One of the characteristics of the Japanese diet is a high consumption of mushrooms(Reference Nanri, Shimazu and Ishihara1). Mushrooms are rich in dietary fibre, the intake of which has been associated with a lower risk of dementia(Reference Yamagishi, Maruyama and Ikeda2). However, so far, only one Japanese cohort study has reported an association between mushroom intake and the risk of dementia, and they found an inverse association only among women(Reference Zhang, Tomata and Sugiyama3). In addition, several cross-sectional studies reported that mushroom intake was associated with improved cognitive performance(Reference Mori, Inatomi and Ouchi4–Reference Nurk, Refsum and Drevon7). However, the reasons for the sex-related discrepancy of association remain unclear. Although different types of dementia (including Alzheimer’s disease and vascular dementia) are assumed to have different mechanisms and risk factors, analyses based on a classification of dementia types have not yet been conducted.
The Circulatory Risk in Communities Study (CIRCS) conducted a 24-h recall dietary survey in several communities in Japan and has been followed up to assess the risk of incident dementia and stroke. In the present study, we sought to investigate whether mushroom intake is inversely associated with the risks of incident disabling dementia and dementia with/without a history of stroke among Japanese men and women.
Methods
Design and study population
CIRCS is an ongoing dynamic community-based prospective study involving five communities in Japan. Details of the CIRCS protocol have been described elsewhere(Reference Yamagishi, Muraki and Kubota8). In the present study, cases of incident disabling dementia were surveyed in three of those five communities: Ikawa (a rural town in Akita Prefecture in northeastern Japan), Kyowa (a rural town in Ibaraki Prefecture in mid-eastern Japan) and Yao (a city in Osaka Prefecture in mid-western Japan).
We performed a prospective study involving 3750 people aged 40–64 years who lived in these three communities and participated in an annual cardiovascular risk survey from 1985 to 1999. Cases of incident disabling dementia were surveyed from 1999 to 2020 (except for the period from April 2005 to April 2008, when the data were unavailable) in Kyowa, from 1999 to 2019 in Ikawa and from 2006 to 2019 in Yao. We did not collect information on the history of dementia during the baseline survey when the participants were generally healthy and could answer questions in the dietary interview. Instead, we excluded those who developed disabling dementia within 5 years of the dietary survey (n 2). Persons with invalid dietary data were also excluded (n 9). This resulted in a total study population of 3739 people (1650 men and 2089 women).
Dietary survey
We conducted a 24-h recall dietary survey which investigated how much the participants had eaten during the 24 h prior to the interview conducted by well-trained dietitians. We recorded the foods consumed and their amounts and then calculated the energy intake and the nutrient intake on the basis of the Standard Tables of Food Composition in Japan, 7th revised edition(9). The major foods recorded as mushrooms were shiitake (Lentinula edodes), shimeji (Hypsizygus tessulatus), enokitake (Flammulina filiformis) and nameko (Pholiota microspora) mushrooms.
Other risk factors
The measurement of potential risk factors for dementia was conducted at the time of the dietary survey, and these factors have been described in a prior publication(Reference Aoki, Yamagishi and Maruyama10). The participants rested for at least 5 min and then had their arterial systolic pressure and fifth-phase diastolic blood pressure measured. Well-trained study physicians or nurses conducted blood pressure measurements using standard mercury sphygmomanometers on the right arm of the participants. The physician conducted the measurement again when the first reading showed a systolic blood pressure greater than or equal to 140 mmHg and/or a diastolic blood pressure greater than or equal to 90 mmHg. In such cases, the second reading was used in the analysis; otherwise, the first reading was used. We measured the height and weight of participants wearing light clothing and with their shoes off and calculated the BMI (weight in kilograms divided by height in metres squared). We collected information on smoking status (never, former or current), number of cigarettes smoked per d, alcohol drinking status (never, former or current), the amount of alcohol consumption per d, prescriptions for antihypertensives, cholesterol-lowering and diabetes drugs, and history of a stroke through face-to-face interviews. We measured serum glucose and total cholesterol without a fasting requirement. The definition of diabetes mellitus was a fasting serum glucose level greater than or equal to 126 mg/dl or a non-fasting serum glucose level greater than or equal to 200 mg/dl and/or being on medication for diabetes.
