysis of covariance with fat type and capsule type as fixed factors and baseline values as covariate, after checking for interaction. To further check for changes during each period in each group, paired t tests were used. Bivariate correlations were done with Pearson's correlation analysis. All data were analysed with the Statistical Package for the Social Sciences software (version 14·0; SPSS Inc., Chicago, IL, USA) and statistical significance was established at P < 0·05.
Results
There were no significant differences in age, anthropometry or self-reported fish consumption between the four groups at baseline (Table 2). Although 30 % of the participants reported occasional (less than weekly) intake of oily fish during the intervention, the restriction on oily fish was generally undertaken. Data from the weighed dietary records (Table 3) showed that the participants' energy and macronutrient intake was close to the Nordic recommendations23 and, apart from a slightly lower fat intake, corresponded well to that of the general Danish male populationReference Fagt, Matthiesen, Trolle, Lyhne, Christensen, Hinsch, Hartkopp, Biltoft-Jensen, Moller and Daae24. The dietary composition is shown without contributions from alcohol. Based on the dietary records, the median alcohol consumption during the intervention was 3, 1, 10 and 24 g/d in the four groups shown in Table 3, but based on the questionnaire it was 10, 0, 0 and 9 g/d. The reported energy intake remained constant throughout the study in all four groups, whereas the relative contribution of energy from fat tended to increase in the intervention period, where the participants received oil capsule supplements (Table 3).
Table 2 Baseline characteristics of the study participants in the four intervention groups*†
(Mean values and standard deviations)
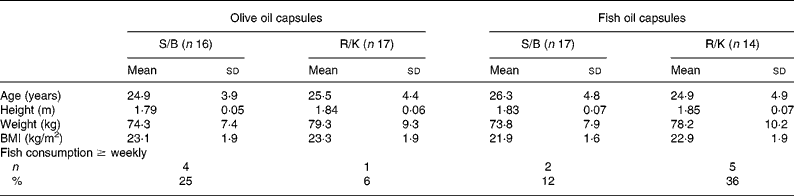
S/B, sunflower oil/Becel margarine; R/K, rapeseed oil/Kaergaarden spread.
* For details of subjects and procedures, see Methods.
† The intervention groups were compared in one-way ANOVA except for fish consumption, which is shown as number of persons who consumed fish at least weekly and was tested with two-sided χ2 test. No significant differences were seen for any of the variables.
Table 3 Intake of macronutrients in the intervention groups at baseline, after the intervention and after the wash-out period (n 63-64)†‡
(Values are means and standard deviations except for 18: 3n-3 and the PUFA ratio, which are given as medians (25th, 75th percentiles))

S/B, sunflower oil/Becel margarine; R/K, rapeseed oil/Kaergaarden spread; E%, percentage of total energy intake.
Changes during each period within each group were tested with paired t test. Mean value was significantly different from that of the preceding visit: *P < 0·05; **P < 0·01; ***P < 0·001.
Baseline values were tested in one-way ANOVA comparing the four groups. After the intervention and wash-out period the groups were compared in analysis of covariance, adjusting for the two interventions and baseline values. P values are shown for the two interventions (capsule and fat).
† Contributions from the oil capsules are included in the numbers; reported alcohol consumption is not included.
‡ For details of subjects and procedures, see Methods.
The fat intervention mainly changed the composition of dietary fat in the S/B groups, resulting in higher 18: 2n-6 and total PUFA intake and lower intake of MUFA relative to the R/K groups (Table 3). 18: 2n-6 constituted 7 (sd 2) E% and 4 (sd 1) E% in the S/B groups and R/K groups, respectively, in the last week of the intervention period. The consumption of 18: 3n-3 tended to increase slightly in all groups, but did not differ between the S/B groups and the R/K groups (Table 3). The FO groups had significantly higher n-3 PUFA intake than the OO groups. Accordingly, the dietary n-6:n-3 PUFA ratio was affected by both interventions. No differences were seen in the intake of oleic acid (18: 1n-9) (Table 3).
No significant interactions were seen between the fat and capsule intervention on n-3 LCPUFA incorporated in PBMC. This indicates that the effect of the FO capsules did not depend on the type of provided dietary fats. As expected, the FO capsules resulted in markedly lower PBMC 20: 4n-6 and higher n-3 LCPUFA than the OO capsules (Table 4). PBMC 22: 5n-3 was slightly higher and 20: 5n-3 tended to be slightly higher (P = 0·06) in the R/K groups, but no differences were seen for 22: 6n-3. The fat intervention gave lower PBMC MUFA in the S/B groups compared with the R/K groups and tended to increase PBMC n-6 PUFA (P = 0·06). In within-group comparisons, 18: 2n-6 was found to increase with S/B, but only when FO capsules were given (Table 4), possibly due to negative feedback inhibition of the Δ6-desaturase by the FOReference Garg, Thomson and Clandinin25. Overall, incorporated n-3 PUFA increased and n-6 PUFA decreased when moving from left to right in Table 4, but the effect of the FO supplement was much stronger than that of changing the 18: 2n-6 intake.
