Non-communicable diseases are responsible for 71 % of annual global deaths(1). The most important risk factors for non-communicable diseases are elevated blood glucose (hyperglycaemia) and excessive body weight(Reference Lim, Vos and Flaxman2). Postprandial hyperglycaemia is defined as blood glucose levels ≥7·8 mmol/l (considered as impaired glucose tolerance) or ≥11·1 mmol/l (considered as diabetes) 180 min after the consumption of a 75 g glucose load(3). Excessive body weight results from an imbalance between energy intake and energy expenditure, and it is diagnosed using BMI of which values ≥25–29·9 or ≥30 kg/m2 are categorised as ‘overweight’ or ‘obese’, respectively(Reference Bray4). The aetiology of hyperglycaemia and increased body weight is multi-factorial, with lifestyle (e.g. diet and exercise) being a significant contributing factor to both(Reference Murea, Ma and Freedman5).
Dietary interventions are therefore employed as a cornerstone strategy for the prevention and management of both hyperglycaemia and increased body weight(Reference Ajala, English and Pinkney6). Of relevance, dietary fibre has been shown to positively modulate both blood glucose levels and energy homoeostasis via a myriad of mechanisms. For example, soluble fibres can delay gastric emptying, glucose absorption in the small intestine and starch degradation; all of which contribute to decreasing blood glucose levels. Also, soluble fibres have been shown to stimulate the secretion of hormones such as glucagon-like peptide 1 (GLP-1) which acts as an incretin (stimulating the secretion of insulin) and promotes pancreatic β-cell growth(Reference Chutkan, Fahey and Wright7). In contrast, insoluble fibres interact with host digestive enzymes to attenuate digestible polysaccharide hydrolysis and consequently dampen the glycaemic response(Reference Dhital, Gidley and Warren8). Furthermore, insoluble fibres can be fermented by the gut microbiota to produce bioactive metabolites – such as the SCFA acetate, butyrate and propionate(Reference Tarini and Wolever9) – which can stimulate incretin effects in the peripheral circulation(Reference Wong, De Souza and Kendall10).
With regard to energy homoeostasis, dietary fibre has been shown to reduce energy intake via various mechanisms(Reference Wanders, van den Borne and de Graaf11). Increased bulk and viscosity(Reference Slavin and Green12), increased gastric distension(Reference De Graaf, Blom and Smeets13) and decreased gastric emptying(Reference Bergmann, Chassany and Petit14) have all been implicated in fibre-induced satiety. Additionally, the release of the anorexigenic hormones GLP-1 and peptide tyrosine tyrosine (PYY) following fibre ingestion (due to mechanisms such as gut-derived SCFA production(Reference Byrne, Chambers and Morrison15)) may also be a key driver of this effect. Overall, these processes result in a decrease in appetite and energy intake which can ultimately facilitate body weight loss.
Dietary protein has also been shown to positively influence blood glucose levels(Reference Nuttall, Mooradian and Gannon16) through mechanisms that are not yet fully defined. Putative mechanisms include the effects of incretin secretion (e.g. GLP-1 and gastric inhibitory peptide(Reference Ma, Stevens and Cukier17)), as well as the insulin secretagogue effects of amino acids(Reference Lindgren, Pacini and Tura18). Moreover, dietary protein can increase satiety and suppress energy intake, possibly via its effect on gluconeogenesis(Reference Mithieux, Misery and Magnan19), diet-induced thermogenesis(Reference Rampone and Reynolds20) and/or release of anorexigenic hormones from the gastrointestinal tract(Reference Belza, Ritz and Sørensen21). Therefore, foods high in both dietary fibre and protein may modulate blood glucose levels, appetite and consequently energy intake to a greater extent than foods that contain either fibre or protein alone.