Definition of disabling dementia
The outcome of the present study was dementia requiring care (‘disabling dementia’), which was based on the National Long-Term Care Insurance System. This insurance system is compulsory for all individuals aged 40 years or more in Japan(Reference Tamiya, Noguchi and Nishi11). Physicians identified disabling dementia according to the physicians’ manual issued by the Ministry of Health, Labour and Welfare of Japan(12). We regarded disabling dementia as the following two conditions: (1) certified for disability under the long-term care insurance programme and (2) with a grade of activities of daily living related to dementia above the class of IIa (i.e. some symptoms/behaviours or communication difficulties that interfere with daily life and need someone’s assistance to live by themselves)(Reference Ikeda, Yamagishi and Tanigawa13). These criteria have been previously validated against the diagnosis by certified psychiatrists with high specificity (96 %) and moderate sensitivity (73 %)(Reference Noda, Yamagishi and Ikeda14), and the grade of activities of daily living related to dementia was found to be highly correlated with the Mini-Mental State Examination score(Reference Hisano15). We did not possess the data on the specific types of dementia (i.e. Alzheimer’s disease and vascular dementia). Instead, we classified disabling dementia into two subtypes: with or without a history of stroke based on a systematic stroke registration during the follow-up period for disabling dementia and the self-reported history of stroke at the time of the dietary survey. The definition of stroke in our systematic registration was a rapid-onset focal neurological disorder persisting for more than 24 h or until death. In the present study, we identified 90 % of stroke occurrences using standardised criteria based on computed tomography or MRI(Reference Iso, Rexrode and Hennekens16), while the remainder were identified using previously reported clinical criteria for diagnosis without imaging(Reference Shimamoto, Komachi and Inada17,Reference Imano, Iso and Kiyama18) . Due to the availability of stroke registry data, the stroke subtype analysis was conducted using the follow-up data until 31 December 2015 for Kyowa, and until 31 December 2018 for Ikawa and Yao.
Statistical analysis
We classified participants into three groups according to mushroom intake per d: no intake (0 g/d), intake below the median (0·1–14·9 g/d) and intake above the median (≥ 15·0 g/d) on the basis of the dietary survey conducted from 1985 to 1999. Age- and sex-adjusted means and percentages of characteristics of participants at the time of the dietary survey were compared between groups. Follow-up started from 1999 (2006 for Yao) and ended at the time of diagnosis of incident dementia, move out to a different community, death or end of follow-up, whichever came first. We calculated hazard ratios (HR) and 95 % CI for incident total dementia and dementia with/without a history of stroke for the two groups of participants with some mushroom intake (mushroom intake between 0·1 and 14·9 g/d, and mushroom intake greater than 15·0 g/d) compared with the group with no mushroom intake using Cox proportional hazard models sex-specifically. The proportional hazards assumption was tested using the interaction term of time by mushroom intake and was not violated for each outcome. We adjusted for age (continuous) and nested by communities in model 1. We further adjusted for energy intake (continuous), smoking status, drinking status, intakes of vegetables (quartile), fruits (quartile), fish (quartile), meat (quartile) and sodium (quartile) in model 2. The soluble fibre intake (quartile) was further adjusted in model 3, and the insoluble fibre intake (quartile) in model 4. We evaluated multiplicative interaction between men and women. The statistical significance of multiplicative interactions of HR were calculated using the Wald test. Analyses were conducted using SAS 9.4 (SAS Institute). All probability values for the statistical tests were two-tailed, and probability values below 0·05 were considered significant.