Table 4 Peripheral blood mononuclear cell fatty acid composition (FA%) in the intervention groups at baseline, after the intervention and after the wash-out period (n 63–64)†‡
(Values are means and standard deviations except for 20: 5n-3, which is shown as median (25th, 75th percentile))

FA%, area percentage of a given fatty acid relative to all fatty acids in a chromatogram; S/B, sunflower oil/Becel margarine; R/K, rapeseed oil/Kaergaarden spread.
Mean values within a row with unlike superscript letters were significantly different (P < 0·05) (Tukey's post hoc test). After the intervention and wash-out period the groups were compared in analysis of covariance, adjusting for the two interventions and baseline values. P values are shown for effect of the two interventions (capsule and fat). Baseline values were tested in one-way ANOVA comparing the four groups. Changes during each period within each group were tested with paired t test.
Mean value was significantly different from that of the preceding visit: *P < 0·05; **P < 0·01; ***P < 0·001.
† No 20: 3n-9 was detected in any of the samples.
‡ For details of subjects and procedures, see Methods.
After the wash-out period the dietary data showed that the participants had actually returned to their habitual diet (Table 3). PBMC 20: 5n-3, 22: 5n-3 and in particular 22: 6n-3 were still increased in the FO groups, compared with values at baseline (Table 4). Slight effects of the fat intervention were also seen on PBMC 20: 4n-6, 20: 5n-3, 22: 5n-3 and the n-6:n-3 PUFA ratio after 8 weeks of wash-out.
The proportion of n-3 LCPUFA in PBMC at the end of the intervention was strongly correlated with the reported intake of n-3 PUFA (r 0·70, P < 0·001, n 64) and also when the numbers were evaluated as changes during the intervention (r 0·69, P < 0·001, n 64). The overall pattern in PBMC n-6:n-3 PUFA (Table 4) corresponded well to the reported ratios in the diet (Table 3).
Discussion
In the present study, we found clear effects of FO on PBMC n-3 LCPUFA, but no profound differences in 22: 6n-3 or other n-3 LCPUFA as a result of the fat intervention. This is in accordance with a recent tracer study by Goyens et al. Reference Goyens, Spilker, Zock, Katan and Mensink17, who investigated the effect of dietary 18: 2n-6:18: 3n-3 ratios between 19:1 and 7:1 at 18: 2n-6 intakes of 3–7 E % in elderly subjects. In their study, 20: 5n-3 in plasma phospholipids increased at both low 18: 2n-6 and high 18: 3n-3 intake, but 22: 6n-3 was only slightly increased and only in the group with high 18: 3n-3, indicating no change in the rate of conversion of 20: 5n-3 to 22: 6n-3. A number of studiesReference Hussein, Ah-Sing, Wilkinson, Leach, Griffin and Millward13–Reference Metcalf, James, Mantzioris and Cleland16 have sought to increase the endogenous synthesis of 22: 6n-3 by increasing the consumption of 18: 3n-3, whereas we aimed at a similar intake of 18: 3n-3 in all groups, since a high consumption of 18: 3n-3 may theoretically inhibit the Δ6-desaturaseReference Blank, Neumann, Makrides and Gibson12. As reviewed by Burdge and CalderReference Burdge and Calder11, most studies have failed to show increases in synthesis and incorporation of 22: 6n-3 by increasing the 18: 3n-3 intake and/or decreasing dietary 18: 2n-6. However, it could be speculated that 22: 6n-3 synthesis would be increased if the 18: 2n-6:18: 3n-3 ratio and the 18: 2n-6 intake were even lower than in the study by Goyens et al. Reference Goyens, Spilker, Zock, Katan and Mensink17. In a study in piglets where the intake of 18: 2n-6 was kept constant, Blank et al. Reference Blank, Neumann, Makrides and Gibson12 showed maximal incorporation of 22: 6n-3 when the 18: 2n-6:18: 3n-3 ratio was 2:1 to 4:1, indicating that a high consumption of both 18: 2n-6 and 18: 3n-3 limited the production and/or incorporation of 22: 6n-3. However, in line with the existing evidence we saw no effect on PBMC 22: 6n-3 despite the low 18: 2n-6:18: 3n-3 ratio (4:1) combined with a low 18: 2n-6 intake (4 E%) in the OO+R/K group. Comparable intakes of 18: 2n-6 and 18: 3n-3 were tested in a recent Canadian cross-over studyReference Liou, King, Zibrik and Innis19 in healthy men, where the dietary 18: 2n-6:18: 3n-3 ratios were manipulated from 4:1 to 10:1 at 18: 2n-6 intakes of 3·8–10·5 E% by providing specially produced foodstuffs to the participants. Again, no effect was seen on 22: 6n-3 in plasma phospholipids. We cannot exclude the possibility that 22: 6n-3 incorporation may be increased at a 18: 2n-6 intake < 4 E%, but this would probably require dietary manipulations that are difficult to achieve with a normal diet and therefore of questionable relevance for recommendations to healthy individuals.
