Vitamin and mineral intakes among young children have been investigated because of their importance in various metabolic and physiological processes during childhood( Reference Singh 1 ), as well as because they are an important part of optimal nutrition at preschool age( Reference Butte, Fox and Brieffel 2 ). Vitamin A, vitamin C, folate, Ca, Fe and Zn deficiencies may result in growth stunting( Reference Allen 3 – Reference Rosado 5 ), increased morbidity( Reference Tomkins, Behrens and Roy 6 , Reference Rosado, López and Muñoz 7 ) and delayed cognitive function( Reference Sandstead, Penland and Alcock 8 – Reference Uauy, Kain and Mericq 10 ).
Changes in children’s dietary patterns in the last decade (especially lower fruit and vegetable consumption) are predictive of inadequate micronutrient intake( 11 – Reference Wolfenden, Wyse and Campbell 14 ). The literature describes an increased consumption of ultra-processed foods (industrial formulations manufactured from substances derived from foods or synthesised from other organic sources( Reference Monteiro, Levy and Claro 15 ) around the world)( Reference Monteiro and Cannon 16 – Reference Moodie, Stuckler and Monteiro 18 ), especially fortified foods (to which nutrients have been added), which have been associated with a decrease in the prevalence of inadequate vitamin and mineral intakes( Reference Shamah and Villalpando 19 – Reference Hennessy, Walton and Flynn 21 ).
In Brazil, thus far, there has been no national population survey that has investigated the percentage of young children below the estimated average requirement (EAR). The only study that estimated the prevalence of inadequate micronutrients intake was a multicentre study including children aged 2–6 years (n 3058) who were enrolled in kindergartens that offered all meals during the day. Such data suggest low prevalence of inadequate intake for folate (<0·001 %), Ca (12·6 %), vitamin A (0·7 %), vitamin C (<0·001 %), Fe (0·4 %) and Zn (<0·001 %)( Reference Bueno, Fisberg and Maximino 22 ). Furthermore, no publications were found describing a prevalence of inadequate micronutrient intake and the contribution of fortified food for children at 2–3 years of age in Brazil, especially in low-income families, as they often consume poor-quality diets and are supposedly at risk for inadequate micronutrient intake( Reference Huffman, Baker and Shumann 23 – Reference Black, Allen and Bhutta 25 ). Therefore, the present study aimed to (1) estimate the prevalence of inadequate micronutrient intake patterns among children from low-income families and (2) to assess micronutrient intake from fortified foods.
Methods
Study design
This study used data of children who participated in a randomised field trial with intervation in healthcare workers on breast-feeding and dietary practices( 26 ). The randomisation units were healthcare centres that provide primary medical services predominantly to low-income residents. The outcomes were assessed for mothers and children who received treatment at these centres. From April to December 2008, all pregnant women at the participating healthcare centres were invited to sign up for outcome tracking by fieldworkers who were blinded to the allocation status of the participants. The births occurred between May 2008 and February 2009.
The sample size initially chosen for the trial was based on the goal of detecting a difference in the prevalence of exclusive breast-feeding at 4 months( Reference Vitolo, Bortolini and Feldens 27 ). A power of 90 %, CI of 95 %, loss prediction of 20 % and design effect of 1·5 were used to calculate the sample size, resulting in the inclusion of 300 mother–baby pairs in the intervention group and another 300 in the control group. Recruitment of 720 individuals was estimated in order to reach the sample size, accounting for an anticipated loss of 20 %. Of the 715 pregnant women who registered initially, 635 of their children were enrolled to the study at 6–9 months of age. At 2–3 years of age, 476 children remained for the follow-up study (Fig. 1). As the present study had a different aim, we calculated that the available sample at 2–3 years of age was sufficient to investigate the prevalence of inadequate micronutrient intake. The estimated prevalence of inadequate nutrient intake was set at 65 %, with a margin of error of 5 % and a 95 % CI( Reference Bueno, Fisberg and Maximino 22 ).

Fig. 1 Flow diagram of the study.