Results
During 16·0 years of average follow-up in 3739 participants, a total of 670 people (260 men and 410 women) developed disabling dementia. The incidence rates per 1000 person-years were 10·2 in men and 12·0 in women, respectively.
Table 1 shows the age- and sex-adjusted baseline characteristics according to mushroom intake. The proportion of women, being prescribed cholesterol-lowering medication, the mean value of diastolic blood pressure, and intakes of total fat, protein, total fibre, vegetables, meat and sodium were positively correlated with mushroom intake (Table 1).
Table 1. Characteristics of participants according to mushrooms intake at dietary surveys

Otherwise adjusted for age and sex.
* Adjusted for sex.
† Adjusted for age.
For women, mushroom intake was inversely associated with the risk of total dementia and dementia without a history of stroke, but not with dementia with a history of stroke (Table 2). Among women, the multivariable HR (95 % CI) in model 2 for total dementia for those with mushroom intake between 0·1 and 14·9 g/d and those with mushroom intake greater than or equal to 15·0 g/d were 0·81 (0·62, 1·06) and 0·56 (0·42, 0·75) (P for trend = 0·003), respectively, while the multivariable HR (95 % CI) for dementia without a history of stroke were 0·66 (0·47, 0·93) and 0·55 (0·38, 0·79) (P for trend = 0·01), respectively, compared with those with no mushroom intake. The multivariable HR (95 % CI) per 10 g/d increment of mushroom intake were 0·89 (0·82, 0·96) for total dementia and 0·88 (0·80, 0·98) for dementia without a history of stroke. However, we did not observe any associations between mushroom intake and the risk of disabling dementia in men. Further adjustment for intakes of soluble and insoluble fibres slightly attenuated the associations of mushroom intake with the risk of total dementia and the risk of dementia without a history of stroke in women, but we still observed significant associations. We adjusted further for hypertension (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, and/or taking antihypertensive medication), diabetes mellitus (fasting glucose ≥ 126 mg/dl or non-fasting glucose ≥ 200 mg/dl and/or taking medication) and obesity (BMI ≥ 25 kg/m2) which could mediate the mushroom–dementia association, but the results did not change. The multivariable HR of total dementia for mushroom intake between 0·1 and 14·9 g/d and mushroom intake greater than or equal to 15·0 g/d were 0·85 (0·65, 1·12) and 0·58 (0·43, 0·78), respectively, in women compared with no mushroom intake (P for trend = 0·006).
