Interest in carotenoids has increased in recent years because of their benefits to human health, specifically the association of higher intakes with lower risks for chronic diseases such as cancer, CVD, cataracts and age-related macular degeneration( Reference Kostic, White and Olson 1 – Reference Bohn 4 ). Lutein and zeaxanthin appear to protect the eye, and are associated with a lower risk of cataracts and macular degeneration. They are the main carotenoids present in the human macula and lenses( Reference Yeum, Booth and Sadowski 2 ), and lutein has recently been shown to be the main carotenoid present in the brain of infants( Reference Vishwanathan, Kuchan and Sen 5 ) and older adults( Reference Johnson, Vishwanathan and Johnson 6 , Reference Craft, Haitema and Garnett 7 ). This finding suggests potential roles of lutein in brain development and cognitive function. Humans cannot synthesise carotenoids, and thus, depend on regular dietary intake. Many vegetables are high in lutein and zeaxanthin, such as spinach, kale, greens, squash, maize and broccoli( 8 ).
With the growing awareness of the association between increased carotenoid intake and lower risks for chronic diseases, the absorption of lutein from the diet is an important determinant of its delivery and physiological action. Lutein is one of the predominant carotenoids in human serum, but factors other than dietary intake can affect its concentration. Various factors have been linked with the absorption of carotenoids. An epidemiological study conducted with 2786 adults found that race/ethnicity, education level and smoking have strong correlations with the serum lutein concentration( Reference Rock, Thornquist and Neuhouser 9 ). Other studies have found relationships of serum lutein or other carotenoid levels with alcohol consumption, seasonality and sex( Reference Forman, Beecher and Lanza 10 – Reference Stryker, Kaplan and Stein 12 ). In addition, various carotenoids such as lutein and β-carotene are known to affect the absorption and/or utilisation of each other in the human body( Reference Kostic, White and Olson 1 ). There are different diet components that influence the bioavailability and/or serum concentration of lutein such as dietary fat, fibre, food source and preparation methods( Reference Rock, Thornquist and Neuhouser 9 , Reference Riedl, Linseisen and Hoffmann 13 ). The bioavailability of β-carotene, lycopene and lutein is markedly reduced in the presence of different types of dietary fibre, and even differs substantially among vegetables (spinach, green beans and broccoli) that contain high amounts of carotenoids( Reference Riedl, Linseisen and Hoffmann 13 – Reference Van het Hof, Tijburg and Pietrzik 15 ). As lutein is a lipophilic carotenoid, the amount and type of dietary fat consumed with lutein can affect its absorption from the gastrointestinal tract( Reference Rock, Thornquist and Neuhouser 16 , Reference Van het Hof, West and Weststrate 17 ). Baskaran et al.( Reference Baskaran, Sugawara and Nagao 3 ) and others( Reference Lakshminarayana, Raju and Krishnakantha 18 – Reference Sugawara, Kushiro and Zhang 20 ) have shown in mice, rats and human Caco-2 cells that the use of glyco- and phospholipids may improve lutein bioavailability, thus suggesting that lutein absorption is dependent on the nature of lipids (polar, fatty acid profile, amount) when consumed together.
As lutein plays a significant role in maintaining and promoting overall health, its inclusion into commercially available multivitamin and mineral supplements is becoming commonplace. Many polar lipid nutrients that are typically included in nutritional supplements are generally less bioavailable than desired and, as such, they are generally over-fortified in the product to ensure that the plasma levels desired by the consumer to achieve the nutritional benefits can be obtained. In some cases, the over-fortification can be from about two to ten times the amount required to achieve the desired benefits. These high fortification rates lead to increased product costs without providing additional consumer benefits. Accordingly, there is a need for nutritional formulations having an improved bioavailability of lutein, so as to provide the same health benefits to consumers while allowing for lower fortification rates of the nutrient.
Currently the most frequently used commercially available form of lutein is a crystalline lutein/zeaxanthin mixture suspended in TAG-based oil. Preclinical testing to explore ways to increase lutein bioavailability led to the finding that the solubility of lutein could be significantly increased by mixing lutein in a specific combination of mono- and diglycerides (MDG) compared with standard TAG-based oils. Using a lymph fistula rat model of absorption, we were able to quantitatively show lutein absorption improvements using MDG as evident by significantly higher cumulative lutein absorption over the 6 h absorption period (AUC) v. lutein suspended in TAG oil( Reference Tso, Vurma and Lee 21 ). These data suggest that a mixture of MDG helps solubilise lutein and facilitate gastrointestinal micelle formation thus improving lymphatic lutein output compared with TAG oils.
A better understanding of the effects of combining MDG and lutein on lutein absorption and changes in plasma levels in healthy adults would be helpful when considering improvements in lutein delivery to target tissues. The primary objective of this study was to identify whether increases in plasma lutein levels could be achieved when lutein is combined with a mixture of MDG compared with TAG oil in healthy adults under controlled conditions.
Methods
Participants
Study subjects were required to have a BMI between 18 and 25 kg/m2, be male or a non-pregnant, non-lactating female practicing birth control during study period, and, if applicable, on stable dosages and regimens of any chronic medications for at least 2 months before enrolment. Prospective subjects were excluded if they were current smokers, were vegetarian or had very selective eating habits, drank more than two alcoholic drinks per d, were on medications or dietary supplements that affect lipid absorption or transport, were on a supplement containing carotenoids, were suffering from any contagious, infectious disease or chronic disease such as gastrointestinal disorders, hyperlipidaemia, diabetes, active malignancy, etc. Written informed consent was obtained from all subjects before study initiation, and study was approved by Quorum Review Institutional Review Board (protocol no. BL14/QR#27725).