This study was approved by the Ethics Committee of the Federal University of Health Sciences in Porto Alegre and the Ethics Committee of the Porto Alegre municipal government and was registered on the ClinicalTrials.gov website under the identification number NCT00635453. A consent form with all of the study’s information was given to the responsible parties, clarified and signed.
Data collection
Trained fieldworkers, masked to allocation status, contacted participants at baseline and approximately 6 months and 3 years after birth. Data on sex and date of birth were collected from the children’s vaccination records, and the mothers were asked about their race, age at child’s birth, maternal education level, maternal employment and annual family income during home visits conducted in 2009. When the children were 2–3 years old (2011–2012), trained fieldworkers performed dietary recalls during home visits, asked about Fe supplement use and collected antropometric data using a digital scale (Techline) to the nearest 0·1 kg and a stadiometer (SECA) to the nearest 0·1 cm. BMI-for-age z-scores were estimated on the basis of World Health Organization standards( 28 ).
Nutrient intakes and nutrient sources from foods
In this study, two 24-h dietary recalls were collected for each child on two non-consecutive days chosen randomly during a period of 2 weeks to 1 month during home visits. Mothers provided information about all foods consumed by their children during the previous 24 h. Details about food types, amounts and preparation methods were recorded. Common household measures (e.g. teaspoons, tablespoons, cups and serving sizes) were used to help mothers report the amounts of food given to their children. Vitamin A (μg/d), vitamin C (mg/d), folate (μg/d), Ca (mg/d), Zn (mg/d) and Fe (mg/d) intake estimates were obtained using Dietwin® software program (version 2008 professional; Dietwin®), which is based on US Department of Agriculture chemical composition tables (USDA, Agricultural Research Service, 1998) to assess nutrient intakes. Manufacturers’ information on industrial products and other available national chemical composition tables were added to the programme( 29 , Reference Philippi 30 ).
Data on the consumption (in grams or millilitres per day) of healthy foods and fortified foods were obtained to identify the children’s dietary micronutrient sources. Fruits, vegetables, beans, meats (beef, chicken, pork, poultry, fish) and dairy products (milk and yogurt) were considered healthy foods and contributed to >30 % of micronutrient intake. Therefore, they were considered the ‘main food sources of micronutrients’( Reference Manios, Moschonis and Grammatikaki 31 ). Ultra-processed foods that were fortified (gelatin desserts, ready-to-eat cereal, soya juice, soya milk, powdered milk, powdered chocolate, baby cereal and petit suisse cheese) were included because of their high consumption prevalence in this sample of children (≥20 %).
According to official guidelines, the amounts of healthy foods were investigated as servings per day and compared with the recommendations for fruits (240 g), vegetables (180 g), beans (25 g), meats (70 g) and dairy products (600 ml)( 26 , Reference Philippi, Latterza and Cruz 32 , 33 ). Consumption of natural fruit juices was not considered to calculate total fruit intake. Potatoes were not considered components of the vegetable group, as they are traditionally included in the carbohydrate-rich food group, in accordance with Brazilian food guides( 26 , Reference Philippi, Latterza and Cruz 32 ).
Data analysis
The multiple source method (MSM) was used to estimate the means and distribution percentiles for usual dietary micronutrient intake and food group consumption. The MSM calculates the dietary consumption of individuals using logistical regression and distributes the individual data adjusted for the population( Reference Harttig, Haubrock and Knüppel 34 , Reference Haubrock, Nöthlings and Volatier 35 ). All participants were considered consumers of micronutrients. For the food and beverage groups, a probability value of 0·5 (50 %) was used to assign the status of habitual consumer, assuming that there was a percentage (50 %) of habitual consumers among the preschool children who did not consume such foods or beverages in at least one of the dietary surveys. Estimated intakes were calculated for those who were chosen in this manner. The presence of outliers was investigated using box-plot graphs.