Table 2. Multivariable hazard ratios and 95 % CI of incident dementia according to mushrooms intake
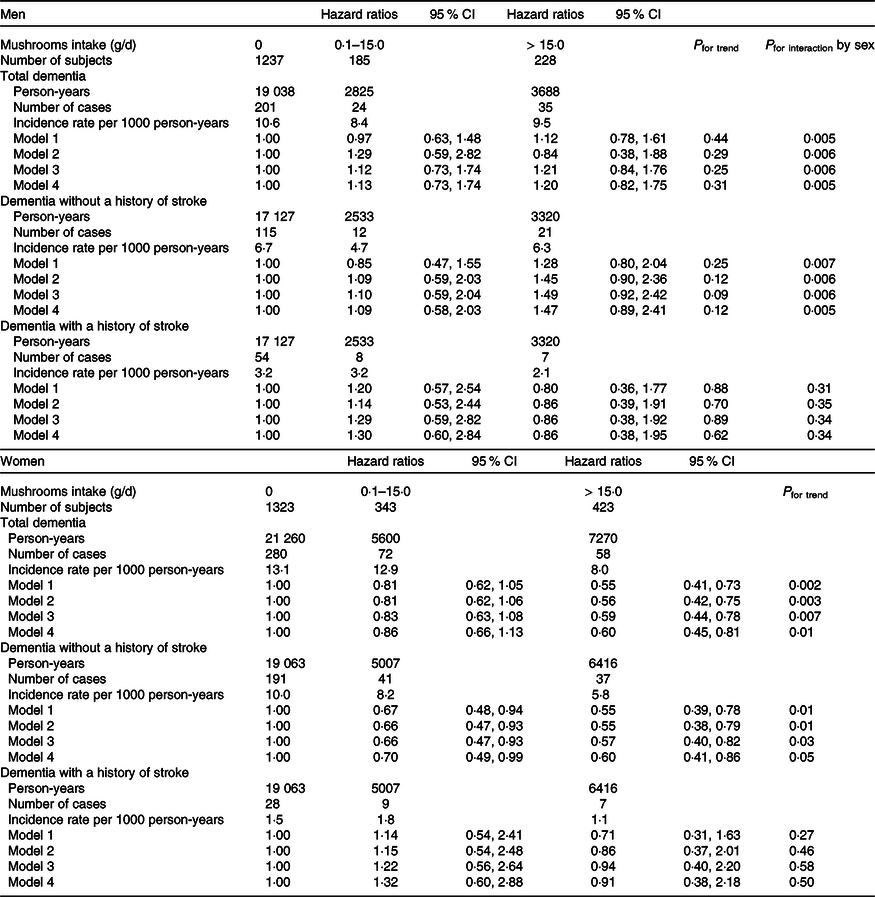
Model 1: Adjusted for age and energy, and stratified by area.
Model 2: Multivariable model adjusted further for smoking status, drinking status, intakes of vegetables, fruits, fish and meat.
Model 3: Multivariable model adjusted further for variables in model 2 and soluble fibre.
Model 4: Multivariable model adjusted further for variables in model 2 and insoluble fibre.
Since the data regarding registration of dementia diagnosis between 2005 and 2008 in Kyowa were unavailable, we performed a sensitivity analysis starting follow-up from 2009 in order to reduce the possibility of reverse causation. The results did not change essentially (online Supplementary Table 1). Of note, when we excluded the participants who had had a history of stroke, the results did not change substantially. The multivariable HR of total dementia for mushroom intake between 0·1 and 14·9 g/d and mushroom intake greater than or equal to 15·0 g/d were 0·82 (0·63, 1·07) and 0·57 (0·43, 0·77), respectively, in women compared with no mushroom intake (P for trend = 0·005). It was also possible that mushroom consumption varied by season, but the results were similar when the analysis was conducted separately for each of the four seasons, although they were not always statistically significant probably because the numbers of participants were limited (data not shown).
Discussion
We found an inverse association between mushroom intake and the risk of incident disabling dementia only in women. The association was confined to dementia without a history of stroke.
This sex-related discrepancy of association was consistent with a previous study. This was a prospective study involving 13 230 Japanese participants aged 65 years or above with a 5·7-year follow-up, which showed an inverse association between mushroom intake and the risk of dementia in women but not in men. The multivariable HR (95 % CI) for persons with a mushroom intake of 1–2 times/week and a mushroom intake greater than or equal to 3 times/week were 1·15 (0·92, 1·43) and 0·97 (0·76, 1·24) (P for trend = 0·83), respectively, in men, and 0·80 (0·66, 0·98) and 0·68 (0·55, 0·85) (P for trend < 0·01), respectively, in women, compared with those with a mushroom intake less than 1 time/week(Reference Zhang, Tomata and Sugiyama3).