Study design
This was a randomised, controlled, double-blind, cross-over, two-treatment study. As an eligible subject was enrolled, a sealed envelope containing a randomised treatment assignment prepared by the sponsor from computer-generated schedules by a pseudo-random block algorithm was opened by personnel at the clinical research centre to determine the subject’s treatment sequence. Subjects were assigned to one of two treatment sequence groups at the screening visit if all eligibility requirements were met. At this visit, height and body weight were measured, a urine pregnancy kit was administered, if applicable, medication and dietary supplement usage was documented and then randomised subjects were scheduled for their first study visit. If a subject was on a medication or supplement that affected plasma lipids or contained carotenoids, a wash-out period of at least 30 d was employed before the first treatment visit. A period of 30 d was selected to account for varying dosages of lutein subjects may be consuming and the unknown impact of lipid medication. In addition, there was an equilibration period with consumption of low-carotenoid foods for at least 14 d before the first treatment visit. A wash-out period of 14 d was chosen as the estimated half-life of lutein is approximately 5 d and at 14 d about 90 % of the lutein would be eliminated( Reference Thϋrmann, Schalch and Aebischer 22 ). A list of high-carotenoid foods to be avoided, such as spinach, kale, greens, pumpkin, carrots and moderate-level carotenoid foods, such as egg yolks, beans, peppers, with daily limited quantities was provided to subjects. The first treatment date was scheduled a minimum of 14 d and maximum of 35 d after the screening visit.
At each of the two treatment visits, subjects came in fasted (8–12 h), and were asked questions about any changes in health status (adverse event assessment), medications, dietary supplements and consumption of high-carotenoid foods since the last visit. A fasting baseline blood sample was drawn for a lipid panel (total cholesterol, TAG, HDL-cholesterol, and calculated LDL-cholesterol), plasma lutein, zeaxanthin, lycopene and β-carotene. The lipid panel was only conducted at baseline and 48 h postprandial, with values averaged. After the baseline sample, subjects consumed the test product with approximately 240 ml water, and start of product consumption was considered time zero. After study product consumption, subjects consumed a standardised breakfast that was low in carotenoids and contained a maximum of 30 % of total energy content as fat. Postprandial blood samples were drawn at 2, 4, 6, 8 and 12 h (± 5 min). During the visit, subjects also received a standardised low-carotenoid lunch and dinner with subjects staying at the centre during the 12 h visit. After the 12 h postprandial blood draw, subjects were sent home and given instructions to return to the centre the following morning after an 8–12 h fast and to only consume water, coffee or tea before the next blood draw at approximately 24 h. Before the 24 (sem 1) h postprandial draw, questions about any changes in health status, medications, dietary supplements and consumption of high-carotenoid foods were asked of subjects. Following the blood draw at this time point, subjects were sent home and provided instructions to return the next day for the 48-h postprandial blood draw after an 8–12 h fast. Standardised low-carotenoid foods were provided in a cooler to eat over the next day as only permitted foods were allowed until 48 h draw, along with water, coffee or tea. Subjects came into the centre fasted for the 48 (sem 1) h blood draw, questions about any changes in health status, medications, dietary supplements and consumption of high-carotenoid foods were asked of subjects, and the postprandial sample was drawn. All standardised meals during the first 48 h of each treatment were assessed by a dietitian and deemed to be low in carotenoids and containing ≤30 % energy content as fat. After the first treatment, subjects were scheduled to return to the centre at day 14 or 336 h postprandial (± 1 d) after an 8–12 h fast, and after consuming a low-carotenoid diet for the interim time period between study visits. The 336-h postprandial blood draw for the first treatment also served as the baseline blood draw for the second treatment visit. Subjects received a reminder phone call before their second treatment visit. All assessments and procedures from the first treatment visit and following 48 h were conducted at the second treatment visit. The subjects were exited from the study after they returned for their second 336 h postprandial blood draw. At this final study visit, any changes in health status, adverse events, medications, dietary supplements and consumption of high-carotenoid foods were documented. During the study, there were no documented deviations for consumption of high-carotenoid foods; and, additionally, each subject served as their own control with a cross-over study design, which helps eliminate individual eating behaviour differences.
Test products
There were two test articles investigated in this study; we compared a control formulation comprising of lutein/zeaxanthin (FloraGLO, 20 % suspension in safflower oil; Kemin Industries, Inc.) mixed in a carrier oil of high-oleic safflower oil (‘SAF’ formulation) to a novel formulation consisting of lutein/zeaxanthin mixed with a blend of 42 % monoglycerides and 47 % diglycerides derived from high-oleic SAF (Abitec) (‘MDG’ formulation). The test articles were filled into 1000 mg clear oblong bovine gelatin capsules by Captek Soft Gel International. Each formulation was designed to provide 3 mg of lutein, 0·25 mg zeaxanthin and approximately 985 mg of the respective carrier oil. Both test articles were assayed for lutein content and uniformity by HPLC analysis before initiation of the study, at 3 and 6 months and at the end of the study. The specifications were ≥3 mg of lutein per capsule. The range of tested values was 3·12–3·47 mg of lutein. A single dose was two capsules.