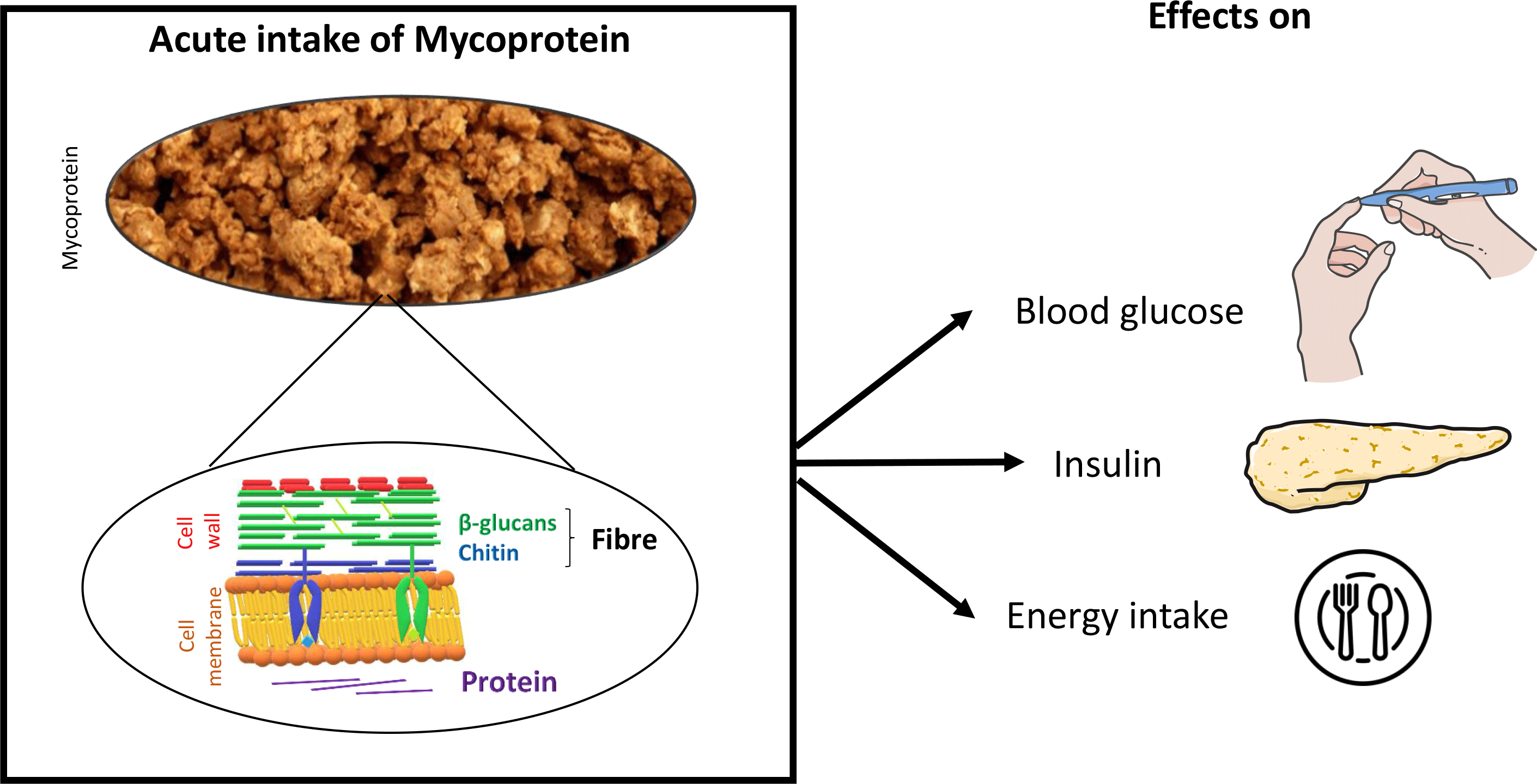
Mycoprotein is the name given to the biomass of fungal filaments produced following the continuous fermentation of a glucose substrate by the microscopic fungus Fusarium venenatum (Reference Finnigan, Needham, Abbott, Nadathur, Wanasundara and Scanlin22). Owing to its fungal nature, mycoprotein is high in both dietary fibre (6 g/100 g wet weight; composed of 1/3 chitin and 2/3 β-glucan) and non-animal-derived protein (11 g/100 g wet weight) (Table 1; Fig. 1). Mycoprotein constitutes an ingredient which is processed into foods that resemble animal-derived meat products (e.g. nuggets, mince, fish fingers) and sold under the brand name of QuornTM. QuornTM foods have similar organoleptic properties to meat (taste and appearance) and contain all nine essential amino acids, making it a popular meat substitute.
Table 1. Macronutrient composition of 100 g of mycoprotein (wet weight)


Fig. 1. (a) Fungal filament of Fusarium venenatum and (b) simplified schematic representation of the fungal cell wall (constituted by mannoproteins – constituting the protein source, β-1,6-glucan, β-1,3-glucans and chitin – constituting the fibre sources), phospholipid bilayer and cytoplasm including microtubules(Reference Riquelme, Aguirre and Bartnicki-García23) – protein source. Figure adapted from Wiebe and Fesel & Zuccaro(Reference Wiebe24,Reference Fesel and Zuccaro25) .
Mycoprotein is commercially available in the USA, Europe, Asia and Australia and becoming increasingly incorporated into the diet of many populations(Reference Finnigan, Needham, Abbott, Nadathur, Wanasundara and Scanlin22). Mycoprotein is, therefore, a significant source of dietary fibre and non-animal-derived protein for numerous consumers worldwide. As such, and as mycoprotein consumption continues to increase, it is of growing interest to understand its role in nutrition and health. Randomised controlled trials (RCT) have been conducted to investigate the effects of mycoprotein on blood glucose and energy intake in healthy humans. However, to the best of our knowledge, such work has not been systematically reviewed, appraised and synthesised to provide a consensus on the role of mycoprotein in glycaemic control and energy intake regulation. In this paper, we aim to systematically review the RCT investigating the effects of mycoprotein on glycaemic control and energy intake in humans.
