Brain and gut form a bi-directional communication axis called the microbiota–gut–brain axis(Reference Mörkl, Butler and Holl1), creating a dynamic interaction that can interfere with energy homoeostasis, mood and well-being(Reference Mayer2–Reference Torres-Fuentes, Schellekens and Dinan5). These communication mechanisms are still under investigation but involve neuro-endocrine and immune pathways(Reference Mörkl, Butler and Holl1).
Some micro-organisms can generate neurotransmitters, neuromodulators(Reference Cryan and Dinan6) and SCFA, affecting brain physiology and behaviour(Reference Kim and Shin7). For instance, the administration of probiotics can favourably stimulate the gut–brain axis, reducing anxiety behaviours and stress levels(Reference Clark and Mach8,Reference Takada, Nishida and Gondo9) . Studies have shown that frequent consumption of probiotics may be associated with modulation of metabolism and secretion of melatonin, a derivative of serotonin (5-HT), involved in the control of circadian rhythm and modulation of the bowel function(Reference Wong, Yang and Song10).
In this context, military personnel are an interesting group for interventions aimed at reducing stress levels and gastrointestinal symptoms, and improving well-being indicators. This is because they are exposed to extreme physical activity conditions, sleep deprivation, hunger and dehydration during combat. To prepare for this, simulation training is commonly used to evoke physiological and psychological tensions present in stressful circumstances(Reference Lieberman, Bathalon and Falco11,Reference Nindl, Barnes and Alemany12) . This type of short-term training is known to challenge the immune system, impair physical performance, raise indices of muscle pain perception, change biochemical markers, induce fatigue and weight loss symptoms, and affect mood and hormonal regulation(Reference Nindl, Barnes and Alemany12–Reference Chicharro, Lucía and Pérez14). Cognitive aspects of military personnel are also critical in tense situations, such as those experienced in missions. These situations require personal defence and strength since psychological factors are often associated with poor performance, difficulty in decision-making and communication problems(Reference Bardwell, Ensign and Mills15).
Probiotics are live micro-organisms that, when administered in adequate amounts, confer a health benefit on the host(Reference Hill, Guarner and Reid16). In contrast, prebiotics selectively stimulate the growth and activation of the metabolism of probiotic strains delivered by products and even endogenous strains of the host(Reference Konar, Toker and Sagdic17). When probiotics and prebiotics are administered together, they are called ‘synbiotics’, defined as a combination of live micro-organisms and substrate or substrates selectively utilised by host micro-organisms which may confer health benefits to the consumer(Reference Swanson, Gibson and Hutkins18). A study, recently published by our group, using the Simulator of the Human Intestinal Microbial Ecosystem (SHIME®) showed that synbiotic ice cream produced with Bifidobacterium animalis subsp. lactis BB-12 and Lactobacillus acidophilus LA-5 and inulin favoured the maintenance and metabolic activity of probiotics added, due to the increase in the proportion of SCFA (acetate, propionate and butyrate)(Reference Rodrigues, Duque and Fino19).
Bifidobacterium animalis subsp. lactis BB-12 is the most documented probiotic Bifidobacterium (Reference Jungersen, Wind and Johansen20). More than 130 clinical studies have been published with the strain BB-12 showing improvement of bowel function, protection against diarrhoea, increased body resistance to common respiratory infections and reduced incidence of acute respiratory tract infections(Reference Jungersen, Wind and Johansen20). Lactobacillus acidophilus LA-5 has been evaluated co-administered with B. animalis BB-12 in some clinical trials, such as the study registered at the US National Library of Medicine (NCT01102036) to evaluate transit time and digestive discomfort in healthy women and men, and studies focusing on irritable bowel syndrome(Reference Bogovič Matijašić, Obermajer and Lipoglavšek21) and remission in ulcerative colitis(Reference Wildt, Nordgaard and Hansen22).
Considering the above, the use of synbiotics may benefit military personnel submitted to an environment of physical and psychological stress that affects their routines, performance and quality of life. Thus, the objective of the present study was to evaluate the effects of 30-d supplementation with synbiotic ice cream (containing L. acidophilus LA-5, B. animalis BB-12 and inulin) on salivary IgA, gastrointestinal symptoms, sleep quality, well-being indicators and gut microbiota in young military participants undergoing a 5-d military field training.
Methodology
Experimental design
This research was conducted at the Army Cadets Preparatory School (EsPCEx), located in Campinas, São Paulo, Brazil. The study population consisted of eighty military personnel, students at EsPCEx, living in boarding school, of both sexes, aged between 18 and 22 years. This was a controlled, parallel, randomised and double-blind clinical trial (Fig. 1), approved by the Research Ethics Committee of UNICAMP (CAAE: 90850718.8.0000.5404) and enrolled in the Brazilian Clinical Trials Registry (ReBec) (ID: RBR-6DV55J).
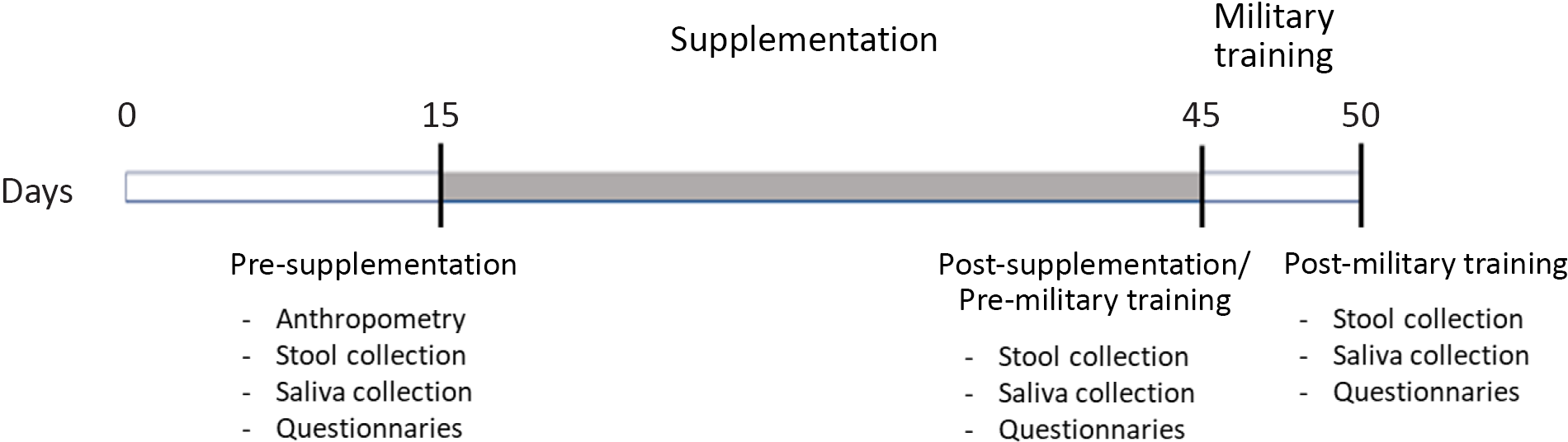
Fig. 1. Experimental design. From time 0 to the 15th d volunteers abstained from consuming products containing probiotics and prebiotics. The supplementation period was 30 d and the field training spanned 5 d.
Study’s exclusion criteria were age below 18 years and above 22 years, lactose intolerance and allergy to milk proteins or to any of the other ingredients used in the product (emulsifier, vanilla extract, inulin), being immunosuppressed or having undergone an organ transplant, as well as any type of heart disease.
Criteria for exclusion were antibiotic use by volunteers within 15 d before the start of supplementation and during the experimental period, need for any type of surgery, tooth extraction or other types of dental manipulation.
Volunteers were randomly allocated into two groups (1:1) to receive either placebo or synbiotic ice cream for 30 d.
This clinical trial supervisor was responsible for distributing identification numbers to the volunteers and randomising them via the Random.org website (www.random.org). Stratified randomisation was used so that the two groups started the protocol with the same sample size and the same sex distribution. This distribution was stored on the computer of the leader for the research until it was disclosed.
The doctoral student in this project was responsible for supplementing and collecting data from the volunteers. This researcher and the volunteers remained blind during the study, so they did not know which ice cream (synbiotic or placebo) was being consumed. The samples provided were coded to ensure a double-blind experiment.
