Obesity is characterised by chronic low-grade inflammation, which has been proposed to be a key factor in the development of obesity-related co-morbidities including insulin resistance and type 2 diabetes mellitus (T2D)( Reference Trayhurn 1 – Reference Pradhan, Manson and Rifai 3 ). Adipose tissues appear to play a crucial role as a source and site of inflammation, as the accumulation of immune cells within the adipose tissue in obese subjects may lead to the process underlying the link between obesity and its associated pathologies( Reference Lolmede, Duffaut and Zakaroff-Girard 4 ). Dietary changes that are targeted to prevent or treat disease in obese persons have exceptional value in that the cost of these modifications is low and may be associated with fewer side effect compared with pharmacological and surgical treatments. As obesity is associated with low-grade inflammation( Reference Trayhurn 1 – Reference Pradhan, Manson and Rifai 3 ), it is of great importance to investigate how to modify or manipulate the response of immune cells to improve host defence mechanisms( Reference Calder 5 ) in such a manner that beneficially affects the pathophysiology of obesity.
The prevalence of T2D is low in populations where the intake of fish is high, suggesting that the intake of fish may protect against T2D( Reference Kromann and Green 6 – Reference Nkondjock and Receveur 8 ). However, findings in prospective studies are inconsistent, as positive association( Reference Kaushik, Mozaffarian and Spiegelman 9 – Reference van Woudenbergh, van Ballegooijen and Kuijsten 11 ), no association( Reference Schulze, Manson and Willett 12 ) and negative association( Reference Villegas, Xiang and Elasy 13 ) between fish intake and risk for T2D have been reported.
Fish consumption has been associated with lower circulating levels of several inflammatory markers in healthy adults( Reference Zampelas, Panagiotakos and Pitsavos 14 ), although the mechanisms of this process are unknown. It has been suggested that the anti-inflammatory effect of fish is a result of reduced production of pro-inflammatory eicosanoids derived from arachidonic acid and an increased conversion of EPA and DHA to anti-inflammatory eicosanoids (e.g. PGE3, TXA3, LTA5) and pro-resolving lipid mediators such as resolvins and protectins( Reference Flock, Rogers and Prabhu 15 ). In addition, other components in fish, such as Se( Reference Duntas 16 ), taurine( Reference Schuller-Levis and Park 17 ) and proteins( Reference Ouellet, Weisnagel and Marois 18 , Reference Pilon, Ruzzin and Rioux 19 ) may also have anti-inflammatory properties and affect immune function.
The aims of the present study were, primarily to study the effects of 8 weeks of high intake of lean fish (cod) or fatty fish (salmon) compared with a control group with no fish intake on glucose tolerance in healthy overweight/obese adults, and second, to investigate the effect of these interventions on leucocyte membrane fatty acid composition and leucocyte function. Our hypothesis was that a high fish intake would improve glucose tolerance, increase the n-3 PUFA content in leucocytes and improve the leucocyte function ex vivo in healthy overweight/obese adults when compared with a control group with no fish intake.
Methods
Participants, study setting and ethics
The study population consisted of overweight or obese adults and all participants were of Norwegian ethnic origin (Caucasian). Participants were recruited through advertisements and publicity in local newspapers, through a local radio station, through the Haukeland University Hospital intranet and through the University of Bergen home page in the period from June to September 2011. Inclusion criteria were having BMI ≥27 kg/m2, fasting blood glucose ≤7·0 mmol/l and being in the age range of 18–69 years. Participants would have to be willing to consume the assigned amount of fish during the intervention. Exclusion criteria were pregnancy, incompatibility with fish consumption (allergies, intolerance and/or dislike), having been diagnosed with diabetes mellitus, heart disease or gastrointestinal diseases, use of medications affecting lipid metabolism or glucose homoeostasis, use of anti-inflammatory medications, use of supplements containing n-3 fatty acids, intentional weight loss and large fluctuation in body weight (>3 kg) over the previous 2 months.
The study was designed as a randomised, controlled intervention study with a parallel group design, with three intervention arms: lean fish (cod) in weekly doses of 750 g, fatty fish (farmed salmon) in weekly doses of 750 g, and a no-fish group as the control. The intervention period was 8 weeks.
In all, seventy-six participants were included in the study and were randomly assigned to the lean fish (n 27), fatty fish (n 27) or the control (n 22) group. The participants were stratified into the different groups by the project manager on the basis of sex, age and BMI. All examinations were conducted at the Haukeland University Hospital, Bergen, Norway.
The study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures were approved by the Regional Committee for Medical and Health Research Ethics of Western Norway (REC no.: 2011/572). Written informed consent was obtained from all subjects. Health professionals performing blood sampling and measuring body composition and height, and personnel conducting the laboratory analyses, were all blinded. All data were analysed anonymously. This trial was registered at clinicaltrials.gov as NCT02350595.