The prevalence of inadequate intake for each micronutrient was estimated using the proportion of individuals below the EAR, which represents the median nutrient requirement. The RDA, which represents a value that meets the needs of approximately 98 % of health people, was used as complementary reference to verify how many children were above the values( 36 – 40 ). The contributions of fortified foods to vitamin A, vitamin C, folate, Ca, Zn and Fe intakes were calculated using the amount each child consumed individually. The contributions of fortified foods were analysed according to the EAR and upper tolerable level (UL). UL is the highest daily intake of a nutrient considered to be safe, above which potential adverse health effects can occur. It is defined as the absolute value of usual micronutrients obtained from fortified food items and supplements( 36 – 40 ).
The database was built using Statistical Package for the Social Science, version 16.0 (SPSS Inc.), and all statistical analyses were performed using the same software. All data were double entered independently by different people for subsequent validation in EPI-INFO, version 6.4 (CDC). The Kolmogorov–Smirnov test was used to assess distribution normality. Variables were described using means and standard deviations, medians and minimum and maximum values and per cent frequency. Statistical significance was set at P<0·05.
Comparisons of maternal and family characteristics between children who were lost to follow-up and those who remained in the study were made using the χ 2 test and Student’s t test, or the Mann–Whitney U test, as deemed appropriate. To eliminate the potential effect of energy intake, we compared the mean daily energy intake between children whose intakes were above the EAR and those who met EAR for each micronutrient using the Mann–Whitney U test. Moreover, comparisons in terms of the prevalence of inadequate intake for each micronutrient between the intervention and control groups were made using the χ 2 test.
Results
Of the 715 pregnant women who registered initially, 635 of their children were enrolled into the study at 6–9 months of age (an 11·1 % loss). The data collected at 2–3 years of age were obtained from the 6–9-month sample, and 476 children remained in the follow-up (a 24·8 % loss). The causes for loss were refusal to participate in the study (n 32), death of the child or mother (n 1), re-location to another city (n 56) and inability to locate the family (n 70) (Fig. 1). Of these 476 children, thirteen were excluded from the analyses because of the presence of genetic diseases and seventeen were excluded because they did not have complete dietary data. Thus, the data of 446 preschool children were analysed for this study. No differences were found for race, sex, weight, length, maternal age at child’s birth, maternal education level, maternal employment and family income between the children who were lost to follow-up and those who remained at 2–3 years of age (P>0·05; data not show).
Regarding maternal and family characteristics, 18·4 % (n 82) of the mothers were under 20 years of age at the child’s birth, 46·4 % (n 207) had 8 years of schooling or less and 65·7 % (n 293) did not have paid employment. Family income was low for most families – 79·6 % (n 344) had a monthly income less than three times the national minimum wage (approximately US$ 565·00/month).
More than half of the children were male (51·5 %, n 238) and 35·3 % (n 162) were non-white. The prevalence of overweight was 43·1 % (n 196), and the mean daily energy intake was 6289 (sd 1347) kJ 1503 (sd 322) kcal, which is higher than the requirement for this age (4874 kJ (1165 kcal)). Fe supplement use was recorded for 2·4 % (n 11) of the children. No difference was found for the micronutrients assessed (vitamin A, vitamin C, folate, Ca, Zn and Fe) and energy intake, neither for intervention nor control groups (P>0·05; data not show).
The mean values of vitamin A, vitamin C, folate, Ca, Zn and Fe intakes were higher than the requirement, as shown in Table 1. A low prevalence of inadequate micronutrient intake was observed for all micronutrients studied, especially Zn, whose intake was above the EAR (Table 1 and Fig. 2). Furthermore, 99·8 % (n 445) of the children presented a consumption above the RDA value for Zn, 92·4 % (n 412) for vitamin C, 78·7 % (n 351) for vitamin A, 71·3 % (n 318) for Ca, 70·2 % (n 313) for folate and 67·9 % (n 303) for Fe (Fig. 2).

Fig. 2 Percentage of children at 2–3 years of age with usual micronutrient intakes below the estimated average requirement (EAR, ![]() ) and above the RDA (
) and above the RDA (![]() ), in Porto Alegre, Brazil (n 446). EAR values: vitamin A (210 μg/d); vitamin C (13 mg/d); folate (120 μg/d); calcium (500 mg/d); iron (3 mg/d); zinc (2·5 mg/d). RDA values: vitamin A (300 μg/d); vitamin C (15 mg/d); folate (150 μg/d); calcium (700 mg/d); iron (7 mg/d); zinc (3 mg/d).