Carotenoid and lipid analyses
Baseline and postprandial blood samples (1–2 ml) were drawn from study subjects into 6 ml Na heparin tubes, gently inverted several times, centrifuged for 10 min at 1000–1300 g within 60 min of draw, and transferred to 2 ml cryovials. Samples were stored at –20°C at the clinical centre and then batch-shipped on dry ice to Craft Technologies, Inc.. Samples were stored at –70°C before being analysed. The carotenoid panel was conducted on all subject plasma samples as previously described( Reference Craft 23 ). The carotenoids are released from biological matrices by denaturing the protein and lipoproteins with ethanol. A quantity of 150 μl of plasma was diluted with 150 μl water containing EDTA and ascorbic acid. The samples were denatured with ethanol containing Tocol as an internal standard. The lipid soluble substances were extracted by vortex-mixing for 3 min with 1 ml of hexane containing BHT. After centrifugation, the hexane layer was transferred to a clean tube and the extraction repeated. The combined extract was evaporated in a centrifugal evaporator. The residue was dissolved in 30 μl ethyl acetate and diluted with 100 μl acetonitrile/isopropanol (90:10) before HPLC analysis. The carotenoids were separated by reverse phase HPLC based on their lipophilicity and detected at 450 nm visible. Tocol was monitored at 296 nm. Carotenoid concentration was calculated by comparison of peak areas of carotenoids in test samples with those of standards and adjusted for Tocol recovery. Plasma concentrations of lutein, zeaxanthin, cis-lutein/zeaxanthin, α-cryptoxanthin, β-cryptoxanthin, trans-lycopene, cis-lycopene, α-carotene, trans-β-carotene, cis-β-carotene, total lutein/zeaxanthin, total lycopene and total β-carotene were included in the full carotenoid panel of analytes. The lipid panel included total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG.
Analytes can be expressed using conventional units or international system (SI) units. The conversion factors from conventional units to SI units for the analytes presented are as follows: lutein and zeaxanthin, 1µg/dl=0·018µmol/l; cholesterol 1mg/dl=0·026mmol/l; and TAG 1mg/dl=0·011mmol/l.
Statistical analysis
Participants
In all, twenty-four healthy adult volunteers (18–45 years in age) were enrolled in the study. The standard deviation of the difference between lutein with β-carotene compared with lutein alone in previous research was used to calculate the variability for the sample size determination( Reference Kostic, White and Olson 1 ). The power calculation was derived from a decision that a 25 % difference in lutein absorption would be clinically significant.
A sample size of sixteen subjects with complete data for both treatments in the cross-over was calculated to have 90 % power to detect a difference in means of 14·9 µg/dl equal to a 25 % increase in lutein absorption, assuming a standard deviation of differences of 16·54 µg/dl, using a paired t test with a 0·05, two-sided significance level (nQuery Advisor 5.0 Software; Statistical Solutions Ltd). A sample of twenty-four subjects was randomised, 50 % males and females, to allow for attrition and to allow meaningful analyses of male and female subgroups.
Absorption of lutein and zeaxanthin were measured by AUC for plasma concentrations from 0 to 48 h and 0 to 336 h postprandially, adjusted for plasma concentration at baseline (baseline concentration was used at 0 h), peak value adjusted for baseline, time to peak, change and percent change in plasma concentration from 0 to 12 h and 0 to 48 h. The AUC was calculated by adding up the areas of trapezoids defined by time intervals and plasma concentrations at those time points (missing if missing plasma concentration at 2 or more points). The adjusted AUC was calculated by subtracting from the AUC the area of the rectangle defined by the baseline value and time duration. Peak value adjusted for the baseline was maximum plasma concentration during 0 to 336 h minus the plasma concentration at baseline (missing if missing plasma concentration at any time point). Time to peak was the first time to maximum plasma concentration during 0 to 336 h. Each continuous variable was analysed using parametric or non-parametric, if declared non-normal, two-treatment, two-period cross-over analysis. Parametric analyses were carried out using three factors, treatment sequence, period and treatment repeated measures ANOVA with compound symmetry covariance structure and Kenward–Roger df. The residuals from the parametric analysis were utilised to check for deviation from normality by Shapiro–Wilk test. A variable was declared non-normal if a P value of the Shapiro–Wilk test was <0·01. For the variables declared non-normal, non-parametric analyses were done to compare two treatment sequences for the sum and difference of two periods for sequence and treatment effects respectively using Wilcoxon’s rank sum test. Sequence effect was declared statistically significant if a P value of an analysis was <0·10. If sequence effect was statistically significant, only period one data was used to test for treatment effect using one factor ANOVA or the Wilcoxon rank sum test. Mean values (or least squares mean) with their standard errors or medians and 25th and 75th percentiles were reported when parametric or non-parametric analyses, respectively were done. The categorical safety variables were analysed using McNemar’s test with exact P value calculation. The hypothesis tests were completed using two-sided α of 0·05 for each test. Statistical software SAS release 9.2 and 9.4 (SAS Institute Inc.) were used for the analyses.