Interventions
Participants in the lean fish group were instructed to eat five dinners per week containing 150 g of lean fish (cod), and participants in the fatty fish group were instructed to eat five dinners per week containing 150 g of fatty fish (salmon). The participants were told not to exceed a total amount of 750 g of fish/week. If 150 g of fish was not sufficient, participants were encouraged to supplement their meal with vegetables, pasta or rice. All participants were allowed to eat meat, but not along with the same meals as those with fish consumption for the study. Participants in the fish-eating groups were instructed not to consume any other types of fish or seafood during the study period. It was emphasised that the fatty fish group should not eat lean fish throughout the study period, and, conversely, that the lean fish group should not eat fatty fish during the study. The participants were instructed to maintain their normal eating habits throughout the study period, apart from eating the mandatory amount of 750 g fish/week. Participants in both fish intervention groups received a booklet with recipes for inspiration and to help them to increase the variation of their meals, as previously described( Reference Hagen, Helland and Bratlie 20 ). The control group was also instructed to continue their normal eating habits, except to avoid fish and seafood intake. No recipes were handed to the participants in the control group.
Subjects were instructed to not change their physical activity level during the intervention period, and were requested not to take any dietary supplements throughout the study period. Their habitual lifestyle was controlled at the baseline and the endpoint, using food record charts and a questionnaire for reporting physical activity. In addition, standardised questions were asked to control for lifestyle alterations and for evaluating the intervention (i.e. whether participants consumed the fish provided, frequency of fish consumption and intake of any additional fish/seafood) at the endpoint to assess compliance.
The fish was provided as frozen-skin and boneless-fillet portions (150 (sd 10) g; Lerøy Seafood Group ASA). The fish fillets were supplied free of charge to the participants, and were distributed at the baseline or at any time during the study period if preferred.
The compositions of the fillets were as follows, presented as mean values and standard deviations for analyses of five to ten samples: proteins: cod 19·7 (sd 1·0) wt%; salmon 19·5 (sd 1·7) wt%, total fats: cod 0·58 (sd 0·13) wt%; salmon 13·0 (sd 3·2) wt%, moisture: cod 79·4 (sd 1·9) wt%; salmon 65·3 (sd 2·8) wt%. The contents of n-3 PUFA in fish fillets were: α-linolenic acid: cod 0·002 (sd 0·004) g/100 g fillet; salmon 0·57 (sd 0·15) g/100 g fillet, EPA: cod 0·08 (sd 0·01) g/100 g fillet; salmon 0·46 (sd 0·10) g/100 g fillet, DPA: cod 0·01 (sd 0·00) g/100 g fillet, salmon 0·24 (sd 0·05) g/100 g fillet, DHA: cod 0·20 (sd 0·05) g/100 g fillet; salmon 0·71 (sd 0·12) g/100 g fillet. The fillets were analysed by Skretting ARC Laboratory using standard laboratory methods. Protein (as N×6·25) was analysed using the Kjeldahl method( 21 ), moisture was analysed gravimetrically after drying to constant weight in an oven at 105°C( 22 ), and fatty acid composition was determined after methylation of the fatty acids in methanolic HCl and extraction in hexane( Reference Grahl-Nielsen and Barnung 23 ). The methyl esters were separated in a gas chromatograph (Thermo Trace GC with Triplus autosampler; Thermo Scientific) and identified by retention time using standard mixtures of methyl esters (Nu-Chek), using 19 : 0 as the internal standard for quantification. Total fat content was calculated as the sum of fatty acids.
Protocol for study visits
The total study period was 8 weeks, with study visits at the baseline and after 8 weeks (endpoint) at the Clinical Research Unit at the Haukeland University Hospital. Examinations were conducted in the morning after an overnight fast. The subjects were instructed not to eat or drink anything except water, or use substances containing nicotine after 22.00 hours the previous day, and to avoid physical exercise and alcohol for 24 h before each sampling day.
Body height was measured at the baseline, using a wall-mounted stadiometer (Seca 222; Seca). Body weight and body composition were measured using a bioelectrical impedance analysis device (InBody 720; Biospace Co. Ltd) at the baseline and the endpoint in a fasted state. The manufacturer’s guidelines for use were followed, and participants were weighed barefoot, wearing light clothing, before blood sampling.
Blood samples were collected at the baseline and the endpoint in a fasting state. Blood was drawn from an antecubital vein by inserting a cannula connected to a three-way tap for repeated measures. The system was flushed with sterile saline (0·9 %) before and after each blood sample was collected. Fasting blood sample were collected in BD Vacutainer SST II Advance gel tubes (Becton, Dickinson and Company) for isolation of serum and Vacuette NH Sodium Heparin tubes (Greiner Bio-One) for isolation of leucocytes. A randomised selection of fourteen participants of both sexes from each group was assigned for the analyses of leucocyte function and membrane composition at the baseline and after 8 weeks.