), in Porto Alegre, Brazil (n 446). EAR values: vitamin A (210 μg/d); vitamin C (13 mg/d); folate (120 μg/d); calcium (500 mg/d); iron (3 mg/d); zinc (2·5 mg/d). RDA values: vitamin A (300 μg/d); vitamin C (15 mg/d); folate (150 μg/d); calcium (700 mg/d); iron (7 mg/d); zinc (3 mg/d).
Table 1 Prevalence of inadequate micronutrient intake among children of low socio-economic status at 2–3 years of age in Porto Alegre, BrazilFootnote * (Mean values and standard deviations; medians, minimum and maximum intake values)
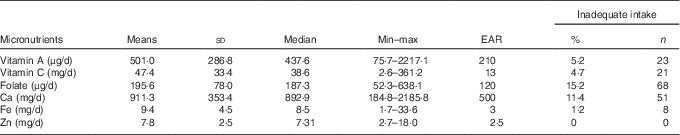
EAR, estimated average requirement.
* Prevalence of inadequate intake: the percentage of a group with usual intake less than the EAR.
Consumption of healthy foods and recommended servings per day, considering the mean consumption obtained by two 24-h recalls, are described in Table 2. Mean numbers of servings per day were below the minimum recommendations for healthy foods, except for beans (80·5 %). The proportions of children who consumed recommended servings of meats, dairy products and fruits were 35·4 % (n 158), 28·3 % (n 126) and 2·5 % (n 11), respectively. None of the children met the recommendations for vegetables.
Table 2 Healthy food groups and the proportion of children who consume such foods and meet the intake recommendations among children of low socio-economic status at 2–3 years of age in Porto Alegre, Brazil (Mean values and standard deviations; medians, minimum and maximum intake values; percentages and numbers)
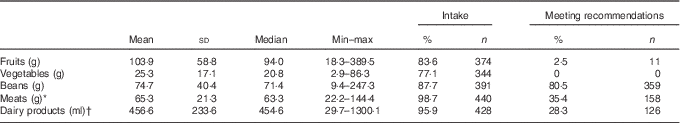
* Meats: beef, chicken, pork, poultry, fish.
† Dairy products: milk and yogurt.
The prevalence of children who consumed fortified foods was 88·1 % (n 393). The percentage of micronutrient intake provided by consuming ultra-processed foods was 38·3 (sd 33·5) for Fe, 20·4 (sd 28·3) for vitamin A, 19·7 (sd 11·8) for Ca, 17·2 (sd 31·4) for vitamin C, 14·6 (sd 19·3) for Zn and 11·3 (sd 22·1) for folate.
Estimates of adequate intake (% ≥EAR) or potentially excessive intake (% ≥UL), considering micronutrients derived from fortified foods, are described in Table 3. In total, 43·0 % (n 193) of the children met the EAR for Fe, 13·9 % (n 62) for vitamin C and 12·3 % (n 55) for Zn using fortified foods only. In addition, 4·0 % (n 18) of the children exceeded the UL for vitamin A, 3·1 % (n 14) for Zn, 1·1 % (n 5) for folic acid and 0·2 % (n 1) for Fe.
Table 3 Percentage of children at 2–3 years of age with usual micronutrient intakes above the estimated average requirement (EAR) and above the upper tolerable level (UL), considering only fortified foods, in Porto Alegre, Brazil (n 446)Footnote * (Percentages and numbers)
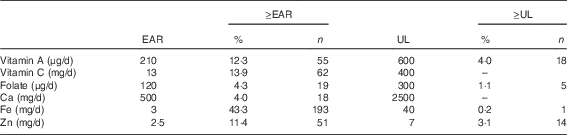
* Fortified foods=gelatin desserts, ready-to-eat cereal, soya juice, soya milk, powdered milk, powdered chocolate, baby cereal, petit suisse cheese.