The military students of the Army Cadet School are evaluated continuously, and their physical and intellectual performance are considered in their classification scores; thus, supplementation of any kind is forbidden, so it does not lead to potential advantages of one on another. If any military personnel has a specific need, the medical team is responsible for the prescription (recorded in medical records) and supply. However, it was not the case for any of the volunteers who took part in the present study. We recommended participants not to consume any foods containing prebiotics and probiotics (e.g. probiotic yogurts, fermented milk) 15 d before the beginning of the research period, particularly over the weekend, when they are released to go home. This consumption was controlled during the week because they live in a boarding school where all food is provided. After this period, participants were assessed for anthropometric measurements. Before and after supplementation and after the 5-d field training, participants were assessed for salivary IgA levels, gastrointestinal symptoms, sleep quality and well-being indicators via specific questionnaires. The stool was also collected for gut microbiota and metabolites analysis (Fig. 1).
Supplementation consisted of a daily serving of either synbiotic or placebo ice cream (60 g)(23) for 30 calendar days with weekend breaks due to logistic reasons, as volunteers went home during weekends.
Military training was carried out in an Army Instruction Camp. It lasted 5 d with instructions given day and night, simulating the physical and psychological exhaustion expected in real combat situations. This training aims to check students’ attitudes and practises based on what they have learned in boarding school. In this training, they receive practical instructions on progression practices on the terrain, first aid in combat, orientation with a compass and topographic chart, transposition of watercourses, stress firing, march on foot from 8 to 20 km, among others. Moreover, military students remain armed and equipped the whole time, with enough material to stay in combat for 48 h (approximately 30 kg overweight). In this training, the military is also subjected to weather conditions (rain, sun, cold) and sleep deprivation. The food consumed four times a day was prepared at the base and transported to the campsite for 4 d, and 1 d an operational ration was provided. The training took place in May, in the fall, with great thermal amplitude (the temperature varies, on average, from 84 to 55 °F during the day).
Experimental procedures
Production and characterisation of the synbiotic and placebo ice creams
First, 120 l of ice cream was produced (considering placebo and synbiotic ice cream) following good manufacturing practices. Analyses were conducted regarding microbiological safety, the number of viable probiotics and added inulin.
The formulations underwent mass balance: 7·93 % fat, 11·71 % non-fatty milk solids, 13 % sugars, 32·84 % total solids for placebo formulation and 37·04 % total solids for the synbiotic formulation, which are results consistent with Marshall and Arbuckle(Reference Marshall and Arbuckle24).
Whole milk powder, mineral water, pasteurised whole milk, sucrose, milk cream, emulsifier and vanilla essence were used to produce the ice cream. Commercial cultures of B. animalis subsp. lactis BB12 and L. acidophilus LA5, both from Chr Hansen, and commercial inulin Orafti®GR (Granulated Inulin of vegetable origin) from Beneo-Orafti were used for the synbiotic ice cream. For the placebo ice cream, we used the same ingredients, with the exception of probiotics and inulin, the latter being replaced by carboxymethylcellulose stabiliser. The placebo formulation was developed to present similar consistency and taste when compared with the synbiotic ice cream.
To ensure ice cream safeness, we performed microbiological analyses of five random samples of synbiotic and placebo ice cream. The results were compared with legislation(25). We analysed counts of Escherichia coli and coagulase-positive staphylococci, according to Downes and Ito(Reference Downes and Ito26), and Salmonella presence according to Hammack and Andrews(Reference Hammack and Andrews27).
For selective counts of L. acidophilus, we utilised de Man, Rogosa and Sharpe (MRS) agar (Acumedia-Neogen) added with 0·05 % stock solution of 0·02 % clindamycin and anaerobic incubation (72 h at 37°C) (GasPak), according to the technical bulletin P-10 by Chr Hansen(28). For B. animalis, we used MRS agar added with 0·5 % dicloxacillin stock solution at 0·01 %, 1 % of 11 % lithium chloride stock solution and 0·5 % of 10 % cysteine hydrochloride stock solution and anaerobic incubation (72 h at 37°C) (GasPak), as specified in technical bulletin P-12 by Chr Hansen(29).
Samples were analysed for fructan content by the colorimetric method developed by McCleary et al. (Reference McCleary, Charmier and McKie30) using a kit (K/Fruc, Megazyme International Ireland Ltd, Co.).
The ice cream presented adequate quality for consumption with E. coli and coagulase-positive staphylococci below the limits established by Brazilian legislation, as well as the absence of Salmonella (25).
High viability of probiotic cultures was obtained, totalling an average of 2·1 × 108 CFU/g for L. acidophilus LA-5 and 2·7 × 109 CFU/g for B. animalis BB-12. After 60 d of shelf life, the counts obtained for the probiotic strains were 2·7 × 108 for LA-5 and 1·0 × 109 CFU/g for BB-12, indicating that during ice cream supplementation (30 d) both probiotic strains could have presented a drop during storage but still remained in the same log cycle counts.
Some countries, such as Italy and Canada, suggest that probiotic products should present 9 log CFU per serving(Reference Hill, Guarner and Reid16); therefore, considering the serving of 60 g of ice cream in our research, 10·3 log CFU for L. acidophilus LA-5 and 11·0 log CFU for B. animalis BB-12 were reached per serving.
The measurement of fructan was 3·85g/100g, which corresponds to 2·31 g of inulin in the 60 g daily portion of ice cream. To obtain a bifidogenic effect, the review written by Meyer and Stasse-Wolthuis(Reference Meyer and Stasse-Wolthuis31) presents studies with ranges from 5 to 9 g/d consumption of inulin for adults. However, we adopted a lower concentration (˜2·5 g/d) to not impair the ice cream’s textural characteristics.
Analysis of salivary IgA
The analysis of salivary IgA was carried out by an outsourced laboratory (Grupo Centrolab Medicina Laboratorial/Campinas/SP/Brazil). Saliva was collected with volunteers fasting, before brushing their teeth and using a sterile swab. We measured salivary IgA concentrations by nephelometry and considered 2–20 mg/dl the normality reference values.
Questionnaires
All questionnaires were administered at three times: pre-supplementation period (baseline), post-supplementation period and post-military training.
The Mood and Wellbeing questionnaires use visual analogue scales and have been used in previous studies(Reference Dougkas and Östman32,Reference Dougkas and Ostman33) . The questionnaire addresses ten feelings reported by participants at the time of completion: mental alertness, sadness, tenseness, effort to perform activities, happiness, weariness, calmness, sleepiness, concentration capability and energy. We assessed the data using 100 mm visual analogue scales, and each feeling generated a variable for comparison between the groups(Reference Jeon, O’Mahony and Kim34). A composite mood and well-being score was calculated at each supplementation period to reflect the ten questions using the following formula: average mood (mm) = (mental alertness + happiness + calmness + concentration capability + energy + (100 − sadness) + (100 − tenseness) + (100 − effort) + (100 − weariness) + (100 − sleepiness))/10.
The Gastrointestinal Symptoms questionnaire has been used in previous studies(Reference Bovenschen, Janssen and Van Oijen35). The research volunteers were asked about the presence of gastrointestinal symptoms such as nausea, vomiting, diarrhoea, abdominal pain, flatulence, loss of appetite, burning and dysphagia during the week before completing the questionnaire. The sum of different symptoms reported by the volunteers was compared between the supplemented and placebo groups.
The Pittsburgh Sleep Quality Index is a validated questionnaire(Reference Buysse, Reynolds and Monk36) that assesses subjective sleep quality during the week before completing the questionnaire. The index has internal consistency and a reliability coefficient (Cronbach’s α) of 0·83. It comprises scores for seven categories: subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medication and daytime dysfunction. The sum of these seven components’ scores yields one global score, ranging from 0 to 21 points. A lower Pittsburgh Sleep Quality Index score (<5) indicates higher sleep quality, while a higher Pittsburgh Sleep Quality Index score (> 5) may point out severe difficulties in all seven components, reflecting poorer sleep quality(Reference Buysse, Reynolds and Monk36,Reference Bertolazi, Fagondes and Hoff37) .