Results
Participants
In all, thirty-five individuals were screened for enrolment into the study. Of the eleven persons not enrolled, one withdrew consent, two were current smokers, two did not meet BMI criteria, and six were extra individuals who were not enrolled once the cells for males and females were filled (Fig. 1). A total of twenty-four subjects (twelve males and twelve females) were randomised, with their enrolment characteristics shown in Table 1. The subjects had a mean age of 29·3 (sem 1·8) years with a mean BMI of 22·4 (sem 0·4) kg/m2. Before the 1st day of treatment (baseline) subjects had mean total plasma lutein and zeaxanthin concentrations of 11·7 (sem 1·6) and 3·4 (sem 0·3) µg/dl, respectively, showing a mean lutein to zeaxanthin ratio of 3·4:1. A total of twenty-three subjects completed the study while one subject suffered an adverse event not related to product intake that resulted in a premature study exit. The adverse event was an injury resulting in a ligament sprain, which resulted in the subject completing only the MDG treatment. There were no serious adverse events reported during the study period. In addition to the ligament injury, five mild to moderate adverse events were recorded. One adverse event in the SAF group was a vasovagal reaction to catheter insertion, four adverse events in the MDG group were two with headache, one with nausea, and one subject with fluid retention, with only one headache event and nausea documented as possibly related to study product. No statistical differences were observed in adverse events between the study treatments or overall safety concerns.
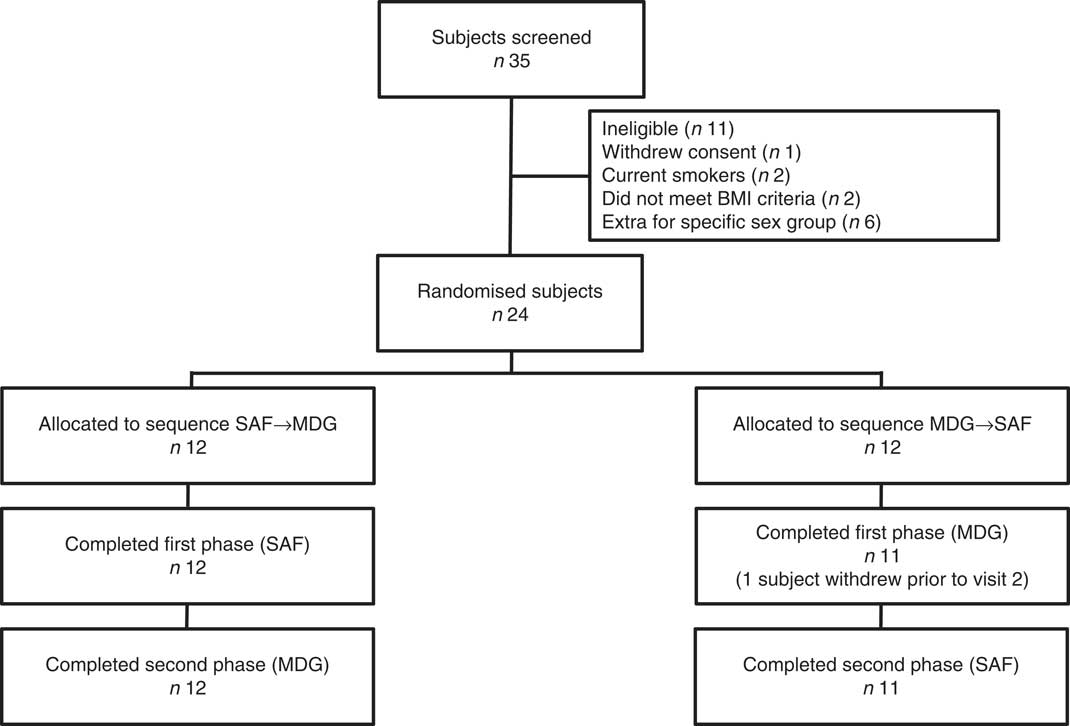
Fig. 1 Consolidated Standards of Reporting Trials (CONSORT) flow diagram. SAF, safflower; MDG, mono- and diglycerides.
Table 1 Characteristics of subjects at enrolment* (Numbers and percentages; mean values with their standard errors)
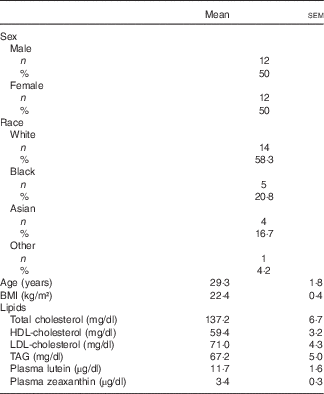
* Analytes can be expressed using conventional units or international system (SI) units. The conversion factors from conventional units to SI units for the analytes presented are as follows: lutein and zeaxanthin, 1 µg/dl = 0·018 µmol/l; cholesterol 1 mg/dl = 0·026 mmol/l; and TAG 1 mg/dl = 0·011 mmol/l.
Absorption of lutein
All results presented were based on analysis of untransformed intent-to-treat data for periods 1 and 2 combined unless otherwise noted. All reported/calculated values for lutein and zeaxanthin represent trans-lutein and trans-zeaxanthin concentrations (Table 2).
Table 2 Lutein and zeaxanthin absorption kinetics† (Least squares means with their standard error (parametric); medians and 25th, 75th percentiles (non-parametric))
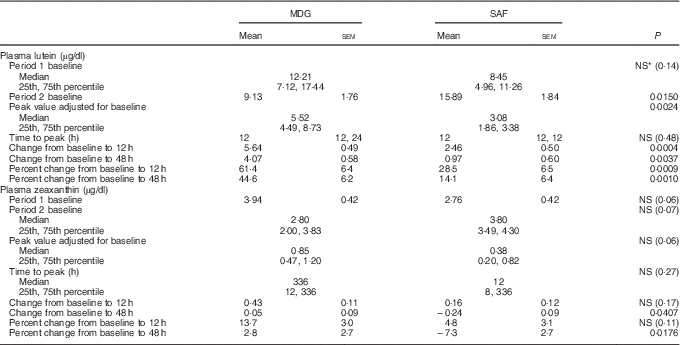
MDG, mono- and diglyceride; SAF, safflower.
† Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for lutein and zeaxanthin is as follows: 1 µg/dl = 0·018 µmol/l.
The two treatment groups for baseline lutein were not significantly different at period 1 (P=0·14) but significantly different at period 2 (P=0·0150, SAF>MDG). Baseline zeaxanthin was not significantly different at period 1 (P=0·06) or period 2 (P=0·07). For the primary study variable, adjusted AUC for plasma lutein from 0 to 336 h, MDG was significantly greater than SAF (P=0·0002, median: SAF=108, MDG=1080, a 900 % increase from SAF to MDG in µg/dl over 336 h) (Fig. 2(a)). In addition, adjusted AUC for the first 48 h (0–48 h) for plasma lutein was significantly higher for subjects given MDG (199 (sem 20) µg×h/dl) compared with controls (60 (sem 21) µg×h/dl) (P=0·0002; 232 % increase) (Fig. 2(b)). Though there was no difference between the MDG and SAF groups for the time to reach peak plasma lutein (12 h), the adjusted peak lutein value for MDG was significantly greater than SAF (P=0·0024, median: SAF=3·08 µg/dl, MDG=5·52 µg/dl, a 79 % increase from SAF to MDG) (Table 2). There were also significant differences between MDG and SAF adjusted plasma lutein absorption at 4, 6, 8, 12, 24, 48 and 336 h postprandial as shown in Fig. 3.
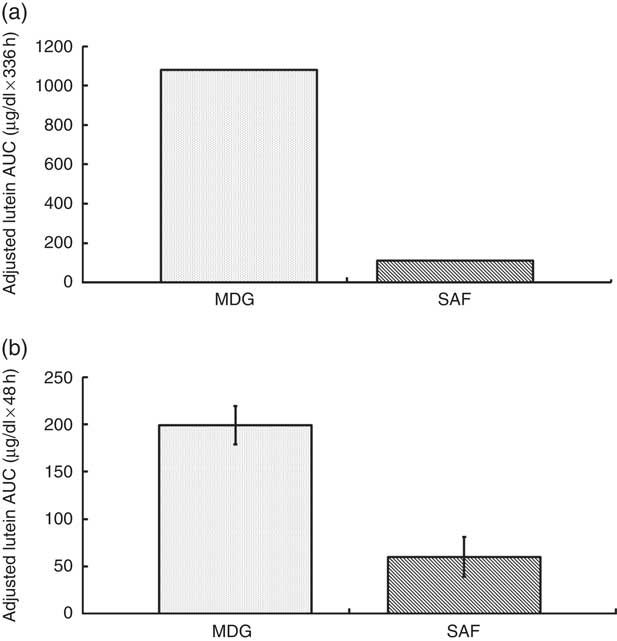
Fig. 2 Adjusted AUC for plasma lutein concentrations (µg/dl) for subjects receiving single-dose mono- and diglyceride (MDG) or safflower (SAF) capsules. (a) Adjusted AUC for plasma lutein from 0 to 336 h, MDG was significantly greater than SAF (P=0·0002, values are medians, non-parametric (25th and 75th percentiles) with SAF=108 (−338; 596) and MDG=1080 (506; 1682), a 900 % increase from SAF to MDG in µg/dl over 336 h). (b) Adjusted AUC for plasma lutein from 0 to 48 h was significantly higher for subjects given MDG (199 (sem 20) µg×h/dl) compared with SAF (60 (sem 21) µg×h/dl) (P=0·0002; 232% increase). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for lutein is as follows: 1 µg/dl=0·018 µmol/l.
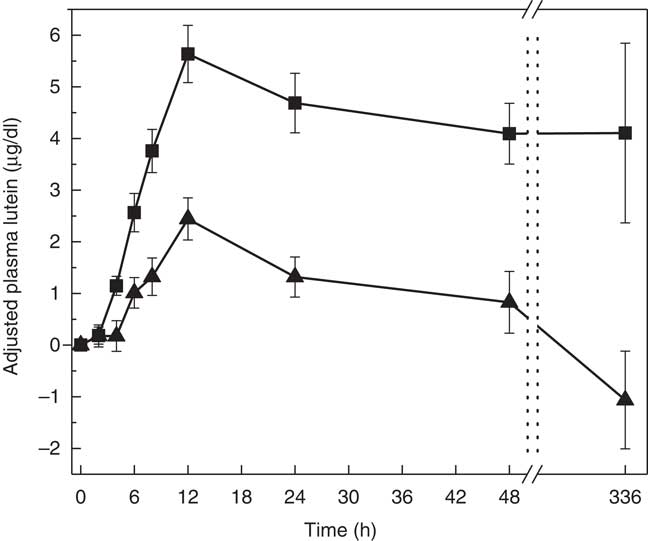
Fig. 3 Adjusted mean plasma total lutein concentrations (µg/dl) for subjects receiving single-dose mono- and diglyceride (MDG, ![]() ) or safflower (SAF,
) or safflower (SAF, ![]() ) capsules over 336 h. Significant differences between MDG and SAF adjusted plasma lutein absorption at 4, 6, 8, 12, 24, 48 and 336 h postprandial (P<0·01 for all time points). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for lutein is as follows: 1 µg/dl=0·018 µmol/l.