After the collection of fasting blood samples, glucose tolerance was tested using a standardised breakfast meal containing fat and protein in addition to carbohydrates. A meal was chosen instead of the traditional oral glucose tolerance test where the subject is given a measured dose (usually 75 g) of glucose, as the former gives a more physiological description of the body’s response to an oral carbohydrate load( Reference Lefebvre and Luyckx 24 ). The breakfast consisted of two slices of white bread, 10 g margarine, 25 g strawberry jam, 20 g white cheese and 0·3 l orange juice, providing a total of 2218 kJ (80 g carbohydrate, 14 g protein and 16 g fat), and had to be consumed within 15 min. The macronutrient and energy content in the breakfast were calculated using ‘Mat på Data 5.1’ (www.matportalen.no/Emner/matpadata)( 25 ). Blood samples were collected at the baseline and the endpoint; in a fasting state, and at 30, 60, 90 and 120 min after the participants had consumed the standardised breakfast.
Estimation of energy and macronutrient intakes from dietary records
Participants completed dietary records of the 5 preceding days before the baseline and the 5 preceding days before the 8-week visit, including at least 1 weekend-day. The intakes of energy, carbohydrates, proteins and fats were calculated from the participants’ dietary record using the ‘Mat på Data 5.1’ software( 25 ). Food records were checked for completeness at both study visits.
Estimation of physical activity level
The participants were asked about the types of physical activity they engaged in, such as whether they worked out in a gym, were members of sports clubs or whether they worked out individually, the type of physical activity (e.g. hiking, running, biking) and the number of hours of light physical activity (not sweaty/not breathless) or hard physical activity (with sweat/breathless). The participants completed the form at the baseline and endpoint visits. The weekly number of hours and the intensities of the physical activities were coded as continuous variables.
Analyses of serum samples
Analyses of glucose, C-reactive protein (CRP), insulin and insulin C-peptide in the blood serum were performed by routine methods at the Laboratory of Clinical Biochemistry and the Hormone Laboratory at the Haukeland University Hospital. CRP and glucose were analysed on a Modular P instrument (Roche Diagnostics GmbH), using the Tina-quant C-Reactive Protein Gen.3 immunoturbidimetric assay (Roche Diagnostics) and Gluco-quant Glucose/HK enzymatic assay (Roche Diagnostics). Insulin and insulin C-peptides were analysed on the Immulite 2000 Immunoassay System (Siemens Healthcare GmbH), using the IML.2000 Insulin kit 600T (Siemens) and the IML.2000 C-peptide kit 200T (Siemens). Serum concentrations of IL-1b, IL-6, IL-8, monocyte chemoattractant protein 1 (MCP-1) and TNFα were measured using the Human Adipokine Magnetic Bead Panel 2 (Milliplex Map kit, HADK2MAG-61K; EMD Millipore Corporation) and analysed using a Luminex 100 instrument (Luminex Corp.) with STarStation version 3 software (Applied Cytometry). For IL-1b, fifty-eight of sixty-five participants had levels below the detection limit (1·3 pg/ml). Also, twenty participants had IL-6 below the detection limit (0·96 pg/ml) at both time points. Therefore, results from IL-1b and IL-6 measurements were not analysed statistically.
Analyses of leucocyte fatty acid composition and function
Randomised selections of fourteen participants of both sexes from each of the three groups was assigned for leucocyte isolation. Of these, thirty-five participants completed the study. Owing to technical difficulties, not all samples were successfully isolated and processed. For the fatty acid composition, thirty-three samples were analysed (eleven from each group). For ex vivo phagocytosis, samples from thirty-one participants were analysed (ten from the lean fish group, ten from the fatty fish group and eleven from the control group), and for chemotaxis, samples from twenty-eight participants were analysed (nine from the lean fish group, ten from the fatty fish group and nine from the control group).
Leucocytes were isolated from heparinised blood as previously described( Reference Lehmann, Sornes and Halstensen 26 ) and adjusted to 1·25×107 non-lymphocytes/ml.
For determination of fatty acid composition, samples of isolated leucocytes were added to heneicosanoic acid as internal standard and were methylated without prior extraction of lipids, as described previously( Reference Meier, Mjøs and Joensen 27 ). After methylation, lipids in the samples were extracted twice with iso-octane. The methyl esters were quantified using an Agilent 7890 gas chromatograph equipped with a flame ionisation detector (Agilent Technologies, Inc.) and a BPX-70 capillary column (SGE Analytical Science) as described in Sciotto & Mjøs( Reference Sciotto and Mjøs 28 ) with minor adjustments of the temperature programme. The compounds were identified by GC-MS using the BPX-70 column and by the methodology described in Wasta & Mjøs( Reference Wasta and Mjøs 29 ).