Microbiota sequencing and bioinformatic analysis
Faecal samples were collected by volunteers in a specific container and kept at room temperature for a maximum of 1 h before being frozen at −20 oC. The samples were stored until the time of sequencing and metabolites analysis. Gut microbiota of the participants was sequenced by the NGS company, according to the protocol published by Callahan et al. (Reference Callahan, McMurdie and Rosen38), and DNA extraction was performed using the MagMax™ CORE Nucleic Acid Purification Kit (Thermofisher). The quality of the DNA extracted was determined by electrophoresis in agarose gel and quantified in Nanodrop®. KingFisher (Thermofisher) equipment was used for extraction.
The researchers also considered Illumina recommendations for library preparation. The gene-specific sequences used in this protocol target the 16S V3 and V4 regions. The primers Forward: 5’-CCTACGGGNGGCWGCAG-3’ and Reverse: 5’-GACTACHVGGGTATCTAATCC-3’ were selected from Klindworth et al. (Reference Klindworth, Pruesse and Schweer39) publication as the most promising bacterial primer pair.
The first PCR was performed for locus-specific amplification. Then, AMPure XP beads were used to purify the PCR reaction, and the size of fragments generated in the PCR reaction was evaluated by electrophoresis in agarose gel. The second PCR was carried out to connect barcodes of the Nextera XT kit and new stages of PCR purification and validation of the libraries. Subsequently, the libraries were quantified so that all samples/libraries were joined together in an equimolar way into a single pool.
The multiplexed readings were attributed to biological samples and DADA2 software (version 1.16) was used for modelling and correcting amplicons, without considering operational taxonomic units. Fastq filtering was performed to cut the sequences of PCR primers and filter the 3' ends of the readings due to quality decay (Q < 30). After filtering, the reads were 2 × 260 pb in size, maintaining the overlay for subsequent joining of readings and reassembling of the V3–V4 region fragment. Then, the researchers carried out the denoising analysis to obtain a detailed list of unique sequences and their abundances, to produce a consensus position quality score for each unique sequence, calculating the average of the positional qualities of the component readings.
After the initial processing of the sequencing data by DADA2 (version 1.16), the taxonomies were assigned to each amplicon sequencing variant (ASV). The Silva 16S rRNA database was used as a reference (version 132).
Taxonomic classifications and their quantifications, generated by DADA2, were entered in Phyloseq software (R package), in which α diversity analyses were performed(Reference McMurdie and Holmes40).
ASV that were not classified into at least the family level were filtered, and ASV marked as the same species were clustered. ASV that were not present in at least 5 % of the samples were also filtered. The Phyloseq file with taxonomy counts was then imported into EdgeR software, a R/Bioconductor package (version 3.11). For differential abundance analysis between groups, we used Limma Voom packages for normalisation, together with EdgeR(Reference Robinson, McCarthy and Smyth41,Reference Gentleman, Carey and Bates42) . This approach simultaneously solves the problems of DNA sequencing libraries of widely different sizes and ASV count ratios that vary more than expected in a Poisson model. Therefore, we used one of the most popular implementations of this approach, currently used in the analysis of RNA-Seq, namely EdgeR, adapted for microbiome data. This approach enables a valid comparison between ASV, substantially improving both power and accuracy in detecting differential abundance.
Finally, we submitted the sequences to the European Nucleotide Archive under access number PRJEB39682 (ERP123229).
Analysis of ammonia and SCFA in faeces
Faecal samples stored at a temperature below –20 °C were analysed for ammonia and SCFA concentrations. Ammonium ions (NH4 +) were quantified according to Bianchi et al. (Reference Bianchi, Rossi and Sakamoto43), using a specific ion meter coupled to an ammonia-selective electrode. We calibrated the electrode with different standards – 10, 100 and 1000 ppm (Thermo) – and then conducted readings of 10 ml from the 1000 mg faecal solution diluted in 100 ml of distilled water, adding 0·2 ml of solution for ionic strength adjustment. The reading was performed with a temperature of 25 °C and under agitation. The results obtained were divided by the molar mass of the ammonium ion (18·04), which is expressed in mmol/l(Reference Lima, Cecatti and Fidélix44).
SCFA were analysed with 200–300 mg of faeces samples, which were homogenised with 1 ml of 0·15 mM H2SO4, centrifuged (14 000 g , 5 min) and 2 ml of the supernatant stored for fatty acid analysis(Reference Dostal, Baumgartner and Riesen45,Reference Duque, Monteiro and Adorno46) . The supernatants were diluted 1:1 with MilliQ water, filtered in Millex® (0·45 µm) and then injected into an Agilent gas chromatograph (model HP-6890), equipped with an Agilent selective mass detector (model HP-5975) using a DB-WAX capillary column (60 m × 0·25 mm × 0·25 µm) under the following conditions: temperature of injector = 220 °C, column = 35 °C, 2 °C/min, 38 °C; 10 °C/min, 75 °C; 35 °C/min, 120 °C (1 min); 10 °C/min, 170 °C (2 min); 40 °C/min, 170 °C (2 min) and detector = 250 °C. Helium was used as a drag gas at a flow rate of 1 ml/min. Analytical curves were constructed using the stock solution of the acids of interest: acetic, propionic and butyric acids. The samples were analysed in duplicate and data expressed in mmol/g(Reference Dostal, Baumgartner and Riesen45,Reference Duque, Monteiro and Adorno46) .
Statistical analyses and data plotting
Sample calculation analysis was performed to estimate an adequate number of volunteers. Sample calculation in G * Power 3.1.9.2 software was based on the following data: 5 % sample error, 95 % CI and 0·72 effect size considering pre- and post-intervention IgA values. The effect size was estimated based on the study by Olivares et al. (Reference Olivares, Díaz-Ropero and Gómez47), who observed changes in IgA (mg/dl in serum) from 137·08 (sd 20·37) to 159·29 (sd 23·00) after 4 weeks of probiotics intake. With this, we obtained the minimum sample number of twenty-eight individuals in each group. Although the study by Olivares et al. (Reference Olivares, Díaz-Ropero and Gómez47) presented very similar data and methodological design to this research, the study evaluated serum IgA. Considering that salivary IgA concentrations may vary due to hydration status, among other factors(Reference Silva, Natali and Paula48), we increased sample size by 10 % (n 31) per group to avoid type II sampling error. Considering potential dropouts, we further increased the sample size, initiating the study with eighty volunteers.
To minimise the impact of inter-individual variability, dependent variables were converted into delta scores (i.e. post-supplementation – pre-supplementation values and post-military – pre-supplementation values). First, the data normality was assessed by analysing mean, standard deviation, skewness and kurtosis values. After, the Shapiro–Wilk test was used. Thereafter, potential changes in these variables (except for the microbiota data) were assessed by the generalised estimating equations approach with group and time as fixed factors and subjects as a random factor. We performed the generalised estimating equations models based on the assumption of a normal distribution, identity link function and an exchangeable working correlation. All generalised estimating equations models were adjusted for pre-supplementation values and sex. Tukey’s test was used for multiple-comparison post-hoc correction.
All analyses were performed using SAS statistical package (version 9.4). The level of significance was set at P ≤ 0·05. Data are presented as mean and 95 % CI, unless otherwise stated.
For the microbiota data, differential abundance and relative abundance were analysed for the phyla, families, genera and species of faecal bacteria. Quasi-likelihood ratio tests were used to test differential abundance between the control and treat contrasts of interest, with P-values being corrected by the false discovery rate criterion to control the number of false positives(Reference Benjamini and Hochberg49). The Kruskal–Wallis test was done in RStudio version (0.99.467) to assess significant differences in α diversity (Shannon and Simpson indexes) between groups. The non-parametric multivariate ANOVA using Bray–Curtis distance measure was used to test the interaction between group and time in α diversity. The non-parametric multivariate ANOVA was done in PAST software.
For microbiota data plotting, the relation between taxonomic affiliation and the samples was visualised through a heatmap using the XLSTAT 2020·1.3 version (Adinsoft). Multivariate statistics analysis (principal component analysis) was used to emphasise dissimilarity and show patterns in datasets between microbiota and metabolites. This analysis utilised taxonomic affiliation that showed relative abundance > 0·1 % (at family and genus level), and the results obtained for volatile fatty acids (acetic, propionic and butyric acids) and ammonia. Improved samples’ separation was obtained by employing varimax rotation using XLSTAT 2020·1.3 (Adinsoft).