) capsules over 336 h. Significant differences between MDG and SAF adjusted plasma lutein absorption at 4, 6, 8, 12, 24, 48 and 336 h postprandial (P<0·01 for all time points). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for lutein is as follows: 1 µg/dl=0·018 µmol/l.
Absorption of zeaxanthin
The pattern of zeaxanthin absorption was similar to lutein (though only the change from baseline to 48 h was statistically significant) as evident by similar changes in plasma levels over the 12, 48 and 336 h study periods. Adjusted AUC for plasma zeaxanthin from 0 to 336 h showed a higher trend for MDG subjects (35·3 (sem 64·2) µg×h/dl) v. SAF (−12·1 (sem 61·5) µg×h/dl) (P=0·06) (Fig. 4(a)). Adjusted AUC for the first 48 h for plasma zeaxanthin showed a higher trend given MDG (8·9 (sem 3·3) µg×h/dl) compared with SAF (−2·5 (sem 3·4) µg×h/dl) (P= 0·051) (Fig. 4(b)). Similar to the changes in plasma lutein, there was no difference between the MDG and SAF groups for the time to reach peak plasma zeaxanthin at approximately 12 h (Fig. 5, Table 2). Postprandial plasma zeaxanthin levels showed significantly higher concentrations at 48 h for MDG subjects v. SAF (Fig. 5). Beginning at 24 h and continuing through 336 h following zeaxanthin administration, the mean plasma zeaxanthin levels for the SAF group decreased to lower levels than those observed at enrolment. Mean plasma zeaxanthin levels for the MDG group remained higher than baseline throughout the 336 h observational period.
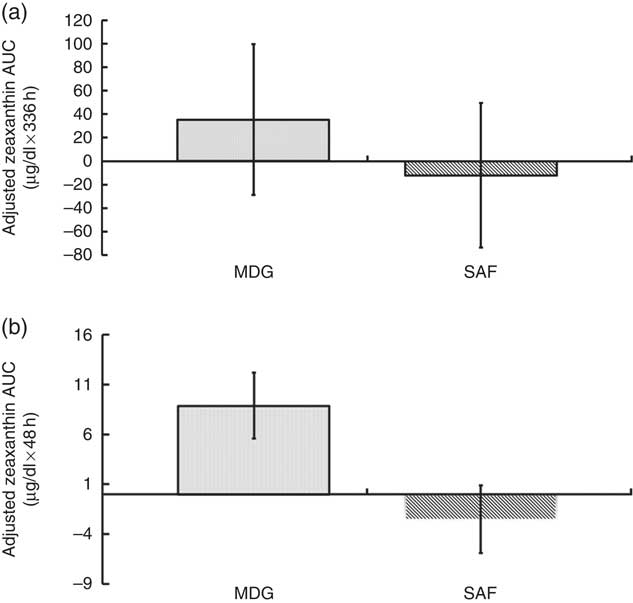
Fig. 4 Adjusted AUC for plasma zeaxanthin concentrations (µg/dl) for subjects receiving single-dose mono- and diglyceride (MDG) or safflower (SAF) capsules. (a) Adjusted AUC for plasma zeaxanthin from 0 to 336 h showed a higher trend for MDG subjects (35·3 (sem 64·2) µg×h/dl) v. SAF (−12·1 (sem 61·5) µg×h/dl) (P=0·06). (b) Adjusted AUC for plasma zeaxanthin from 0 to 48 h showed a trend for subjects given MDG (8·9 (sem 3·3) µg×h/dl) compared with SAF (−2·5 (sem 3·4) µg×h/dl) (P= 0·051). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for zeaxanthin is as follows: 1 µg/dl=0·018 µmol/l.
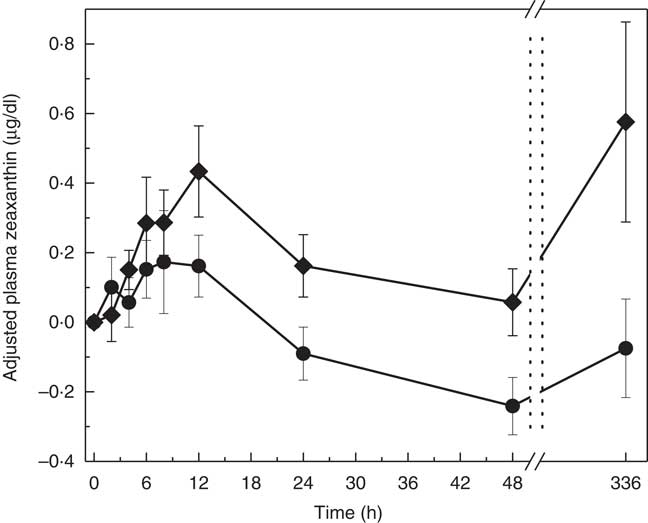
Fig. 5 Adjusted mean plasma total zeaxanthin concentrations (µg/dl) for subjects receiving single-dose mono- and diglyceride (MDG, ![]() ) or safflower (SAF,
) or safflower (SAF, ![]() ) capsules over 336 h. Postprandial plasma zeaxanthin levels showed significantly higher concentrations at 48 h for MDG subjects v. SAF (P=0·0407). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for zeaxanthin is as follows: 1 µg/dl=0·018 µmol/l.