Phagocytosis was studied within 2 h as described previously( Reference Lehmann, Sornes and Halstensen 26 ), using fluorescent bacteria (Staphylococcus aureus Cowan III NCTC 8532 labelled with Rhodamine Green X, 2·5×108 bacteria/ml); the bacteria:phagocyte ratio was 20:1. After incubation, the reaction was terminated by adding ice-cold PBS with 0·02 % EDTA. The samples were analysed using a flow cytometer (Cytomics FCS 500MPL; Beckman Coulter, Inc.) equipped with an argon laser (488 nm), and the fluorescence from the granulocyte population was collected in the Fl 1 detector (BP filter 525 +/−15 nm).
Chemotaxis was examined as previously described( Reference Naess, Stenhaug Kilhus and Nystad 30 ), using Transwell® 3415 polycarbonate membrane polypropylene microtiter plates (Corning). Zymosan-activated serum (5 %) in Dulbecco’s PBS with glucose, albumin, CaCl2 and MgSO4 (DBPSGACM) was used as a chemoattractant and DBPSGACM was used as a negative control. Leucocytes (5×106 /ml) were then added to the upper chambers. After incubation at 37°C in a humid atmosphere with 5 % CO2, ice-cold Dulbecco’s PBS with 0·2 % EDTA was added to the wells to remove adherent cells from the bottom of the wells and the membrane. The suspensions were placed on ice for at least 30 min, thoroughly mixed and reference beads were added. Migrated cells were counted using flow cytometry and are presented relative to the reference beads.
Outcome measurements
The primary outcome of the present study was the changes in serum postprandial concentrations of glucose after a weekly intake of 750 g fillet from either lean or fatty fish for 8 weeks. Secondary outcomes were changes in insulin, insulin C-peptide, leucocyte fatty acids, leucocyte function, body weight and composition, and intake of energy and macronutrients within the groups over time.
Statistical analyses
Sample size calculation was performed on the basis of previously published studies in overweight( Reference Vikøren, Nygard and Lied 31 ) and normal-weight( Reference Hagen, Helland and Bratlie 20 ) adults. Postprandial glucose regulation after a standardised breakfast was of primary interest, and the sample size calculation was therefore conducted for changes in serum glucose after a standardised meal. A change in glucose from the baseline to the endpoint was calculated for glucose measured 120 min after a meal, using 0·8 as sd and 0·7 as the mean difference in change between the fish/fish protein intervention groups and the control group( Reference Hagen, Helland and Bratlie 20 , Reference Vikøren, Nygard and Lied 31 ). The calculation showed that twenty persons were needed in each intervention arm to observe an effect of fish intake on glucose regulation with a power of 80 % and α of 0·05. On the basis of previous experience( Reference Hagen, Helland and Bratlie 20 ), the drop-out rate was expected to be below 10 %, therefore the sample size should be twenty-three persons in each arm, and based on this calculation, the sample size required was sixty-nine subjects. However, this sample size calculation was based on studies with a shorter intervention length (4 weeks in Hagen et al.( Reference Hagen, Helland and Bratlie 20 ) instead of 8 weeks in the present study) or with less impact on everyday life (8 weeks intervention with tablets in Vikøren et al.( Reference Vikøren, Nygard and Lied 31 ) instead of five dinners per week in the present study). Therefore, we were not sure if the compliance would be satisfactory in all participants who completed the study period; thus, we decided to include 15 % more participants. The final sample size was therefore determined to be seventy-six participants.
Statistical analyses were conducted using SPSS Statistics 23 (SPSS, Inc., IBM Company). Subjects who did not complete the study were excluded from statistical analyses. For analytes in serum, and for estimated energy and macronutrient intake from dietary records and reported physical activity, most data were not normally distributed according to the Shapiro–Wilk test, and non-parametric tests were used to investigate changes within groups (the Wilcoxon-signed ranks test). For these non-parametric data, the Kruskal–Wallis Test was used to compare values between the three groups at baseline. Changes within the groups were compared using the Kruskal–Wallis Test, followed by the Mann–Whitney Test whenever between-group differences were detected. Data were not corrected for multiple testing post hoc, in line with the recommendation by Streiner( Reference Streiner 32 ) for tests that are not independent of each other. Data are expressed as medians and 25th, 75th percentiles. Sex-based distribution in the groups was compared using the Pearson’s χ 2 test. All comparisons were two-sided, and P<0·05 was considered statistically significant.