Results
Participants
Volunteers were recruited in March 2019 and followed up until June 2019.
The sample was initially composed of eighty military personnel, sixty-five of whom completed the study, since fifteen military personnel were excluded due to antibiotic use and non-participation in field training activities. Adherence to the supplementation protocol was 100 %, as observed by the researcher that accompanied the supplementations daily.
Table 1 shows the demographic characteristics at the pre-supplementation period. The groups were similar regarding sample size (placebo group: n 33, supplemented group: n 32). No between-group differences were observed at this time for any of the parameters.
Table 1. Distribution and characterisation of the volunteer group, according to supplementation
(Mean values and standard deviations)
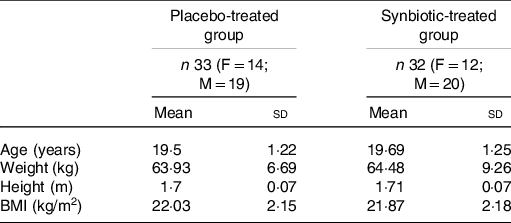
F, females; M, males.
Salivary IgA, gastrointestinal symptoms, well-being and sleep quality
Table 2 shows the effect of supplementation with symbiotic ice cream on salivary IgA concentration, gastrointestinal symptoms, well-being and sleep quality.
Table 2. Comparison between the synbiotic-treated and placebo group as to salivary IgA, number of gastrointestinal symptoms, sleep quality score, average mood, tenseness, sadness, sleepiness, concentration capability, energy, effort, mental alertness, happiness, weariness and calmness, in the pre-supplementation, post-supplementation and post-military training periods‡
(Mean values and standard deviations; odd ratio and 95 % confidence intervals)
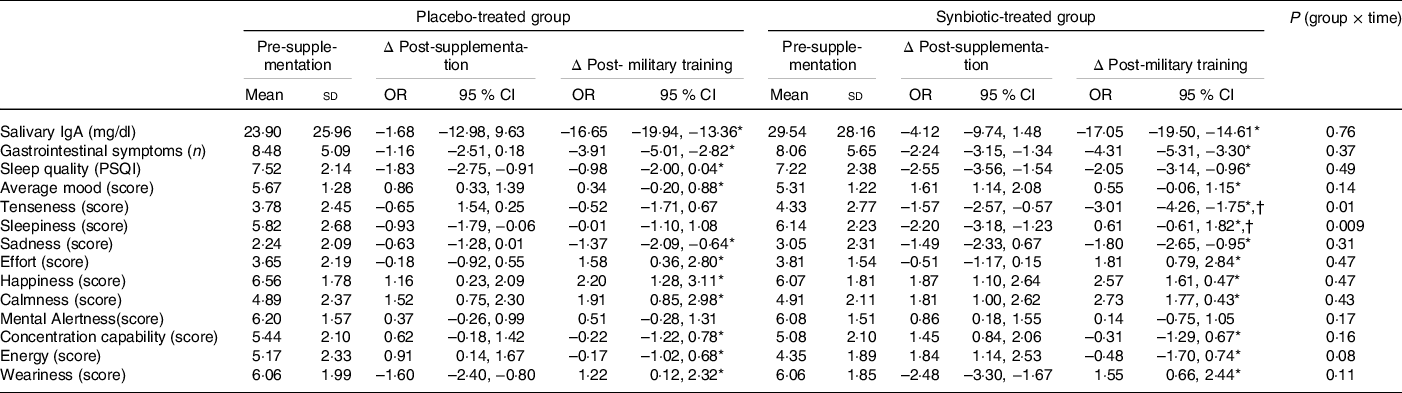
* Means P < 0·05, Δ post-supplementation v. Δ post-military training.
† Means P < 0·05, placebo-treated group v. synbiotic-treated group.
‡ Delta change (Δ) relative to pre-supplementation and 95 % CI, and level of significance (P; group × time effect) calculated using the generalised estimating equations model.
Salivary IgA concentration decreased at the post-military training period in both groups (P = 0·003; main effect of time); however, the supplementation with synbiotic ice cream did not mitigate this effect (P = 0·76; group × time effect) (Table 2).
Both groups showed a decreased number of gastrointestinal symptoms at post-military training (P < 0·001; main effect of time) with no differences between them (P = 0·37; group × time effect) (Table 2).
Mood scores increased at post-supplementation and then decreased at post-military training in both groups (P < 0·001; main effect of time) with no differences between them (P = 0·14; group × time effect) (Table 2).
Tenseness was decreased in the synbiotic-treated group (P = 0·013, within-group difference) but not in the placebo group (P = 0·99, within-group difference) at post-military training (P = 0·03; between-group difference; P = 0·01; group × time effect). Sleepiness was also decreased in the synbiotic-treated group (P < 0·001; within-group difference) but not in the placebo group (P = 0·21; within-group difference) at post-supplementation (P = 0·009; group × time effect) (Table 2).
Sadness was decreased in both groups at post-military training (P = 0·019; main effect of time) with no differences between them (P = 0·31; group × time effect). In contrast, effort, happiness and calmness were increased in both groups at post-military training (P < 0·001, P = 0·009 and P = 0·04, respectively; main effect of time) with no differences between them (P = 0·47, 0·47 and 0·43, respectively; group × time effect). Mental alertness was unaffected by the interventions (P = 0·36; main effect of time; P = 0·17; group × time effect) (Table 2).
Finally, concentration capability and energy were increased at post-supplementation and then decreased at post-military training in both groups (P < 0·001 and 0·001; main effect of time) with no differences between them (P = 0·16 and 0·08; group × time effect). In contrast, weariness was decreased at post-supplementation and then increased at post-military training in both groups (P < 0·0001; main effect of time) with no differences between them (P = 0·11; group × time effect) (Table 2).
Regarding sleep quality evaluated in the pre-supplementation period, we observed that both groups fell into the ‘poor’ category (score between 5 and 10) at the beginning of the study, that is, before the intervention. Sleep quality scores decreased in both groups at post-supplementation; however, only the synbiotic group showed a score (<5) that indicates higher sleep quality. Moreover, this score increased in both groups at post-military training (P = 0·008; main effect of time) with no differences between them (P = 0·49; group × time effect) (Table 2).
Microbiota and metabolites
When analysing the microbiota of the faeces, we found more than 120 genera of bacteria. No significant differences in α diversity between groups or periods (Table 3), either by the Shannon index (P = 0·2559) or the Simpson index (P = 0·2382), were observed. No interaction was also observed between time and groups.
Table 3. α Diversity of the gut microbiota of both the placebo- and synbiotic-treated groups during the follow-up periods of the study through Shannon and Simpson indices
(Mean values and standard deviations)
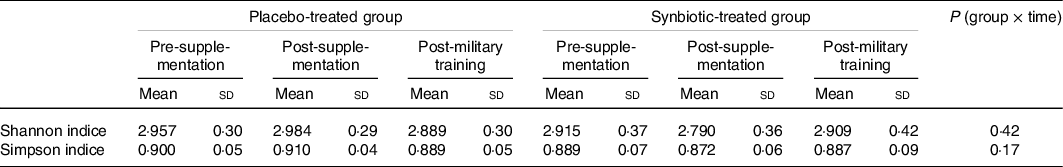
For differential abundance analysis, the values collected at the baseline were used as an adjustment in the post-supplementation and post-military training periods in the comparison between groups for the two periods under evaluation.
Beginning with a higher taxonomic level when observing the relative phyla abundance, we identified Firmicutes, Bacteroidetes, Actinobacteria, Proteobacteria, Verrucomicrobia and Euryarchaeota (data not shown). By the differential abundance analysis, we observed no significant differences in the proportions of each phyla when comparing the two groups, except for the post-military training period in which the percentage of Firmicutes was higher in the group supplemented with synbiotic ice cream (62·8 %) compared with the placebo group (56·6 %), with statistically significant difference between them (P = 0·026) (data not shown).
Observing the differential abundance for families/genera after the supplementation period, as shown in Fig. 2(a), there was a lower percentage of Erysipelotrichaceae and Lachnoclostridium in the faeces of volunteers of the synbiotic-treated group when compared with the placebo group (P = 0·046 and 0·035, respectively), and Peptococcus was found in a larger quantity (P = 0·017). Both the Bifidobacterium and Lactobacillus genera were found in a higher proportion in the group supplemented with synbiotic ice cream (log Fold Change: 1·10 and 1·23, respectively, i.e., doubled in value), tending to statistical significance (P = 0·052 and 0·059, respectively).