No other cohort study has examined the association between mushroom intake and the risk of dementia. A randomised control study of thirty Japanese aged 50–80 years, which divided participants into two 15-person groups, showed that oral administration of four 250 mg tablets containing 96 % of yamabushitake mushroom (Hericium erinaceus) dry powder three times a day for 16 weeks increased scores on the cognitive function scale compared with the placebo group (P < 0·001)(Reference Mori, Inatomi and Ouchi4). A cross-sectional study involving 663 people aged 60 years or above in Singapore showed that mushroom consumption was associated with a lower prevalence of mild cognitive impairment. The multivariable OR (95 % CI) for mild cognitive impairment in those with mushroom consumption greater than two portions per week was 0·43 (0·23, 0·78) compared with those with mushroom consumption of less than one portion per week (P for trend = 0·006)(Reference Feng, Cheah and Ng5). Another cross-sectional study of 2840 people aged 60 years or more in the USA reported that greater mushroom intake was associated with higher scores on cognitive performance tests evaluating new verbal learning, immediate and delayed memory, verbal semantic fluency, attention, and processing speed(Reference Nurk, Refsum and Drevon7). Yet another cross-sectional study of 2031 people aged 70–74 years in Norway reported a significant association between a high mushroom intake (> 10th percentile or more) and better cognitive performance, including episodic memory, executive function, perceptual speed, visuo-spatial skills, an improved score on a modified version of the Mini-Mental State Examination and enhanced access to semantic memory(Reference Nurk, Refsum and Drevon7). However, the above-mentioned four studies did not show sex-specific results.
The potential effects of dietary fibre contained in mushrooms are involved in the pathophysiology of cognitive decline and dementia. The CIRCS previously showed that fibre intake, especially soluble fibre intake, was inversely associated with the risk of disabling dementia(Reference Yamagishi, Maruyama and Ikeda2). An animal study showed that the administration of soluble fibre changed the gut microbiome, which contributed to the production of butyrate, acetate, and total SCFA in the gut and to the prevention of inflammation of microglia(Reference Matt, Allen and Lawson19). Dietary fibre-deficient mice presented decreased Bacteroidetes and increased Proteobacteria in the gut and developed cognitive impairment, including decline of spatial memory and temporal order memory, as well as disabilities in daily living performance(Reference Shi, Ge and Ma20). However, we observed a similar association between mushroom intake and the risk of disabling dementia even after further adjusting for soluble and insoluble fibre intake. Therefore, some nutrients other than dietary fibre such as vitamin B, vitamin D, polyphenol and ergothioneine could contribute to this association. It has been reported that accumulation of amyloid led to neural alterations in young and middle-aged adults(Reference Kennedy, Rodrigue and Devous21), and that antioxidants obtained from foods may slow the neuronal damage related to amyloid β-peptide-induced oxidative stress(Reference Butterfield, Castegna and Pocernich22). Diet patterns containing anti-inflammatory nutrients such as vitamin B, vitamin D3 and polyphenol decrease the levels of pro-inflammatory prostaglandins and eicosanoids and could counteract Alzheimer’s disease pathologies(Reference Szczechowiak, Diniz and Leszek23). Ergothioneine is an amino acid with potent antioxidant activity produced by fungi and actinobacteria(Reference Borodina, Kenny and McCarthy24). An animal study reported that mice orally fed ergothioneine showed improved memory and learning abilities compared with the controls(Reference Yang, Lin and Wu25). A human study reported that plasma levels of ergothioneine were lower in patients with mild cognitive impairment than age-matched controls without cognitive impairment(Reference Cheah, Feng and Tang26). These studies support our findings that mushroom intake was associated with the risk of dementia without a history of stroke (which is likely to be Alzheimer’s disease), but not with the risk of dementia with a history of stroke (which is likely to be vascular dementia). However, in the present study, we calculated the HR for the risk of disabling dementia adjusted for vitamin B1, vitamin B2, niacin, vitamin B6, folate, vitamin D, and vitamin K, respectively, but the results did not change. Since we did not collect information on antioxidant nutrition including polyphenol and ergothioneine, it remains unclear which specific nutrition factor contributed to the association between mushroom intake and the risk of disabling dementia.