Results
Participant characteristics
Overall, seventy-six participants were included in the study and completed the first study visit. The participants were randomised into the control group, the lean fish group or the fatty fish group. Of these, sixty-eight participants completed the trial. One participant (a woman in the fatty fish group) was excluded from statistical analysis after analyses of postprandial blood glucose revealed she had prediabetes, and two participants (one woman in the lean fish group and one man in the fatty fish group) were withdrawn from analysis because they did not comply with the protocol. In total, sixty-five subjects (twenty-nine men and thirty-six women) were included in the statistical analyses. All participants were overweight or obese (median BMI 32·3 (29·6–35·7) kg/m2), with median age 45·5 (36·2–53·2) years. The baseline characteristics for the groups are presented in Table 1. No statistically significant differences were found between the groups at the baseline for sex distribution, age, BMI, percentage body fat or percentage muscle mass. After 8 weeks, no changes were seen in BMI, percentage body fat or muscle mass within the groups (data not presented).
Table 1 Participant characteristics at the baseline (Medians and 25th, 75th percentiles)
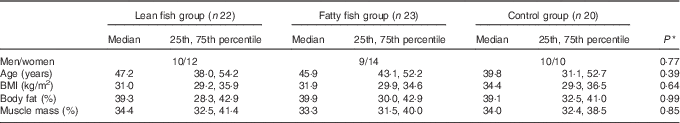
* Groups were compared at the baseline using the Pearson’s χ 2 (categorical data) or the Kruskal–Wallis test (continuous data).
Estimated dietary intake and physical activity
The median intakes of energy and macronutrients (as a percentage of total daily energy intake) were similar between the groups at the baseline and after 8 weeks, and there were no changes in energy and macronutrient intakes within any of the groups during the course of the study (Table 2).
Table 2 Estimated daily dietary intake of energy and macronutrients (as percentage of energy intake) based on 5 d dietary records at the baseline and after 8 weeks, and reported physical activity one week before the baseline and 8 week visitsFootnote * (Medians and 25th, 75th percentiles)

* No differences were seen between the groups at the baseline (Kruskal–Wallis test). Results are presented for twenty-two participants in the lean fish group, twenty-three participants in the fatty fish group and twenty participants in the control group.
† Within-group changes are tested using Wilcoxon’s signed-ranks test.
‡ Changes within the lean fish, fatty fish and control groups are compared using the Kruskal–Wallis test.
The reported physical activity at the baseline was similar between the groups (Table 2). No statistically significant changes in the number of hours of light physical activity (not sweaty/not breathless) or in the number of hours with hard physical activity (with sweat/breathless) over time were seen within any intervention groups.
Glucose regulation
All groups were similar at the baseline regarding fasting serum concentrations of glucose, insulin and insulin C-peptide. The fasting concentrations of glucose, insulin and insulin C-peptide did not change from the baseline to the 8-week endpoint within any of the experimental groups (Table 3).
Table 3 Serum concentrations of glucose, insulin and insulin C-peptideFootnote * (Medians and 25th, 75th percentiles)

* No differences were observed between the groups at the baseline (Kruskal–Wallis test). Results are presented for twenty-two participants in the lean fish group, twenty-three participants in the fatty fish group and twenty participants in the control group.
† Within-group changes are tested using Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and control groups are compared using the Kruskal–Wallis test.
§ Changes within the lean fish group are compared with the control group (A), changes within the fatty fish group are compared with the control group (B), changes within the lean fish group are compared with the fatty fish group (C) using the Mann–Whitney test when the Kruskal–Wallis test showed differences between the groups.
The postprandial concentrations of glucose, insulin and insulin C-peptide were compared with fasting concentrations. In the fatty fish group, the relative increases in glucose concentrations after 90 and 120 min were significantly smaller after 8 weeks when compared with the control group (P=0·012 for both comparisons), whereas lean fish intake seemed not to affect postprandial glucose concentrations (Table 3, Fig. 1). Fish intake did not affect the 120 min postprandial insulin concentration relative to fasting concentration. Fatty fish intake, but not lean fish intake, gave a significantly smaller increase in the 120 min postprandial insulin C-peptide concentration relative to the fasting level when compared with the control group.

Fig. 1 Glucose response after intake of a standardised breakfast in the lean fish group (a), fatty fish group (b) and the control group (c), shown relative to fasting glucose concentrations. Glucose response was measured at the baseline (![]() ) and after 8 weeks (
) and after 8 weeks (![]() ). Results are presented for twenty-two participants in the lean fish group, twenty-three participants in the fatty fish group and twenty participants in the control group, and are presented as medians and 25th, 75th percentiles. * Changes within lean fish and fatty fish groups were compared with control group using the Mann–Whitney test when the Kruskal–Wallis test showed differences between the groups.
). Results are presented for twenty-two participants in the lean fish group, twenty-three participants in the fatty fish group and twenty participants in the control group, and are presented as medians and 25th, 75th percentiles. * Changes within lean fish and fatty fish groups were compared with control group using the Mann–Whitney test when the Kruskal–Wallis test showed differences between the groups.