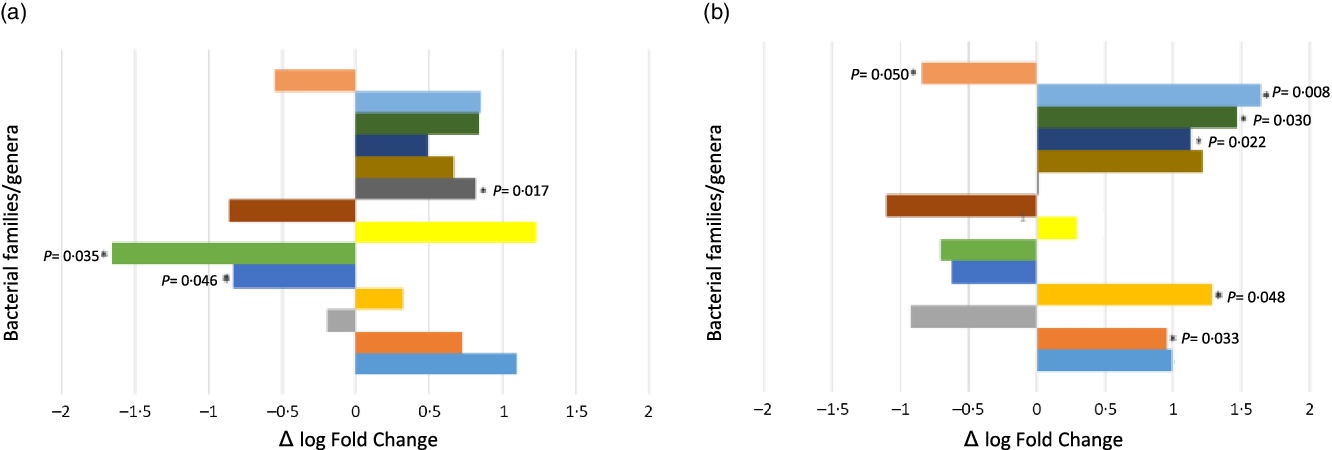
Fig. 2. Differential abundance of the main genera that had relevant variation in the microbiota of the military personnel comparing the synbiotic-treated group with the placebo-treated group. (a) Δ Pre-military training period and (b) Δ Post-military training period. Values express the difference between the synbiotic group and the placebo group and P value expresses group × time effect. Data expressed as mean; Δ means delta change. ![]() , Tannerellaceae “Parabacteroides”;
, Tannerellaceae “Parabacteroides”; ![]() , Ruminococcaceae “Ruminococcaceae_UCG-009”;
, Ruminococcaceae “Ruminococcaceae_UCG-009”; ![]() , Marinifilaceae “Odoribacter”;
, Marinifilaceae “Odoribacter”; ![]() , Erysipelotrichaceae NA;
, Erysipelotrichaceae NA; ![]() , Eggerthellaceae “Eggerthella”;
, Eggerthellaceae “Eggerthella”; ![]() , Streptococcaceae “Streptococcus”;
, Streptococcaceae “Streptococcus”; ![]() , Ruminococcaceae “Ruminiclostridium_5”;
, Ruminococcaceae “Ruminiclostridium_5”; ![]() , Lactobacillaceae “Lactobacillus”;
, Lactobacillaceae “Lactobacillus”; ![]() , Erysipelotrichaceae “Holdemania”;
, Erysipelotrichaceae “Holdemania”; ![]() , Bifidobacteriaceae “Bifidobacterium”;
, Bifidobacteriaceae “Bifidobacterium”; ![]() , Ruminococcaceae “UBA1819”;
, Ruminococcaceae “UBA1819”; ![]() , Peptococcaceae “Peptococcus”;
, Peptococcaceae “Peptococcus”; ![]() , Lachnospiraceae “Lachnoclostridium”;
, Lachnospiraceae “Lachnoclostridium”; ![]() , Eggerthellaceae “Gordonibacter”.
, Eggerthellaceae “Gordonibacter”.
In the post-military training period (Fig. 2(b)), we found a different pattern of differential proportions, especially for the Parabacteroides genus, which presented a reduction (P = 0·050) for the synbiotic-treated group. Conversely, the Eggerthella (P = 0·033), Holdemania (P = 0·048), Ruminococcaceae_UCG-009 (P = 0·022), Ruminococcaceae UBA1819 (P = 0·030) and Streptococcus (P = 0·008) genera/families presented higher proportions in this same group. Bifidobacterium and Lactobacillus also had higher proportions in the synbiotic-treated group (log Fold Change: 0·99 and 0·29, respectively), but without significant difference (P = 0·08 and 0·65, respectively).
High-throughput 16S rRNA gene sequencing with low resolution cannot discriminate species(Reference Jovel, Patterson and Wang50). Fortunately, in the present research, we were able to identify B. animalis, which presented a significant rise in the synbiotic group after supplementation (Fold Change = 5·78 and P < 0·001) (data not shown). This Fold Change value represents approximately a 32-fold increase of B. animalis compared with the pre-supplementation period.
As shown in Fig. 3, the heatmap analysis resulted in two prominent groupings: one group composed of placebo group post-supplementation and placebo group pre-supplementation treatments, and another group composed of placebo group post-military training, synbiotic-treated group post-military training and synbiotic-treated group pre-supplementation. The synbiotic-treated group post-supplementation treatment clustered completely separately from the other treatments, and some genera presented greater relative abundance only in this treatment, such as Agathobacter, Bifidobacterium, Alistipes, Prevotella, Collinsella and Dialister according to heatmap analysis. This result confirms that supplementation with synbiotic ice cream modulated the gut microbiota of the research volunteers. In addition, we observed different patterns in the relative abundance of the microbial community after field training.