Methods
A systematic review of peer-reviewed literature published up to the 3 November 2019 was performed. The systematic review was conducted, in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) guidelines(Reference Moher, Liberati and Tetzlaff26). The protocol for this review has been registered in PROSPERO 2018 (CRD42018114566) (online Supplementary Table S1) and is available from https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=114566.
Eligibility criteria
The PICOS (patients, intervention, comparator, outcome, study design) criteria were used to establish study eligibility and focus the research question (Table 2).
Table 2. PICOS (patients, intervention, comparator, outcome, study design) criteria for inclusion and exclusion of studies

Search strategy
The conducted search strategy was performed within the PubMed, Embase, Web of Science and Google Scholar database. The search used a combination of terms of both title headings, keywords or free-text words detailed in online Supplementary Table S2, with the English language and a date cut-off of 3 November 2019. The following PubMed search algorithm was used: (mycoprotein[All Fields] OR myco-protein[All Fields] OR fungi-derived protein[All Fields] OR quorn[All Fields]) AND (glucose[MeSH Terms] OR A1c[All Fields] OR glycated-haemoglobin[All Fields] OR hba1c [All Fields] OR haemoglobin a [All Fields] OR dysglycemia [All Fields] OR dysglycaemia [All Fields] OR glycaemia[All Fields] OR glycemia [All Fields] OR glycaemic[All Fields] OR glycemic[All Fields] OR insulin*[All Fields] OR insulin [All Fields] OR fasting [All Fields] OR postprandial [All Fields] OR homeostatic model assessment [All Fields] OR oral glucose tolerance test [All Fields] OR oral glucose tolerance test [All Fields] OR appetite [All Fields] OR energy intake [All Fields] OR food intake [All Fields] OR food behaviour [All Fields] OR food behavior [All Fields] OR eating behaviour [All Fields] OR eating behavior [All Fields] OR hunger [All Fields] OR food consumption [All Fields]).
Also, manual searches of bibliographies of original research and previous reviews were performed, to identify additional relevant articles.
Study selection
All articles identified by the search strategy were reviewed by two reviewers independently (A. C.-M. and A. M. L.). Title and abstract of each group of articles were screened by A. C.-M. and A. M. L. Post-screening, full-text articles indicated as potentially relevant were retrieved and study eligibility was assessed. Study eligibility was conducted, through assessing the title, abstract and full text against the defined inclusion and exclusion criteria (see Table 2). For both screening and assessment of study eligibility, all unclear eligibility of papers was resolved by consulting a third party (E. S. C.). Identified duplicates were removed.
Data extraction and management
Articles deemed eligible for inclusion were assigned to subject categories, reflective of the two outcomes of interest (glycaemic control and energy intake), dependent on the outcome of the article reported. Articles could be assigned to more than one subject category if they reported outcomes relevant to two of the subject categories. Tabulated summaries were derived from the extracted data of articles assigned to the subject category (Tables 3 and 4). The tabulated summary for studies assessing mycoprotein intake on glycaemic control, energy intake and appetite contained the following descriptors:
Reference – presents the bibliographic reference of the study.
Participants – presents the characteristics of the population (sample size (n), sex, age, BMI, healthy and smoker status).
Study information – presents the study design (randomised, controlled, blinded, crossover), the test food (form, quantity of mycoprotein, control type and amount of carbohydrate given with the meal) and the protocol of the study.
Outcome – presents the result of the outcome of interest in the study. For energy intake and appetite, the methods of assessment are described in each.
Table 3. Summary of the acute randomised trials on glycaemic control
(Numbers and percentages; mean values and standard deviations)

CHO, carbohydrate; RCT, randomised controlled trial; NSSD, no statistically significant difference; iAUC, incremental AUC.
*Glucose and insulin AUC are expressed as a percentage change from control food. Age and BMI expressed as mean values and standard deviations.
Table 4. Summary of the acute randomised trials on energy intake and appetite*
(Numbers and percentages; mean values and standard deviations)

CHO, carbohydrate; RCT, randomised controlled trial; VAS, visual analogue scale; NSSD, no statistically significant difference; MSSP, macronutrient self-selection paradigm; UEM, universal eating monitor; SSD, statistically significantly different; N/A, not applicable.
* VAS score is expressed as mm. Energy intakes at ad libitum and post-24 h are expressed as percentage change in kJ from control food. Age and BMI are expressed as mean values and standard deviations.
† In Williamson et al. (Reference Williamson, Geiselman and Lovejoy31), blinding is not mentioned, although authors report that meals were prepared to be as equal as possible in palatability and appearance.