Our second aim was to test the widespread view held by Hibbeln and othersReference Hibbeln, Nieminen, Blasbalg, Riggs and Lands2 that a high 18: 2n-6 intake in the diet affects the incorporation of preformed dietary n-3 LCPUFA, in this case from FO. We did not find support for this hypothesis, as no interaction between the interventions were observed and only minor differences in PBMC n-3 LCPUFA content were seen between the two FO-supplemented groups. To our knowledge, the combination of FO supplementation and a high 18: 2n-6 diet has not been investigated in other randomized human trials. One study in rats fed either purified 20: 5n-3 or 22: 6n-3 showed a decrease in tissue phospholipid 20: 5n-3, but virtually no change in 22: 6n-6 when increasing amounts of 18: 2n-6 rich maize oil were included in the background dietReference Raederstorff26.
The slight increase in PBMC 22: 5n-3 in the R/K groups is in line with the findings of human trials investigating the effects of increasing the 18: 3n-3 intake (see ref. Reference Burdge and Calder11) or lowering the 18: 2n-6 intakeReference Chan, McDonald, Gerrard, Bruce, Weaver and Holub14, Reference Goyens, Spilker, Zock, Katan and Mensink17. In contrast to a study by Chan et al. Reference Chan, McDonald, Gerrard, Bruce, Weaver and Holub14, we did not see any marked differences between the fat groups in tissue 20: 5n-3, possibly because a larger difference between the groups were provoked in their study, or because 20: 5n-3 was further converted to 22: 5n-3 in the current study. According to the present data, the most efficient way of achieving a higher proportion of 22: 6n-3 and other n-3 LCPUFA in tissues is by increasing the intake of preformed 20: 5n-3 and 22: 6n-3 from oily fish or FO. It should be noted, however, that some effects of our fat intervention were seen 8 weeks after the participants had returned to their normal diet. This may be important for n-3 PUFA status over time.
For logistic reasons, the encapsulated FO was provided as NEFA, whereas the placebo capsules contained OO in the form of TAG. This is a limitation in the present study but we believe that it did not have any considerable effect on our results since the absorption and incorporation of these two forms of PUFA have been shown to be very similarReference De Schrijver, Vermeulen and Backx27–Reference Vidgren, Agren, Schwab, Rissanen, Hanninen and Uusitupa29. The strength of the present study is that it was conducted in a real-life-setting, which manipulated only the intake of commercially available fats and oils. On the other hand, this gives rise to limitations with regard to determination of the intake of specific fatty acids. The data from the 4-d weighed dietary records are accurate with regard to macronutrient intake but although we added the fatty acid composition of the provided fats and oils to the database, the National Danish Food Composition Tables do not provide information on fatty acid composition of all foodstuffs that were eaten. After subtracting 5 % assumed to be glycerol backbones, about 10 % of the total fat intake was still unexplained in terms of fatty acids. Therefore, the intake numbers on individual fatty acids should be interpreted with caution. The effects on tissue incorporation were determined in PBMC, since another purpose of the study was to explore inflammatory aspects of the interventions. Due to the short leukocyte lifetime, the PBMC fatty acid composition is believed to reflect tissue changes in the relevant period, with maximal incorporation after about 2 weeksReference Gibney and Hunter30. Since consumption of FO affects the proportion of 22: 6n-3 and in particular 20: 5n-3 in a dose–responsive manner, PBMC was also a highly relevant marker of compliance with the FO capsules.
In conclusion, increasing the dietary 18: 2n-6 intake by providing different commercially available fats and oils to healthy men did not affect either the 22: 6n-3 content in PBMC nor the incorporation of n-3 LCPUFA from FO supplements in these cells.
Acknowledgements
The study was funded by The National Danish Research Council for Agriculture and by the Danish Heart Foundation. We gratefully acknowledge Elin Skytte, Anders D. Andersen, Michael Seest, Stine Bartelt, Hanne Jensen and Berit Hoielt for their work with data collection and Pia Madsen for conducting the fatty acid analyses. We also thank Harald S. Hansen for constructive comments during the writing of the manuscript.