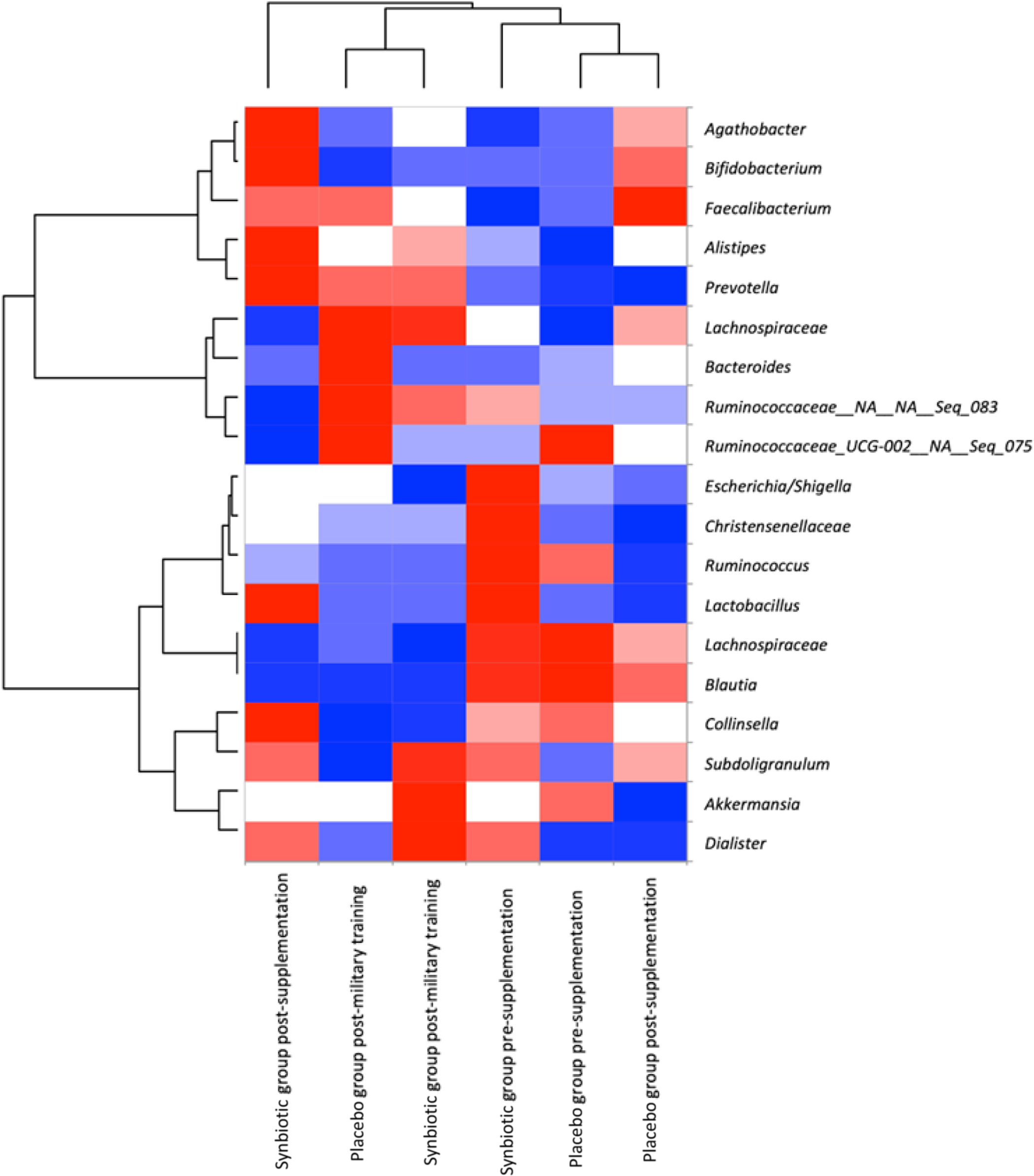
Fig. 3. Heatmap analysis of the microbiota of the military personnel in pre-supplementation, post-supplementation and post-military training periods, containing more abundant families/genera. The colour key represents the scaled relative abundance of each variable, with red indicating high relative abundance and blue indicating low relative abundance, clustered independently using ascendant hierarchical clustering based on Euclidian distances. Data expressed as mean. ![]() , <-1;
, <-1; ![]() , −1 to −0·71;
, −1 to −0·71; ![]() , −0·71 to −0·43;
, −0·71 to −0·43; ![]() , −0·43 to −0·14;
, −0·43 to −0·14; ![]() , −0·14 to 0·14;
, −0·14 to 0·14; ![]() , 0·14 to 0·43;
, 0·14 to 0·43; ![]() , 0·43 to 0·71;
, 0·43 to 0·71; ![]() , 0·71 to 1;
, 0·71 to 1; ![]() , >1.
, >1.
Higher proportions of the most abundant micro-organisms were observed for the synbiotic-treated volunteers compared with the placebo group (Fig. 3). However, field training resulted in a reduction in the abundance of several members of the microbiota, both for the military supplemented with synbiotic ice cream and for the group that received placebo ice cream. The reduction was more salient for the control group. Even so, this variation was not enough to result in a significant difference in α diversity between treatments (Table 3).
The principal component analysis (Fig. 4) elucidated 95·5 % of the dissimilarity from data, 92·66 % (first dimension) and 2·89 % (second dimension). Both pre-supplementation groups clustered with the genera Blautia and Bacteroides and the family Christensenellaceae. However, the 30-d supplementation with synbiotic ice cream clustered with Bifidobacterium, Faecalibacterium and Agathobacter. The first is related to health benefits such as immune enhancement, has anti-carcinogenic properties and produces some vitamins; the second has anti-inflammatory properties and produces butyrate(Reference Karl, Hatch and Arcidiacono51). The genus Agathobacter is part of the Lachnospiraceae family and is an important butyrate producer(Reference Engels, Ruscheweyh and Beerenwinkel52).
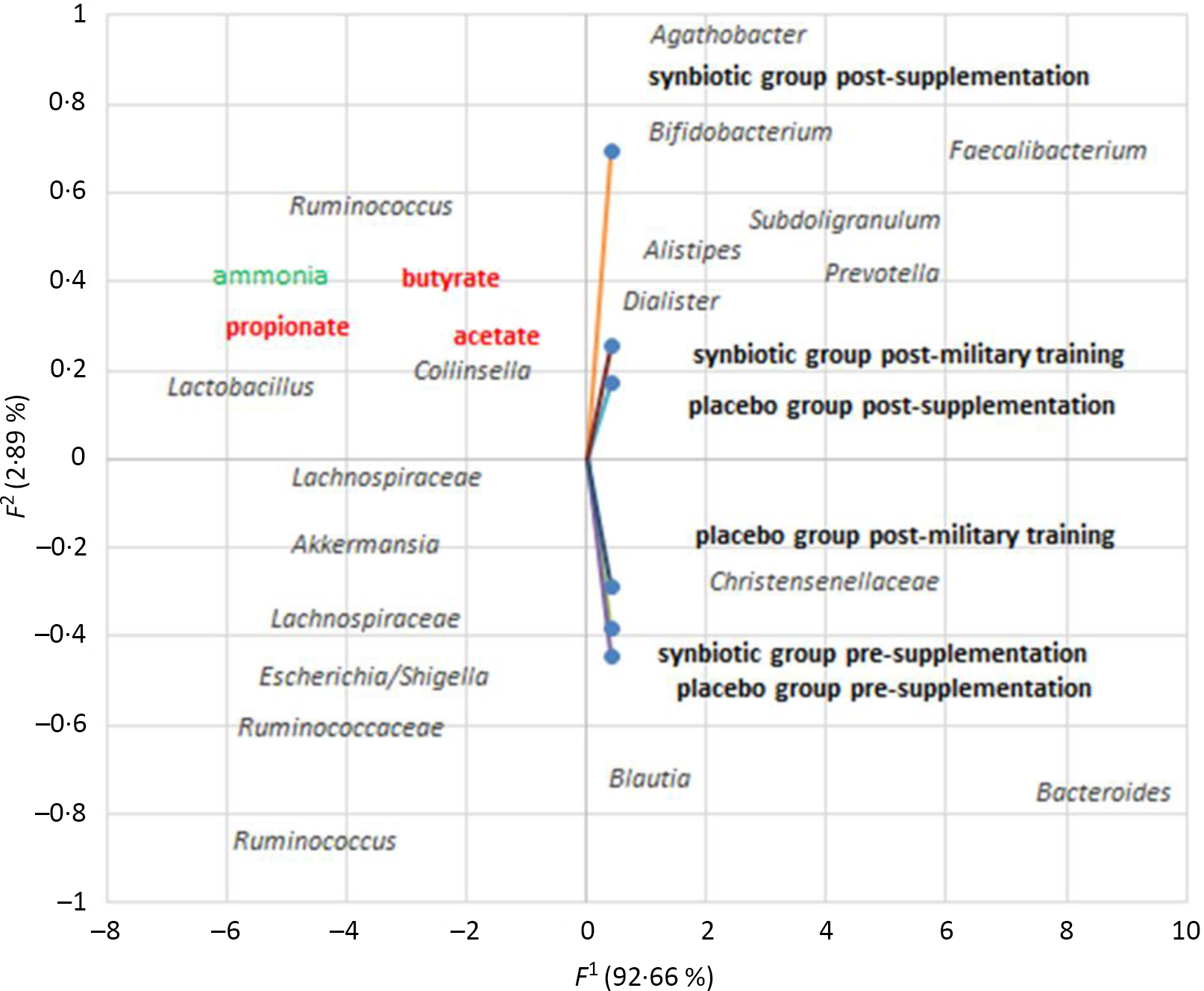
Fig. 4. Principal component analysis (PCA) analysis of the similarity of faecal microbiota (genera/family/metabolite) between the pre-supplementation, post-supplementation and post-military training periods. Genera/family or metabolite closer to the each treatment + time are more closely related. Data for pre-supplementation, post-supplementation and post-military training periods groups were plotted on the first two principal components of the genera profiles. The first two components explained 95·55 % of all results. Only genera with abundance values above 0·1 % in least all samples are shown. Data expressed as mean of the treatments.
Unfortunately, SCFA (butyrate, propionate and acetate) did not cluster with the synbiotic treatment but clustered with Lactobacillus, Ruminococcus and Collinsella.