Risk-of-bias assessment
Studies were assessed for risk of bias by two independent reviewers (A. C. M. and A. M. L.) following the Cochrane Risk of Bias Assessment Tool. The five methodological features assessed were selection, performance, detection, attrition and reporting bias. Studies were classified as ‘high risk’ if they contained methodological flaws that may have influenced the results, ‘low risk’ if the flaw was not deemed to have affected the results and ‘unclear risk’ if not enough information was provided to perform a judgement. Disagreements in the classification were resolved by consulting a third party (E. S. C.).
Data analysis
Demographic data and described outcome values were reported as mean values and standard deviations.
Glycaemic control was defined as blood glucose and insulin AUC or incremental AUC. In addition, for one study(Reference Dunlop, Kilroe and Bowtell29), mean glucose levels (mmol/l) were reported at fasting (0 h), early (0–2 h) and late (2–4 h) postprandial stages. Energy intake was defined as acute energy intake (≤5 h post-ingestion) which included ad libitum meal and as short-term energy intake (>5 h post-ingestion) which included food diaries. Energy intake at ad libitum was assessed via buffet meal, macronutrient self-selection paradigm and universal eating monitor, and energy intake post-visit was assessed using self-reported food diaries. Appetite was measured via subjective appetite feelings scoring (mm) using a visual analogue scale. When possible, all outcomes were calculated as the percentage of change compared with control.
A statistical meta-analysis of the results was not possible due to the heterogeneity in the reported glycaemic outcomes and the insufficient power of studies assessing the energy intake outcomes measurement. Therefore, for all outcomes, a systematic, narrative approach was conducted according to the Cochrane handbook for systematic review of interventions(Reference Higgins32). Results were synthesised according to outcome measure.
Results
Overview of studies identified
The conducted search strategy yielded a total of twenty-three potentially relevant articles. Of which, eleven duplicates were identified and removed. Of the remaining twelve potentially relevant articles, six were excluded post-abstract screening as they were a review (n 1), European Food Safety Authority report (n 1), conference paper (n 2), an in vitro study (n 1) and irrelevant outcome measure (n 1). A total of six potentially relevant full-text articles were therefore assessed for eligibility, during which one was excluded for not meeting the defined inclusion and exclusion criteria: not being an RCT (n 1). A total of five articles were deemed eligible for inclusion and therefore included in the final synthesis (see Fig. 2). All of these studies were of an acute nature (measurements were performed for a minimum of 180 min to a maximum of 72 h post-mycoprotein ingestion).

Fig. 2. Preferred Reporting Items for Systematic Reviews and Meta-analysis flow diagram of the literature search and screening process.
Risk of bias
Individual studies were judged as having a high, low or unclear risk of bias for the five methodological features measured (see online Supplementary Table S3).
For studies assessing glycaemic control, for the methodological features of random sequence generation (selection bias), the majority of the studies (66 %) were judged as having an unclear risk of bias, whereas the remaining 33 % was given a low risk of bias. For the allocation concealment (selection bias), all studies (100 %) had an unclear risk of bias. For the method of blinding (performance bias), 100 % of the studies had a low risk of bias. For the blinding of the outcome assessment (detection bias), 100 % of the studies had an unclear risk of bias. For the attrition bias, all studies (100 %) had a high risk of bias. Selective reporting bias was unclear for all studies (100 %).
For studies assessing energy intake, all studies (100 %) were judged as having an unclear risk for both selection bias (random sequence generation and allocation concealment), a low (33 %), high (33 %) and unclear (33 %) risk was given for both performance bias and detection bias, a high risk was given for attrition bias to all studies (100 %) and an unclear risk for selective reporting bias was given for all studies (100 %).
For studies assessing both outcomes (glycaemic control and energy intake), in the methodological features for selection bias (random sequence generation), half of the studies (50 %) were judged as having a low and unclear risk of bias. For the selection bias (allocation concealment), all studies (100 %) had an unclear risk of bias. For the performance bias, all of the studies (100 %) had a low risk of bias. For the detection bias, 100 % of the studies had an unclear risk of bias. For the attrition bias, all studies (100 %) were given a high risk of bias. Selective reporting bias was unclear for all studies (100 %).