Analysis of leucocyte fatty acid composition and function
The total amounts of SFA, MUFA and PUFA (online Supplemental Table S1), and the amounts of individual SFA and MUFA (data not presented) in the leucocyte membranes were not affected in any group after 8 weeks. Lean fish intake increased the membrane content of DHA when compared with the control group, but no changes were seen in the contents of other n-3 and n-6 PUFA after lean fish intake (Table 4). Fatty fish intake increased the total amount of n-3 PUFA in leucocytes when compared with lean fish intake and controls, due to increased amounts of EPA and DPA. The leucocyte DHA amount was also higher after fatty fish intake when compared with controls. Compared with lean fish intake and controls, fatty fish intake reduced the total amount of n-6 PUFA in leucocytes, due to lower amounts of 20 : 4n-6 and 22 : 4n-6. The ratios n-3:n-6 PUFA and EPA:AA in leucocyte membranes were significantly increased after fatty fish intake when compared with both the lean fish and the control group.
Table 4 PUFA in leucocyte membranes at the baseline and after 8 weeksFootnote * (Medians and 25th, 75th percentiles)

* No differences were observed between the groups at the baseline (Kruskal–Wallis test). Results are presented for eleven participants in the lean fish group, eleven participants in the fatty fish group and eleven participants in the control group. Only fatty acids with group medians>0·05 g/100 g fatty acids in at least one group is included in the calculation of total n-3 and n-6 PUFA.
† Within-group changes are tested using Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and control groups are compared using the Kruskal–Wallis test.
§ Changes within the lean fish group are compared with the control group (A), changes within the fatty fish group are compared with the control group (B), changes within the lean fish group are compared with the fatty fish group (C) using the Mann–Whitney test when the Kruskal–Wallis test showed differences between the groups.
Intake of lean or fatty fish did not affect chemotaxis (data not presented). Fatty fish intake, but not lean fish intake, resulted in a significant increase in ex vivo phagocytosis from the baseline to 8 weeks; however, this within-group change was not significant when compared with changes within the lean fish and control groups (Table 5).
Table 5 Neutrophil phagocytosis ex vivo Footnote * (Medians and 25th, 75th percentiles)

* No differences were observed between the groups at the baseline (Kruskal–Wallis test). Results are presented for ten participants in the lean fish group, ten participants in the fatty fish group and eleven participants in the control group.
† Within-group changes are tested using Wilcoxon’s signed-rank test.
‡ Changes within lean fish, fatty fish and control groups are compared using the Kruskal–Wallis test.
Inflammatory markers
Serum concentrations of CRP, TNFα, IL-8 and MCP-1 were low but detectable at the baseline, and no differences were seen between the groups. Concentrations of CRP, TNFα, IL-8 and MCP-1 were not changed within any of the groups during the course of the study (data not presented).
Discussion
The present study investigated the effects of a high intake of lean fish or fatty fish on serum glucose regulation, leucocyte membrane fatty acid composition and leucocyte function in healthy adults with BMI≥27 kg/m2. Our results suggest that a high intake of fatty fish, but not of lean fish, for 8 weeks as part of the participants’ normal diet lowered postprandial glucose and insulin C-peptide serum concentrations, and increased the content of n-3 PUFA at the expense of n-6 PUFA in leucocyte membranes. No within-group changes were seen in self-reported energy and macronutrient intakes or in physical activity during the intervention period, or in body weight and body composition, suggesting that the participants did not change their lifestyle during this period.
Reports on the effect of fish intake on the risk for developing insulin resistance and T2D are ambiguous, as the prevalence of T2D is low in populations where the intake of fish is high( Reference Kromann and Green 6 – Reference Nkondjock and Receveur 8 ), whereas prospective studies have demonstrated both a positive association( Reference Kaushik, Mozaffarian and Spiegelman 9 – Reference van Woudenbergh, van Ballegooijen and Kuijsten 11 ), no association( Reference Schulze, Manson and Willett 12 , Reference Patel, Forouhi and Kuijsten 33 ) and a negative association( Reference Villegas, Xiang and Elasy 13 ) between fish intake and the risk for T2D. Of these reports, only two studies distinguished between lean and fatty fish intake( Reference van Woudenbergh, van Ballegooijen and Kuijsten 11 , Reference Patel, Forouhi and Kuijsten 33 ). Thus, more information is needed regarding the effects of lean fish and fatty fish intake as part of a normal diet in groups with increased risk for developing T2D.
Intake of fish, mainly fatty fish, has been shown to beneficially influence body weight and glucose homoeostasis, which could protect against T2D( Reference Feskens, Bowles and Kromhout 7 , Reference Nkondjock and Receveur 8 , Reference Patel, Forouhi and Kuijsten 33 – Reference Ouellet, Marois and Weisnagel 35 ). Also fish proteins seem to improve glucose regulation in overweight/obese subjects( Reference Vikøren, Nygard and Lied 31 , Reference Ouellet, Marois and Weisnagel 35 ) and in rats ( Reference Pilon, Ruzzin and Rioux 19 , Reference Madani, Louchami and Sener 36 – Reference Drotningsvik, Mjøs and Pampanin 40 ). We recently found that a high intake of lean or fatty fish (750 g/week) for 4 weeks did not affect fasting or postprandial glucose regulation in healthy young normal-weight adults( Reference Hagen, Helland and Bratlie 20 ).