) capsules over 336 h. Postprandial plasma zeaxanthin levels showed significantly higher concentrations at 48 h for MDG subjects v. SAF (P=0·0407). Values are means with their standard errors. Analytes can be expressed using conventional units or international system (SI) units. The conversion factor from conventional units to SI units for zeaxanthin is as follows: 1 µg/dl=0·018 µmol/l.
The absorption of zeaxanthin showed differences between the two treatments at 48 h postprandial with the adjusted plasma value for MDG greater than SAF (P=0·041). (Note: total lycopene (adjusted peak value): MDG>SAF, P=0·0231 (period 1 only), total β-carotene µg/dl (adjusted value at 24 h): MDG>SAF, P=0·0439.)
Sex
Treatment×sex interaction was not tested because the study was not designed to have sufficient statistical power to detect such an interaction. However, the adjusted AUC values (0 to 48 hrs, 0 to 336 h) for plasma lutein and plasma zeaxanthin were analysed within male and female subgroups. The plasma lutein results within male and within female were statistically significant (P<0·05, MDG>SAF) and the plasma zeaxanthin results within male and within female were not significant (P>0·05). These results are concordant with the combined sex results.
Adjustment for plasma lipids
AUC values for lutein and zeaxanthin were adjusted for plasma levels of these carotenoids at time 0 on each test day. Differences in plasma lipoprotein lipid parameters (total cholesterol, HDL-cholesterol, LDL-cholesterol and TAG) from 0 to 48 h within test conditions, and between the two test conditions at 0 and 48 h, were not statistically significant, nor clinically meaningful. Accordingly, no adjustment of plasma carotenoid levels for circulation cholesterol content was performed.
Discussion
One goal of this study was to gain a better understanding of how a new formulation technology, based on facilitating lutein solubility and gastrointestinal micelle formation, affected lutein absorption compared with a standard lutein supplement suspended in TAG oil. To assess this, this proof-of-principle cross-over study in healthy adults was performed to compare the effect of two different delivery vehicles on the bioavailability of lutein and zeaxanthin after a single oral dose. Subjects consumed capsules containing a total of 6 mg lutein in either SAF or MDG oils and changes in plasma levels of lutein over a 336 h period were observed.
The results showed that a single dose of MDG lutein resulted in a 129 % increase in plasma lutein within the first 12 h compared with control. This increase in plasma lutein was maintained throughout the 48 and 336 h monitoring periods, showing significant increases in adjusted AUC (0–48 h) and adjusted AUC (0–336 h). The significantly higher adjusted AUC throughout the study period demonstrate improvements in lutein absorption with MDG over TAG oil.
Additional analysis of the 48 h absorption kinetics of lutein showed that the mean adjusted maximum concentration of lutein was significantly higher with the MDG group compared with SAF. The time to reach maximum lutein concentration was the same for both groups indicating that the use of MDG did not change the normal pattern of lutein absorption. However, the MDG group showed a more rapid rise in plasma lutein as compared with control. Subjects given the MDG lutein had a higher and more sustained plasma lutein level which did not approach baseline lutein concentrations from 48 to 336 h. This sustained elevated plasma lutein during the later phase of the study may be due to a combination of continued absorption of intestinal bound lutein (binding sites along the small bowel mucosal cells were saturated with lutein)( Reference Vurma, DeMichele and Lee 24 ) and increased hepatic secretion.
Lutein is commercially available as a mixture of 90 % lutein and 10 % zeaxanthin. This gave an opportunity for the first time to assess the change in absorption pattern of zeaxanthin when combined with MDG. The pattern of zeaxanthin absorption was similar to lutein as evident by similar changes in plasma levels over the 12, 48 and 336 h study periods. The magnitude of these changes were much lower due to the lower dose of zeaxanthin that was administered. The similar bioavailability patterns of lutein and zeaxanthin is expected given the similar polarities and chemical nature of the two carotenoids. The assessment of zeaxanthin absorption is helpful as the literature typically reports results from combined analyses of lutein and zeaxanthin. Of note, the mean plasma zeaxanthin levels, for the SAF group only, went below enrolment concentrations from 24 h through 336 h. This finding was also observed by Evans et al.( Reference Evans, Beck and Elliott 25 ) during their bioavailability assessment of two different lutein formulations in healthy subjects.
The observed improvements in both lutein and zeaxanthin bioavailability may be explained in part from recent preclinical research assessing the mechanism of action of the use of MDG for absorption enhancing properties( Reference Tso, Vurma and Lee 21 , Reference Vurma, DeMichele and Lee 24 , Reference Vurma, DeMichele and Lee 26 ). Our next step was to identify a vehicle that would have properties to increase lutein absorption when equal amounts of lutein are delivered to the stomach. Preclinical testing led to the finding that the solubility of lutein could be significantly increased by mixing lutein with a combination of MDG and the amount of crystalline lutein needed could be reduced( Reference Tso, Vurma and Lee 21 , Reference Vurma, DeMichele and Lee 26 ).
Currently, the most frequently used commercially available form of lutein is a crystalline lutein/zeaxanthin mixture suspended in a TAG-based oil. The results from the current study concur with those from previous studies showing that lutein in its crystalline form is poorly absorbed, even in the presence of long-chain TAG oils( Reference Tso, Vurma and Lee 21 , Reference Vurma, DeMichele and Lee 24 , Reference Vurma, DeMichele and Lee 26 ). In addition to lutein solubility improvements, preliminary in vivo research has shown the ability of MDG to improve micellization of lutein and zeaxanthin before being transferred to and absorbed by small bowel enterocytes. The combination of lutein and MDG also aid in the formation and secretion of TAG and carotenoid rich chylomicrons into lymph( Reference Vurma, DeMichele and Lee 24 ). These combined benefits of MDG may explain the observed initial 48 h absorption increases v. lutein in TAG oil.