Studies on glycaemic control
Table 3 shows the effect of mycoprotein on glycaemic control. In total, three randomised controlled and crossover trials assessed glycaemic control. Two studies(Reference Bottin, Swann and Cropp28,Reference Dunlop, Kilroe and Bowtell29) were conducted in healthy (no chronic disease such as type 2 diabetes), overweight (BMI 25–29·9 kg/m2) and obese (BMI ≥ 30 kg/m2) adults and one study(Reference Turnbull and Ward27) with healthy lean (BMI 18·5–24·9 kg/m2) adults. Two studies were conducted using mycoprotein as liquid(Reference Turnbull and Ward27,Reference Dunlop, Kilroe and Bowtell29) , whereas one used mycoprotein as solid(Reference Bottin, Swann and Cropp28). The amounts of mycoprotein ranged from 20 to 132 g. The controls used were chicken(Reference Bottin, Swann and Cropp28), soya-based protein(Reference Turnbull and Ward27) and milk protein(Reference Dunlop, Kilroe and Bowtell29) drink. All three studies assessed insulin AUC and two studies assessed glucose AUC(Reference Turnbull and Ward27,Reference Bottin, Swann and Cropp28) . One study assessed mean glucose levels at fasting, early (0–2 h) and late (2–4 h) postprandial state(Reference Dunlop, Kilroe and Bowtell29).
Studies on energy intake and appetite
Table 4 shows the effect of mycoprotein on energy intake and appetite. In total, four randomised controlled and crossover trials assessed energy intake and appetite. All studies were conducted in healthy humans of which three studies(Reference Bottin, Swann and Cropp28,Reference Dunlop, Kilroe and Bowtell29,Reference Williamson, Geiselman and Lovejoy31) included adults with a BMI ≥ 25 kg/m2 and one study(Reference Turnbull, Walton and Leeds30) with a BMI < 25 kg/m2. Three studies were conducted using solid mycoprotein(Reference Bottin, Swann and Cropp28,Reference Turnbull, Walton and Leeds30,Reference Williamson, Geiselman and Lovejoy31) and one as liquid(Reference Dunlop, Kilroe and Bowtell29). The amounts used ranged from 44 to 132 g of mycoprotein. The controls used were chicken in two studies(Reference Bottin, Swann and Cropp28,Reference Turnbull, Walton and Leeds30) , milk protein in one study(Reference Dunlop, Kilroe and Bowtell29) and both chicken and tofu in one study(Reference Williamson, Geiselman and Lovejoy31). Two studies(Reference Bottin, Swann and Cropp28,Reference Williamson, Geiselman and Lovejoy31) measured acute energy intake (<5 h post-ingestion) via ad libitum intake, of which one study used universal eating monitor and macronutrient self-selection paradigm(Reference Williamson, Geiselman and Lovejoy31) and one study using a buffet meal(Reference Bottin, Swann and Cropp28). Two studies measured energy intake post-24 h using 3-d food diaries(Reference Bottin, Swann and Cropp28,Reference Turnbull, Walton and Leeds30) . All four studies explored appetite feelings using subjective appetite scoring via visual analogue scale rating some of the following items: appetite, fullness, prospective food intake, hunger and desire to eat(Reference Bottin, Swann and Cropp28–Reference Williamson, Geiselman and Lovejoy31). All studies assessed feelings of nausea and eating behaviour questionnaires were completed by participants in three studies(Reference Bottin, Swann and Cropp28,Reference Turnbull, Walton and Leeds30,Reference Williamson, Geiselman and Lovejoy31) .
Discussion
Low intake of total dietary fibre increases the relative risk of developing non-communicable diseases such as type 2 diabetes(Reference Reynolds, Mann and Cummings33), and high intake of animal-derived protein is also associated with type 2 diabetes risk(Reference Sluijs, Beulens and Spijkerman34). Therefore, strategies to increase dietary fibre and reduce animal-derived protein are of growing interest. Mycoprotein constitutes a food rich in both dietary fibre and non-animal-derived protein which is being increasingly consumed in the diet of many cultures; however, its effects on blood glucose levels and energy intake have not yet been fully established. This is the first systematic review of RCT investigating the effects of mycoprotein on glycaemia, insulinaemia and energy intake in humans. Overall, five RCT evaluating the effects of mycoprotein on glycaemia, energy intake/appetite or both were identified.
Effect of mycoprotein on glycaemic control
Three studies reported the effects of mycoprotein on glycaemia and insulinaemia (Table 3), of which one was performed in healthy lean adults(Reference Turnbull and Ward27) and two in healthy overweight and obese(Reference Bottin, Swann and Cropp28,Reference Dunlop, Kilroe and Bowtell29) .
Glycaemia
Blood glucose AUC following the intake of a 20 g mycoprotein drink showed a reduction in the first 60 min but not overall (AUC0–120 min) in healthy lean adults, compared with energy and macronutrient-matched soya-based protein drink(Reference Turnbull and Ward27). A similar experiment in healthy overweight and obese adults showed no differences in blood glucose incremental AUC0–180 following the intake of mycoprotein meal at any dose (44, 88 and 132 g) compared with an energy and macronutrient-matched chicken meal(Reference Bottin, Swann and Cropp28). These similar effects on blood glucose are interesting as both studies are conducted in different populations (lean v. overweight and obese) with varying doses of mycoprotein. When different doses of mycoprotein drink are compared over time, mean glucose levels over the first interval (0–120 min) are unchanged relative to baseline levels but are decreased in the second interval (120–240 min) relative to the first interval (0–120 min) with 20, 40 and 60 g of mycoprotein. However, this decrease during the second interval (relative to both baseline and the first interval) is also observed following the ingestion of 20 g of milk protein drink(Reference Dunlop, Kilroe and Bowtell29).