Lean fish did not seem to affect glucose regulation in the present study, which was surprising as we have previously found that cod protein supplementation improved glucose regulation in healthy overweight adults( Reference Vikøren, Nygard and Lied 31 ). In the present study, participants ate 150 g of fish for dinners 5 d/week, corresponding to an average daily intake of 21 g of fish protein, whereas in the Vikøren study( Reference Vikøren, Nygard and Lied 31 ), participants consumed 6 g of cod proteins divided into three doses per day. One may speculate that taking a lower dose of fish protein several times per day can have more potent effects when compared with taking a single dose per day, as the former may result in more rapid achievement and maintenance of steady-state concentrations for nutrients, their metabolites, or of any components produced as a result of intake of fish proteins. This is supported by our findings in obese Zucker rats, where a continuous intake of cod protein (as 25 % of protein intake) during the day and night improved glucose regulation( Reference Drotningsvik, Mjøs and Høgøy 39 ).
There is controversy as to the glucose-regulating effects of fatty fish or fish oil( Reference Pilon, Ruzzin and Rioux 19 , Reference Mori, Bao and Burke 34 , Reference Bhathena, Berlin and Judd 41 – Reference Lara, Economou and Wallace 45 ). We recently found that a protein hydrolysate made from salmon bone frames with muscle remnants after filleting improved postprandial glucose regulation in obese Zucker fa/fa rats, without affecting fasting glucose and insulin concentrations. The beneficial effect of the salmon protein hydrolysate may be due to the presence of bioactive motifs with antidiabetic effects( Reference Drotningsvik, Mjøs and Pampanin 40 ). In the present study, fatty fish intake led to a smaller relative increase in serum glucose concentration from fasting to 90 and 120 min after a meal when compared with the control group, suggesting an improved postprandial glucose control after fatty fish intake which may be caused by long-chain n-3 PUFA, fish proteins or by other components in the fatty fish fillet. The improved postprandial glucose regulation was probably not caused by increased insulin secretion, as the relative increase from fasting to postprandial insulin C-peptide concentration was also lower after fatty fish intake, strongly suggesting that fatty fish intake improved glucose regulation by enhancing the insulin sensitivity after a meal.
Intake of long-chain n-3 PUFA from supplements has been shown to increase the content of EPA and DHA in cells( Reference Calder 46 ), however, to our knowledge, the effect of fish intake on leucocyte membrane fatty acid composition has never been explored. We therefore investigated whether a high fish intake would induce alterations in membrane fatty acid composition of leucocytes. Although no changes were seen in the total amounts of SFA, MUFA and PUFA in leucocyte membranes after a high fish intake, it was evident that especially fatty fish intake resulted in a replacement of n-6 PUFA with n-3 PUFA. The increased content of EPA and DHA, and the lower content of AA in leucocyte membranes after fatty fish intake suggest that the inflammatory state may be improved in this group. Lean fish intake increased the membrane content of DHA, probably as a result of a relatively high proportion of DHA in phospholipids in the cod fillet consumed in the present study (32·4 % of total FA). Incorporation of increased amounts of EPA and DHA into cell membranes has been shown to decrease the production of arachidonic acid-derived eicosanoid mediators( Reference Calder 46 ), and EPA and DHA are precursors of anti-inflammatory eicosanoids (e.g. PGE3, TXA3, LTA5) and pro-resolving lipid mediators such as resolvins and protectins, respectively( Reference Flock, Rogers and Prabhu 15 ). Despite the higher ALA content in fatty fish compared with lean fish, no differences were seen between leucocyte membrane ALA content between these groups, which could be expected since food sources other than fish, such as seed oils, dairy products and meat, also contain ALA.
A substitution of EPA and DHA for AA may increase the membrane fluidity of the leucocytes as the latter has a higher number of double bonds, and affect the structure and function of the membrane( Reference Calder 46 ). Phagocytosis is a membrane-dependent process and is thus susceptible to changes in the membrane fluidity, and it plays an essential role in the first line of defence in the immune response to inactivate or destroy foreign invaders. In the present study, ex vivo phagocytosis was not affected by the intake of fatty fish or lean fish despite increased levels of long-chain n-3 PUFA in leucocyte membranes seen especially after fatty fish intake. Future studies should investigate the effects of fish intake on phagocytosis in obese individuals, as the incidence and severity of certain infectious diseases are found to be higher in this population( Reference Lamas, Marti and Martinez 47 , Reference Milner and Beck 48 ).