Interestingly, preclinical work has shown that lutein is not transported to the liver during the absorptive phase, as non-detectable levels of lutein in portal blood were found for a range of doses of lutein infused. This suggests that the transport of lutein from the gastrointestinal tract is via chylomicron particles, generated after small bowel digestion. In addition, in vivo studies by Vurma et al.( Reference Vurma, DeMichele and Lee 24 ) have shown that gastrointestinal mucosal bound lutein and zeaxanthin were higher following lutein/MDG infusions compared with lutein in TAG oil. The mucosal bound lutein will eventually be packaged into chylomicrons for absorption, thus resulting in a sustained absorption of lutein. This finding may explain why the plasma lutein and zeaxanthin levels remained higher in the MDG group during the later post-absorptive phase (48–336 h) of the study.
The use of a specific combination of MDG is a different approach from the previous known ways to increase lutein absorption. As lutein is a lipophilic carotenoid, the amount and type of dietary fat consumed with lutein can affect its absorption from the gastrointestinal tract( Reference Rock, Thornquist and Neuhouser 16 , Reference Van het Hof, West and Weststrate 17 ). Several researchers have shown in mice, rats and human Caco-2 cells that the use of glyco- and phospholipids may improve lutein bioavailability thus suggesting that lutein absorption is dependent on the nature of lipids (polar, fatty acid profile, amount) when consumed together( Reference Baskaran, Sugawara and Nagao 3 , Reference Lakshminarayana, Raju and Krishnakantha 18 – Reference Sugawara, Kushiro and Zhang 20 ). Serum lutein levels are higher following egg consumption compared with a comparable amount of green vegetables further suggesting that lutein absorption is complex and dependent on the amount ingested, the matrix in which it is contained and the type and amount of lipid present( Reference Yeum, Booth and Sadowski 2 , Reference Handelman, Nightinggale and Lichtenstein 27 – Reference Surai, MacPherson and Speake 29 ). However, previous research utilised a change in plasma lutein as a measure of intestinal absorption, and may have limitations for interpretation given the role of the liver in recirculating lutein after incorporation with lipoproteins. The approach here is based upon a direct measure of intestinal absorption.
The results presented provide a better understanding towards the importance of assessing how formulation changes affect the bioavailability and kinetics of lutein. As lutein plays a significant role in maintaining and promoting overall health, its inclusion into commercially available multivitamin and mineral supplements is becoming commonplace. Many polar lipid nutrients that are typically included in nutritional products are generally less bioavailable upon consumption than desired and, as such, they may be over-fortified in the nutritional product to ensure that the consumer achieves the desired nutritional benefits. These data provide an opportunity for improved nutritional formulations containing lutein/MDG.
The present study has potential limitations. The baseline level of plasma lutein was elevated in the second treatment period in the SAF group that was initially randomised to MDG It was not anticipated that the plasma levels of lutein in subjects given MDG and lutein would not return to baseline levels as those in the SAF group. One possible explanation is that preclinical studies suggest that there may be a continued absorption of lutein due to the higher amounts of lutein bound to the gastrointestinal mucosa waiting to be transported via chylomicron formation. Though this finding was statistically significant, it did not impact the results or its interpretation of lutein changes during the second period. As the timing of the initial lutein dose and its subsequent dose was 14 d later, we do not feel that there would have been any residual gut related issues to affect lutein absorption after such a period of time. As we performed this research using a single dose of lutein (6 mg), possible next steps would include assessing lutein/zeaxanthin absorption using doses lower and higher than 6 mg, as well as multiple dosing regimens to fully determine the scope of MDG’s effect on absorption. Future experiments could assess the absorption kinetics of other fat soluble nutrients (vitamin D, tocopherol), with other competing carotenoids and in combination with a mixed meal. Verification of these absorption benefits would need to be demonstrated when combinations are incorporated into complex nutritional supplement products.
In summary, the data show that a single dose of lutein in MDG oil resulted in significant increases in lutein absorption over the early and later absorptive course of the study compared with lutein in TAG oil. The most probable explanations for this absorption benefits come from data generated from preclinical studies showing that the use of specific mixtures of MDG helps solubilise lutein/zeaxanthin and facilitate gastrointestinal micelle formation, thus improving their lymphatic output compared with TAG oils. Next steps are to assess whether the absorptive benefits of lutein/MDG can lead to increased tissue uptake of these important carotenoids. This information should be useful for designing clinical studies to assess the effectiveness of lutein in MDG oil on some of lutein’s documented physiological benefits such those related to eye and brain health, as well as antioxidant properties.
Acknowledgements
The authors gratefully acknowledge the volunteers who participated in this study.
Funding support for this study was provided by Abbott Nutrition.
B. J. M., J. A. W., M. V. and S. J. D. contributed to the design of the study. B. J. M., J. A. W. and K. C. M. executed the study. Y. S. C. provided statistical support. All authors contributed to the writing of the article.
B. J. M., J. A. W., Y. S. C., M. V and S. J. D. are employees of Abbott Nutrition. K. C. M. is the Chief Science Officer for Midwest Biomedical Research.