Insulinaemia
Insulin AUC was decreased with a drink containing 20 g of mycoprotein at the first 60 min but not overall (AUC0–120 min) in healthy lean individuals compared with a soya-based protein drink(Reference Turnbull and Ward27). A similar effect in insulin incremental AUC0–180 was shown in overweight and obese individuals when 44, 88 and 132 g of mycoprotein were compared with an energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28). These findings, coupled with the observations on blood glucose, suggest that mycoprotein may increase peripheral insulin sensitivity. This is supported by the work of Bottin and colleagues who reported an increased Matsuda index (a measure of insulin sensitivity with higher values indicating improved sensitivity) and reduced insulinogenic index (a measure of insulin output with lower values indicating reduced output) of mycoprotein compared with intake of energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28). This study is of particular importance as it was performed in overweight and obese individuals, a population that is at greater risk of developing non-communicable diseases in which hyperglycaemia and hyperinsulinaemia are implicated, such as type 2 diabetes(Reference Zheng, Ley and Hu35) and CVD(Reference Coutinho, Gerstein and Wang36).
The effect of increasing doses (20, 40, 60 and 80 g) of mycoprotein on insulin AUC0–240 was compared with a milk protein drink, in which 40 g of mycoprotein drink (containing 18 g of protein) induced a similar insulin AUC0–240 response to 20 g of the milk protein drink (containing 16 g of protein), suggesting that these protein sources produce similar effects on insulinaemia when matched for protein content. Doses of 40, 60 and 80 g of mycoprotein increased insulin AUC0–240 in a dose-dependent manner, in which these three doses were significantly different from 20 g of mycoprotein. This response is expected as the insulinotropic effect of dietary protein is proportional to the dose(Reference Gunnerud, Östman and Björck37).
In summary, the effects of mycoprotein on glycaemia are unclear. However, the data suggest that the acute intake of mycoprotein may decrease insulin output in healthy lean and overweight adults. Such conclusions, however, are limited, given they are drawn from a limited number (n 3) of highly heterogeneous acute studies. Further understanding and validation of the acute effects of mycoprotein on glycaemia coupled with translation into real-world settings via well-controlled studies investigating chronic effects are required.
Possible mechanisms underlying the effect of mycoprotein on glycaemic control
The mechanisms involved in the effects of mycoprotein on glycaemia and insulinaemia may be due to a combination of different factors, acting independently or in a concerted manner.
A delay in gastric emptying is often attributed to the reduction in blood glucose observed following protein(Reference Ma, Stevens and Cukier17) and fibre(Reference Jenkins and Jenkins38) ingestion. Nevertheless, 132 g of mycoprotein showed no effect on gastric emptying compared with energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28). It should, however, be noted that the methodology (paracetamol) employed in this study to measure gastric emptying when solid foods are consumed is not considered suitable. This is because paracetamol follows a liquid-phase gastric emptying(Reference Wagner and Nelson39), and liquid and solid phases empty at different rates and in patterns(Reference Horowitz, Collins and Shearman40); therefore, a better methodology should be employed to measure gastric emptying in solid mycoprotein. Thus, an effect of mycoprotein on gastric emptying cannot yet be ruled out.
It is possible that characteristics inherent to mycoprotein (e.g. hyphae, protein and fibre composition) modify the rate of gastric emptying. It has been hypothesised that mycoprotein could delay gastric emptying via the conversion of chitin to its soluble form (namely chitosan) via alkaline deacetylation, although this remains speculative as this process has only been demonstrated in vitro (Reference Turnbull41). Other mechanisms, such as the secretion of GLP-1 and PYY (two hormones that are involved in gastric emptying via activation of the ileal brake), are also possible. However, Bottin et al. showed no significant differences in neither GLP-1 nor PYY following 132 g of acute mycoprotein relative to energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28).
Lastly, the protein characteristics of mycoprotein may be responsible – at least in part – for its effect on postprandial insulin concentrations. Mycoprotein possesses a lower insulinotropic amino acid profile as well as a lower 120-min postprandial amino acid appearance relative to protein-matched milk(Reference Dunlop, Kilroe and Bowtell29) and therefore may explain the reduction in insulin output reported in the literature(Reference Turnbull and Ward27,Reference Bottin, Swann and Cropp28) .
In summary, the mechanisms underpinning the blood glucose and insulin response of mycoprotein intake are largely unclear. Mechanisms such as altered gastric emptying and the secretion of gastrointestinal hormones warrant further investigation.