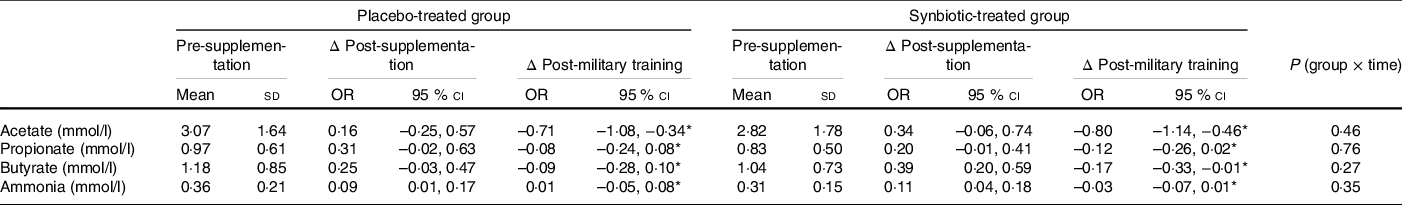
Regarding the metabolites produced by the gut microbiota, we observed that the concentration of ammonia in the faeces was increased at post-supplementation and then decreased at post-military training (P = 0·002; main effect of time) with no differences between the groups (P = 0·35; group × time effect) (Table 4).
Table 4. Comparison of ammonia and SCFA present in the faeces of military personnel in the pre-supplementation, post-supplementation and post-military training periods for the placebo- and synbiotic-treated groups†
(Mean values and standard deviations; odds ratio and 95 % confidence intervals)
* Means P < 0·05, Δ post-supplementation v. Δ post-military training.
† Delta change (Δ) relative to pre-supplementation and 95 % CI, and level of significance (P; group × time effect) calculated using the generalised estimating equations model.
Regarding SCFA, acetic levels were decreased at post-military training in both groups (P < 0·001; main effect of time) with no differences between them (P = 0·46; group × time effect). Propionic acid and butyric acid were increased at post-supplementation and then decreased at post-military training in both groups (P = 0·004 and 0·002, respectively; main effect of time) with no differences between them (P = 0·76 and 0·27, respectively; group × time effect) (Table 4).
Discussion
The impact of long-term supplementation with synbiotic ice cream on immune function, microbiota and its metabolites, gastrointestinal health and sleep in healthy young personnel undergoing military training was investigated in this randomised controlled clinical trial.
Intense physical activity, in addition to stress and lack of sleep, may have harmful effects on immune function(Reference Campbell, Turner and Campbell53). This is in line with the present study, in which we observed a substantial decrease in IgA levels after the military training, an effect not mitigated by the synbiotic ice cream supplementation (Table 2).
Previous studies with probiotic supplementation have shown conflicting results. Olivares et al. (Reference Olivares, Díaz-Ropero and Gómez47) supplemented healthy people with Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711 (present in a fermented product) and found an increase in serum IgA concentrations – the effect was higher after 2 weeks of treatment. Although serum IgA may represent a more sensitive biomarker than salivary IgA, we chose to evaluate the latter because it is obtained by a non-invasive method. Moreover, the IgA rise observed by Olivares et al. (Reference Olivares, Díaz-Ropero and Gómez47) may be due to the specific strains evaluated.
Probiotics associated with prebiotics have been used to reduce susceptibility to gastrointestinal tract disorders. The reduction of these occurrences may be of relevance for practitioners of intense physical activities as they are at higher risk of increased intestinal permeability, culminating in translocation of gut metabolites into circulation(Reference Karl, Hatch and Arcidiacono51,Reference West, Pyne and Cripps54) . Continuous supplementation with probiotics, even in strenuous exercises, can favour the increase in the expression of claudin-1 and zonula occludens 1, which are transmembrane proteins that are part of tight junctions, helping to strengthen the intestinal barrier function(Reference Chaves, Baptista and Simabuco55). We, however, did not observe any effects of the synbiotic supplementation on gastrointestinal symptoms (Table 2).
Indicators of mood and well-being were also investigated in the present study, considering the challenge imposed by field training and potential effects of pre and probiotics on the microbiota–gut–brain axis. According to the review by Purvis et al. (Reference Purvis, Gonsalves and Deuster56), symptoms such as mood disorders, fatigue, insomnia and changes in appetite are widely used as markers of stress in the context of excessive physical activity (coupled with inadequate rest). Clark and Mach(Reference Clark and Mach8) point out that probiotics may improve symptoms of stress, depression and mood disorders induced by strenuous exercises.
In the present study, average mood scores increased at post-supplementation and then decreased at post-military training in both groups (Table 2). The euphoria in preparation for the training activity, besides the motivation provided by their superiors and instructors, may be associated with the improvement of mood and well-being as energy and concentration capability were increased. In contrast, weariness was decreased in both groups after supplementation. Completing the field training possibly generated satisfaction in the volunteers, which may explain the reduced sadness and increased happiness and calmness, despite the increased weariness and decreased concentration capability and energy in both groups. Notably, the synbiotic did not impact these parameters.
Supplementation with the synbiotic ice cream did, however, significantly reduce tenseness and sleepiness in the synbiotic-treated group after the military field training (Table 2). Military training was composed of intense physical activity, which along with sleep deprivation, food restriction and psychological tension was probably a high source of stress to participants. Notably, the negative impact on tenseness and sleepiness imposed by the training was effectively mitigated by the synbiotic supplementation.
Tillisch et al. (Reference Tillisch, Labus and Kilpatrick57) showed activation of brain regions responsible for processing emotions and sensations by the consumption of fermented milk with B. animalis subsp. lactis (strain number I-2494 in French National Collection of Cultures of Micro-organisms (CNCM, Paris, France), Streptococcus thermophilus (CNCM strain number I-1630), Lactobacillus bulgaricus (CNCM strain numbers I-1632 and I-1519) and Lactococcus lactis subsp. lactis (CNCM strain number I-1631) for 4 weeks. Thus, we may infer that supplementation with inulin and probiotics favoured microbiota modulation with signs of homoeostasis due to the bilateral communication established by the microbiota–gut–brain axis, which induced feelings of reduced tenseness after training in the military personnel.
Intestinal modulation exerted by frequent consumption of probiotics and gut–brain communication may be associated with metabolism and secretion of the hormone melatonin, present in the sleep and wakefulness cycle(Reference Wong, Yang and Song10). According to the Pittsburgh questionnaire administered in the present study, the military personnel’s sleep quality was considered poor for both groups in the pre-supplementation period (Table 2). Although both groups showed improved scores, only the group supplemented with synbiotic ice cream showed sleep quality classified as good in the post-supplementation period. Moreover, sleepiness was reduced in the synbiotic-treated group only after the military training (Table 2).
Wong et al. (Reference Wong, Yang and Song10) observed that probiotics might be associated with alteration in melatonin metabolism and secretion. According to the authors, gut microbiota can affect tryptophan’s metabolism, a precursor of many biologically active agents, including melatonin(Reference Wong, Yang and Song10). Marotta et al. (Reference Marotta, Sarno and DelCasale60) observed that improvement in sleep quality fits well with the reduction in depressive mood state, anger and fatigue observed in the experimental group, which was supplemented with probiotics for 6 weeks.
Targeting the intestinal microbiota can provide important information on how psychological stress, sleep restriction, environmental stressors and strenuous physical activity can lead to imbalance (dysbiosis) in this community(Reference Karl, Hatch and Arcidiacono51). In the present study, we did not observe differences in the α diversity of the intestinal microbiota of the military group who volunteered to participate in the study due to the supplementation or field training activity (Table 3). However, Karl et al. (Reference Karl, Margolis and Madslien61), evaluating the microbiota by metagenomics of a military group after field training (4-d cross-country ski march), observed an increase in the Shannon α diversity, although the hypothesis raised by the authors was the opposite, considering the effect of the combination of multiple stressors on the microbiota of the military.
The relative abundances of the different taxonomic levels obtained in the baseline period were adjusted by the statistical analysis in the comparison of differential abundance between groups (Fig. 2). Stress and deprivation caused by field training resulted in statistically significant modifications in six families/genera with a reduction in Parabacteroides and an increase in Eggerthella, Holdemania, Ruminococcaceae_UCG-009, Ruminococcaceae UBA1819 and Streptococcus. The literature reports stress situation-related changes in the proportion of some members of the microbiota, such as Eggerthella (E. lenta) with reduction in altitude situations(Reference Kleessen, Schroedl and Stueck62), and Ruminococcaceae with increased relative abundance after 4 weeks of sleep fragmentation(Reference Poroyko, Carreras and Khalyfa63). Benedict et al.(Reference Benedict, Vogel and Jonas64) observed an increase of Erysipelotrichaceae related to circadian disruption and sleep restriction. In the present study, however, we observed a significant increase in the proportion of the Erysipelotrichaceae family due to the supplementation with synbiotic ice cream and not due to the effect of field training.