Increased fat mass is associated with increased circulating levels of adipokines, including TNFα, Il-1b, IL-6, IL-8 and MCP-1, and with insulin resistance( Reference Antuna-Puente, Feve and Fellahi 49 – Reference Kalupahana, Moustaid-Moussa and Claycombe 51 ). Several studies have shown independent associations between fish intake and lower levels of circulating inflammatory markers, such as CRP, IL-6 and TNFα and thus demonstrate an inverse relationship between fish consumption and low-grade inflammation( Reference Zampelas, Panagiotakos and Pitsavos 14 , Reference Ouellet, Weisnagel and Marois 18 , Reference Pilon, Ruzzin and Rioux 19 , Reference He 52 – Reference Smith, Barraj and Kantor 55 ), whereas others report no effect of intake of fish on CRP and IL-6 in overweight men( Reference Lindqvist, Langkilde and Undeland 56 ) and on CRP in normal-weight adults( Reference Hagen, Helland and Bratlie 20 ). Some studies suggest that dietary EPA may be immunosuppressive through the down-regulation of cytokine production and lymphocyte proliferation in older women and therefore may have detrimental effects( Reference Meydani, Endres and Woods 57 ). In the present study, serum concentrations of CRP and adipokines were generally very low for the overweight but healthy participants of the study, and no effects of lean or fatty fish intakes were seen in the serum concentrations of CRP, TNFα, IL-8 and MCP-1.
There are some limitations to this study. The sample size was estimated on the basis of studies of effects on glucose regulation after interventions using lean or fatty fish, or fish proteins, in populations with normal BMI or overweight/obesity with/without insulin resistance( Reference Hagen, Helland and Bratlie 20 , Reference Vikøren, Nygard and Lied 31 , Reference Mori, Bao and Burke 34 , Reference Ouellet, Marois and Weisnagel 35 ). Therefore, we consider this study to be hypothesis-generating and an important foundation for sample size calculations for future studies with similar designs. Participants in the lean fish and fatty fish groups received a booklet with recipes for inspiration and to help them to increase the variation of their meals, and they were encouraged to vary between the different recipes. Participants in the control group did not receive any recipes, but were instructed to continue their normal eating habits except to avoid fish and seafood intake. Dinner preparation methods and choice of side dishes and accessories were not reported throughout the study period, but participants completed dietary records of the 5 preceding days before the baseline and the 5 preceding days before the 8 week visit. As the groups reported similar intake of energy and macronutrients at these two time points, and as no changes were seen in body weight and body composition during the course of the study, the interpretation that the high intake of fatty fish itself caused the observed effects on glucose homoeostasis is strengthened. No physiological marker for compliance was used in this study, but based on interviews with the participants about their fish intake during the intervention period, the fish intake was well tolerated and participants (both in the fish intervention groups and in the control group) included in the statistical analyses reported good compliance.
Conclusion
The hypothesis of this study was that a high fish intake would improve postprandial glucose tolerance, increase the n-3 PUFA content in leucocytes and improve the leucocyte function ex vivo in overweight/obese adults. Contrary to our hypothesis, our findings suggest that only fatty fish improved postprandial glucose tolerance in healthy overweight/obese adults, whereas lean fish seemed to have no effect on glucose homoeostasis in this population. In addition, intake of fatty fish, but not of lean fish, resulted in a higher content of n-3 PUFA and a lower content of n-6 PUFA in leucocyte membrane. Lean fish increased the leucocyte membrane content of DHA. Fish intake did not affect phagocytic activity.
Acknowledgements
The authors thank all participants who have contributed to the current study. The kind contribution of fish for the intervention trial by Lerøy Seafood Group ASA (Bergen, Norway) is highly appreciated.
The present research has been supported by funding from the Bergen Medical Research Foundation and KG Jebsen Center for Diabetes Research.
A. I. H., H. S., G. R., G. M. and O. A. G. designed the research. A. H., M. B., I. V. H., and O. A. G. conducted the research. A. H., M. B., I. V. H., S. A. M., S. S., K. A. B. and O. A. G. analysed the data and performed statistical analyses. O. A. G. drafted the paper and had primary responsibility for the final content. All authors have contributed to the writing and approved the final manuscript.
H. S. and G. R. are employed in Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA, respectively. Skretting Aquaculture Research Centre AS is a global leader in providing innovative and sustainable nutritional solutions for the aquaculture industry. Lerøy Seafood Group ASA is the leading exporter of seafood from Norway and the world’s second largest producer of Atlantic Salmon. A. I. H. is a part owner of the white fish trawler Granit, owned by Halstensen Granit AS. Skretting Aquaculture Research Centre AS and Lerøy Seafood Group ASA were not involved in on-site data collection. A. H., M. B., I. V. H., S. A. M., S. S., K. A. B., G. M. and O. A. G. declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S0007114517001234