Discussion
This study highlights a low prevalence of inadequate micronutrient intake in children as a result of decreased consumption of healthy foods and increased consumption of ultra-processed foods – the major contributors to micronutrient intake. These data are in accordance with the change in dietary patterns that has occurred in the last few years, which is displacing the traditional Brazilian diet that is based on wholesome foods and meals( Reference Levy, Claro and Mondini 41 ). The drastic decrease in the consumption of healthy foods( Reference Lock, Joceline and Louise 12 – Reference Wolfenden, Wyse and Campbell 14 ) and concomitant increase in the consumption of ultra-processed foods in developed and developing countries such as Brazil can explain this new paradigm( Reference Monteiro, Levy and Claro 42 – Reference Martins, Levy and Claro 45 ). Many ultra-processed foods are fortified with vitamins and minerals, particularly those targeted at children. To our knowledge, there are no studies, in our country, that have evaluated the impact of ultra-processed foods on micronutrient intake among children. However, studies in the US have shown that fortified foods are the main contributors to nutrient intake in the diets of US children and adolescents( Reference Berner, Keast and Bailey 46 , Reference Fulgoni, Keast and Bailey 47 ), especially ready-to-eat cereals( Reference Fulgoni and Buckley 48 ). These study results showed that, without fortification and supplementation, many Americans do not achieve the recommended micronutrient intake levels set forth by the dietary reference intakes. Mandatory fortification of flour with Fe and folic acid was implemented as national policy in Brazil since 2002( 49 ), although it did not impact on these micronutrient intakes among preschool children( Reference Assunção, Formoso and Barros 50 ).
The consumption of ultra-processed foods poses the dilemma of how to minimise the proportion of individuals whose intakes are below the requirements without increasing the risk of consuming amounts above the UL( Reference Bruins, Mugambi and Verkaik-Kloosterman 51 ). The bioavailability of micronutrients depends on food composition and possibly on its encapsulation method. For example, ingredients for cereal fortification recommended by the WHO include ferrous sulphate, ferrous fumarate, ferric pyrophosphate and electrolytic Fe powder, which have good bioavailability( Reference Allen, de Benoist and Dary 52 ). Furthermore, ultra-processed foods do not provide the same health benefits as natural foods( Reference Jacobs and Tapsell 53 ). Beyond the addition of synthetic micronutrients that can exceed the UL, ultra-processed foods are energy-dense, with high levels of fat, sugar and/or Na( Reference Monteiro and Cannon 16 ), contributing to the increased prevalence of obesity, whereas childhood malnutrition has declined dramatically( Reference Popkin 54 , Reference Batista Filho and Rissin 55 ).
Some potential limitations should be discussed to fully appreciate our results. First, our results cannot be generalised to large populations because we only included children from low-income groups. Second, the authors understand that the mean of two dietary recalls may not represent the entire distribution of usual intake due to the intra-individual variance component. Therefore, the dietary intake data have been corrected for inter- and intra-individual variability using the MSM method( Reference Harttig, Haubrock and Knüppel 34 , Reference Haubrock, Nöthlings and Volatier 35 ). Third, there are no national dietary reference intakes, and therefore we applied the American references of Institute of Medicine( 36 – 40 ), which could overestimate the recommended intake for Brazilian children. However, our results indicated low percentages of children below the EAR.
In conclusion, children of low-income families enrolled in this study did not meet the consumption recommendations for healthy foods. On the other hand, ultra-processed foods had a notable influence on the low prevalence of micronutrient intake. Therefore, developing countries with widespread micronutrient deficiencies should confront new dietary patterns as a challenge and work to prevent such deficiencies without running the risk of excessive intakes. Investigations including blood tests for micronutrient deficiencies should be planned to confirm our hypothesis.
Acknowledgements
We thank the healthcare centres, healthcare workers and families who participated in the study.
This research received no specific grant from any funding agency or from commercial or not-for-profit sectors.
C. N. S. contributed to the data analyses, interpretation of the results, drafting and critical revision of the manuscript; F. R. contributed to the drafting and critical revision of the manuscript; M. R. V. contributed to the research proposal, interpretation of the results, drafting and critical revision of the manuscript.
The authors declare that there are no conflicts of interest.