A study conducted by Feng et al. (Reference Feng, Duan and Xu65) used two datasets published by the American Gut Project and a gut metagenomic dataset (NBT) to analyse the relationship between the genera Bifidobacterium and Lactobacillus and the community structure of the gut microbiota. They observed that the relative abundance of Bifidobacterium was significantly increased when Lactobacillus was present. Considering the interinfluence between these two genera, they proposed that they have a close connection with other dominant genera and observed that Bifidobacterium and Lactobacillus showed a positive correlation with Faecalibacterium, Agathobacter and other potential butyrate producers. Those results are in line with our findings, according to Figs. 3 and 4.
In the present study, we evaluated inulin associated with probiotics, and this prebiotic could also be responsible for the increase in the Agathobacter genus in the supplemented group. This observation agrees with other reported evidence, where different types of prebiotics and their effect on gut microbiota were observed(Reference Walker, Ince and Duncan66).
We also observed that before supplementation (baseline period) both groups clustered with Christensenellaceae, indicating a common characteristic of the military volunteers (Fig. 4). According to Zhu et al. (Reference Zhu, Jiang and Du67) and Goodrich et al. (Reference Goodrich, Waters and Poole68), Christensenellaceae is associated with exercise, health improvement and low BMI (< 25 kg/m2).
The gut microbiota can synthesise and stimulate the endogenous secretion of hormones, neurotransmitters, such as serotonin, dopamine and histamine, and produce SCFA(Reference Karl, Hatch and Arcidiacono51). γ-Aminobutyric acid is a neuroactive amino acid produced by some micro-organisms, including Lactobacillus spp. and Bifidobacterium spp.(Reference Barrett, Ross and Toole69). A decrease in anxiety was observed with the ingestion of Lactobacillus rhamnosus (JB-1), probably due to modification of the mRNA expression in brain regions of γ-aminobutyric acid receptors(Reference Bravo, Forsythe and Chew70). SCFA (butyrate, acetate and propionate) are the principal metabolites from soluble fibre fermentation by some micro-organisms such as Bifidobacterium and Lactobacillus and can figure as neurohormonal signalling molecules(Reference Kelly, Kennedy and Cryan71,Reference Macfarlane and Macfarlane72) .
Acetate, butyrate and propionate levels were not different between the groups (Table 4). This result differed from the previous result of our research group(Reference Rodrigues, Duque and Fino19) when we observed an increase in these metabolites using a colonic digestion model (Simulator of the Human Intestinal Microbial Ecosystem – SHIME®) with 14 d supplementation with ice cream containing the same prebiotic (inulin) and the same probiotic strains (BB-12 and LA-5) of the present study. In the previously published study, SCFA analysis was carried out in a model that does not include the absorption of the compounds but can simulate and demonstrate what happens in loco. In the present study, the analysis was performed on the volunteers’ faeces. We suppose that the amount of SCFA in the samples is directly proportional to the amount of these metabolites produced in the gut, although the quantity of butyrate, propionate and acetate absorbed during the digestion process was not measured. Several clinical studies used a faecal SCFA as a good parameter to evaluate the intestinal microbiota metabolism(Reference Karu, Deng and Slae73–Reference Primec, Mičetić-Turk and Langerholc75).
An unfavourable result observed in the present study was that after the 30-d period of consumption of synbiotic ice cream or placebo, we found an increase in the content of ammonia ions (Table 4) in the faeces of the participants. Changes in the diet (increase in protein consumption, e.g.) before the field training could have impacted these results. Nonetheless, it should be noted that ice cream is less explored as a food matrix for the delivery of pre and probiotics compared with fermented milk. Although the ice cream was an efficient vehicle for delivering the probiotic strains evaluated, the daily consumption of this dairy product should be carefully evaluated, since the ammonia metabolite can affect the energy metabolism of colonic epithelial cells and present toxicity depending on its concentration(Reference Davila, Blachier and Gotteland76). Therefore, ice cream with prebiotics and probiotics may be an additional option to diversify the forms of delivery of probiotics, but it should remain a product of occasional consumption.
The present study was not without limitations. First, we did not evaluate the individual effect of each probiotic strain and inulin which may have limited the comparison of the present data with previous studies. Second, for some comparisons, our sample may have been underpowered. We observed low power for the variables IgA (30 %), mental alertness (23 %), concentration capability (65 %), energy (64 %) and weariness (61 %). For all other variables, however, power was higher than 80 %. Third, no physiological markers of stress were evaluated. Finally, subjects in the present study were young and healthy at baseline, precluding the generalisation of these data to other populations which could respond differently.
The results can be useful and recommended not only for military personnel but also for young professionals equally exposed to high-stress rates, such as flight operators, intensive care physicians, police officers and high-performance athletes, among others.
Conclusion
We observed that 30 d of synbiotic ice cream supplementation containing inulin, L. acidophilus LA-5 and B. animalis BB-12 favourably modulated gut microbiota and improved tenseness and sleepiness in healthy young military undergoing a 5-d field training, whereas it did not impact gastrointestinal symptoms, immune function and other aspects of well-being.
These improvements may be of high relevance to this population as they may influence decision-making process in an environment of high physical and psychological stress, where the military need to make crucial decisions related to their survival, the lives of their companions, subordinates and innocents.
Acknowledgements
The authors are grateful to Captain Jayme Campos da Silveira (Brazilian Army – Campinas/SP/Brazil) and Dr. José Evandro de Moraes (Instituto de Zootecnia – Nova Odessa/SP/Brazil) for their assistance. In addition, the authors thank the Army Cadets Preparatory School – Brazilian Army (EsPCEx – EB), General Marcus Alexandre Fernandes de Araújo – former Commander of EsPCEx, Colonel Alexandre de Oliveira Moço – former Subcommander of EsPCEx, and the volunteer cadets who participated in this project.
This work was supported by the São Paulo Research Foundation (FAPESP) – Project number 2017/25007–9 and 2018/18366–5; the Coordination for the Improvement of Higher Education Personnel – Brazil (CAPES) – Financial Code 001; and the Support Fund for Teaching, Research and Extension (FAEPEX) – Number 519 292. The present study was also financed in part by CNPq (403328/2016-0; 301496/2019-6) and FAPESP (2015/50333-1; 2018/11069-5; 2015/13320-9). M. R. M. J. thanks Red Iberomericana de Alimentos Autoctonos Subutilizados (ALSUB-CYTED, 118RT0543). The authors thank Espaço da Escrita – Pró-Reitoria de Pesquisa – UNICAMP – for the language services provided. None of the funders had a role in the design, analysis or writing of this article.
M. C. P. R. V.: Doctoral student involved with designing the study, collecting and analysing data and writing the article; I. A. V.: Scientific Initiation Student, contributed with data tabulation and bibliographic review and SCFA analysis; L. C. F.: responsible for ammonium ions and microbiological analysis; D. A. G.: responsible for the ice cream production; A. M. E.: analysis on sleep quality; D. T. d. C.: responsible for the statistical analysis and critical review of the manuscript; L. L. C.: α diversity, heatmaps and principal component analysis; F. B. B.: involved with statistical analysis, analysing data and critical review of the manuscript ; M. R. M. J.: analysis on mood and well-being and study design; A. G. B.: analysis on mood and well-being; R. S.: responsible for fructan analysis; G. M. P.: responsible for fructan analysis and study design; A. S.: responsible for the SCFA analysis; K. S.: ammonium ions analysis, responsible for data analysis and study design; P. C. T.: microbiota sequencing and bioinformatics analysis; L. L. C.: microbiota sequencing and bioinformatics analysis; A. E. C. A.: Scientific Adviser Professor, involved with study design, research coordination, analysing data and writing the article. All authors contributed to and agreed with the final version of the article.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.