Effect of mycoprotein on energy intake
Four studies have reported the acute effects of mycoprotein on energy intake (Table 4) in healthy lean, overweight and obese humans, with two studies observing a decrease in energy intake at an ad libitum meal(Reference Bottin, Swann and Cropp28,Reference Williamson, Geiselman and Lovejoy31) , two in post-24 h energy intake(Reference Bottin, Swann and Cropp28,Reference Turnbull, Walton and Leeds30) and one in energy intake during the day(Reference Turnbull, Walton and Leeds30). All studies assessed participant's eating behaviour at screening to exclude abnormal responses.
Acute energy intake
Acute energy intake (≤5 h post-ingestion) at a single ad libitum meal was measured in healthy overweight and obese individuals following mycoprotein ingestion and consistently showed an overall reduction relative to energy and macronutrient-matched protein sources.
In healthy overweight and obese individuals, a reduction of ad libitum energy intake by 12 % (167 kJ) was achieved 20 min following 44·3 g of mycoprotein ingestion compared with energy and macronutrient-matched chicken, and similarly to energy and macronutrient-matched tofu(Reference Williamson, Geiselman and Lovejoy31). In this study, a second ad libitum meal served 4·5 h post-ingestion showed no differences in energy intake with mycoprotein compared with energy and macronutrient-matched chicken and tofu. This suggests that intake of mycoprotein did not induce a compensatory response in energy intake at 4·5 h despite a reduced ingestion post-20 min. Interestingly, food intake using the macronutrient self-selection paradigm showed that mycoprotein promoted an increased intake of high fat/high sugar foods compared with energy and macronutrient-matched tofu(Reference Williamson, Geiselman and Lovejoy31). The magnitude of this effect was however not reported by the authors, and therefore, the potential implications of this finding are difficult to evaluate.
In a more recent study with overweight and obese individuals(Reference Bottin, Swann and Cropp28), Bottin et al. tested the effect of 44 and 88 g of mycoprotein on energy intake at an ad libitum meal 180 min post-ingestion relative to energy and macronutrient-matched chicken, reporting no differences between protein sources. However, when 132 g of mycoprotein was tested, an 8 % (201 kJ) reduction in ad libitum energy intake was observed when compared with energy and macronutrient-matched chicken.
The differences in the ad libitum energy intake reduction observed between the two studies could be related to differences in the mycoprotein dose and inter-meal interval employed (i.e. the time between protein ingestion and presentation of the ad libitum meal). However, the small number of studies and the heterogeneous methods employed prevents any definitive conclusions from being made.
Short-term energy intake
Short-term (>5 h post-ingestion) energy intake was assessed in both healthy lean(Reference Turnbull, Walton and Leeds30), overweight and obese population(Reference Bottin, Swann and Cropp28), showing an overall decrease in energy intake relative to other energy and macronutrient-matched protein sources. In healthy lean individuals, 130 g of mycoprotein reduced energy intake by 24 % (987 kJ) on the day and by 16 % (1180 kJ) on the next day, relative to energy and macronutrient-matched chicken(Reference Turnbull, Walton and Leeds30). This suggests that mycoprotein did not only induce a compensatory eating response on the day after but also decreased energy intake during the time measured (2 d). Foods rich in dietary fibre and/or protein have been shown to acutely and chronically decrease energy intake(Reference Wanders, van den Borne and de Graaf11,Reference Halton and Hu42–Reference Lee, Mori and Sipsas44) . Considering the literature on other foods high in fibre and/or protein, and the acute data on mycoprotein, we could hypothesise that chronic intake of mycoprotein results in a net reduction in energy intake, although this remains to be investigated.
In a separate study, healthy overweight and obese people showed a smaller decrease in energy intake (13 %; 1042 kJ) on the next day following 44 g of mycoprotein compared with energy and protein-matched chicken, even though 88 and 132 g of mycoprotein did not significantly decrease energy intake(Reference Bottin, Swann and Cropp28). This difference in energy intake is possibly due to the heterogeneity of the studies (i.e. different BMI, proportion males/females that could have affected appetite feelings due to menses) or due to the reporting method used in one study(Reference Bottin, Swann and Cropp28) using self-reported food diaries (v. weighted food diaries using digital scales) whose validity has been questioned(Reference Wolper, Heshka, Heymsfield and Allison45).
In summary, the limited data suggest that acute mycoprotein may reduce energy intake at an ad libitum meal and over the short-term (>5 h post-ingestion) in healthy lean, overweight and obese individuals. Such conclusions, however, are limited, given they are drawn from a limited number (n 4) of highly heterogeneous acute studies. Further understanding and validation of the acute effects of mycoprotein on energy intake coupled with translation into real-world settings via well-controlled studies investigating the chronic effects are required.
Possible mechanisms underlying the effect of mycoprotein on energy intake
Different mechanisms, acting separately or together, may have been involved in the effect of mycoprotein on energy intake.
Subjective appetite has been demonstrated to correlate with subsequent energy intake(Reference Parker, Sturm and MacIntosh46). Mycoprotein showed a decrease in energy intake at an ad libitum meal following a decrease in subjective appetite(Reference Bottin, Swann and Cropp28,Reference Dunlop, Kilroe and Bowtell29) . Furthermore, this reduction in subjective appetite and energy intake may be mediated by changes in gastrointestinal hormones related to satiety. Despite mycoprotein showing no differences in GLP-1 and PYY concentrations compared with energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28), the role of other appetite-regulating gut hormones (e.g. cholecystokinin or ghrelin) has not been investigated. Metabolomics analysis also indicates that mycoprotein intake increases the presence of guanidine acetic acid and β-hydroxybutyrate, which are known to suppress appetite via direct interaction with the hypothalamus(Reference Bottin, Swann and Cropp28,Reference Jordi, Herzog and Camargo47–Reference Stubbs, Cox and Evans49) . Additionally, while a reduction in gastric emptying and/or transit time correlates with appetite(Reference Bergmann, Chassany and Petit14), the effect of mycoprotein on gastric emptying is unclear. At present, only one study has measured gastric emptying following mycoprotein ingestion, showing no effect compared with energy and macronutrient-matched chicken(Reference Bottin, Swann and Cropp28). However, as previously mentioned, the paracetamol method is not an appropriate method to measure gastric emptying in solid foods. In support of this, the presence of insoluble fibre within mycoprotein (of which certain types can increase gut transit time(Reference Burkitt, Walker and Painter50)), as well as data showing a steady plasma amino acid appearance following mycoprotein intake(Reference Dunlop, Kilroe and Bowtell29) (compared with an acute appearance with protein-matched milk protein), suggests that mycoprotein may decrease the rate of gastric emptying.
The effects of mycoprotein on short-term (>5 h post-ingestion) energy reduction could be in part due to fibre fermentation by the resident gut microbiota and the subsequent production of the SCFA acetate, butyrate and propionate. A recent study using in vitro batch culture fermentation with human faeces showed that mycoprotein fibre increased the production of propionate, without affecting acetate or butyrate levels(Reference Harris, Edwards and Morrison51). Propionate has been shown to increase GLP-1 and PYY production and to decrease acute energy intake in overweight humans(Reference Chambers, Viardot and Psichas52). Therefore, it is plausible that the intake of mycoprotein in humans may have stimulated similar pathways to induce positive effects on appetite and ultimately energy intake.
Although these are all conceivable possibilities, the evidence is limited and hence further research is needed to uncover the mechanisms relating to mycoprotein-induced decreases in subjective appetite, acute and short-term energy intake.
Conclusion
Mycoprotein is a food high in both dietary fibre and non-animal-derived protein whose consumption is increasing across the world. This systematic review reports that the acute effects of mycoprotein on glycaemia are currently unclear, but likely decrease insulinaemia and energy intake (at an ad libitum meal and post-24 h) in healthy lean and overweight and obese humans acutely. The mechanisms underpinning these effects are not yet elucidated and should be further explored. In addition to clarifying the acute effects of mycoprotein on glycaemia, the translation of these findings into real-world settings via well-controlled chronic investigations is a logical next step.
Acknowledgements
The Section for Nutrition Research, Department of Metabolism, Digestion and Reproduction is funded by grants from the MRC, BBSRC, NIHR, an Integrative Mammalian Biology (IMB) Capacity Building Award, an FP7- HEALTH- 2009- 241592 EuroCHIP grant and is supported by the NIHR Biomedical Research Centre Funding Scheme. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This work was supported by an educational no string grant from Marlow Foods Ltd, Stokesley, UK. The funder did not contribute to the manuscript of this paper.
The authors’ contributions were as follows: All authors (A. C.-M., A. M. L., J. F., E. S. C., T. J. A. F. and G. S. F.) wrote the introduction; A. C.-M., G. S. F. and A. M. L. designed the research; A. C.-M., A. M. L. and E. S. C. did the screening; A. C.-M. did the data extraction; A. C.-M., A. M. L., J. F. and E. S. C. did the interpretation of findings and discussion. A. C.-M. has primary responsibility for final content. All authors read and approved the final manuscript.
A. C.-M. is funded by Marlow Foods Ltd. G. S. F. is currently giving and has given consultant advice to Marlow Foods Ltd. T. J. A. F. works for Marlow Foods Ltd. A. M. L., J. F. and E. S. C. have no conflicts of interest. Marlow Foods Ltd was not involved in the screening, data extraction, interpretation and discussion.
Supplementary material
For supplementary material referred to in this article, please visit https://doi.org/10.1017/S0007114520000756