We found the association between mushroom intake and incident disabling dementia only in women. The reason for the difference between men and women is uncertain, but the sex-dependent antioxidant metabolism in the brain affected by Alzheimer’s disease may be involved; the levels of 4-hydroxynonenal (a marker of oxidative damage) were reported to be higher in female Alzheimer’s disease patients than in male patients(Reference Schuessel, Leutner and Cairns27), which may reflect a higher susceptibility against oxidative stress than that of men. As mentioned above, mushrooms contain several antioxidant nutrients, which could protect against oxidative stress and prevent dementia more effectively in women. Although it is unclear whether mushroom intake affects sex hormone production in humans, an animal study reported that adenosine contained in mushrooms contributed to increased testosterone production(Reference Iguchi, Nagashima and Mochizuki28). A meta-analysis showed that a low testosterone level was associated with a higher risk of Alzheimer’s disease(Reference Lv, Du and Liu29). On the other hand, a randomised control study involving 788 men aged 65 years or more reported that 1-year testosterone treatment did not improve cognitive impairment(Reference Yeap and Flicker30). Testosterone level may be a useful biomarker for dementia, but increasing production of testosterone may not contribute to the risk of dementia. As for our study, we did not find any mushroom–dementia association in men.
The strengths of this study are that participants were relatively young (59·7 years of age on average) community-dwelling people, in contrast to a prior study (aged 65 years or more)(Reference Zhang, Tomata and Sugiyama3) allowing us to examine the association of eating habits in midlife with the risk of dementia in later life, and that the target group consisted of members of the Japanese general population, who commonly consume mushrooms. We were also able to secure a long follow-up period (24·1 years on average).
Several limitations should be noted. First, we did not survey the socio-economic status of participants, which could be a residual confounding factor. National surveys of Japanese representative population samples have showed that mushroom intake was positively correlated with household income in 2014(31) and with household expenditure between 2003 and 2007(Reference Nishi, Horikawa and Murayama32). Second, we collected diet information based on a 24-h recall dietary survey, so it is likely that misclassification of dietary intake occurred. However, it is unlikely that participants who rarely ate mushrooms would happen to eat them on the day of the survey. In contrast, it is possible that a participant who occasionally ate mushrooms happened not to eat them on the day of the survey. When we collapsed those with mushroom intake between 0·1 and 14·9 g/d and those with mushroom intake greater than or equal to 15·0 g/d into one group, the multivariable HR of disabling dementia for ‘0·1 g/day or more intake’ versus ‘no intake’ were 0·68 (0·55, 0·84) for total dementia and 0·60 (0·46, 0·79) for dementia without a history of stroke in women. We did not observe any associations between mushroom intake and risk of disabling dementia in men. Third, lifestyle changes including mushroom intake might have occurred during the follow-up period. In a subsample of participants, we examined the repeatability of mushroom intake among those who completed the dietary survey twice or more at intervals 10 (±3)-year apart (n 201). The 10 (±3)-year repeatability of mushroom intake was as follows: among these 201 participants, 147 were classified as having ‘no mushroom intake’ (0 g/d) in the first survey, and 94 (64 %) of these 147 participants were classified as having ‘no mushroom intake’ (0 g/d) in the second survey (kappa statistic = 0·11). Fourth, we did not have data on dementia types. We assumed that dementia with a history of stroke was likely to be vascular dementia and that dementia without a history of stroke was likely to be Alzheimer’s disease. Last, we did not collect data on the history of dementia at the baseline survey. Instead, we excluded those who developed disabling dementia within 5 years of the baseline survey. Furthermore, since cases of incident dementia were not registered from 2005 to 2008 in Kyowa and from 1999 to 2005 in Yao, we performed a sensitivity analysis starting the follow-up from 2009, and we generally observed the same results (online Supplementary Table 1).
In conclusion, mushroom intake was associated with a lower risk of incident disabling dementia, especially dementia without a history of stroke, among Japanese women.
Acknowledgement
The authors thank Florescu Mihail Cosmin, Medical English Communications Center, University of Tsukuba, for language revision. This study was supported by Health and Labour Science Research Grants for Dementia (grant numbers: H21-Ninchisho-Wakate-007 and H24-Ninchisho-Wakate-003), Ministry of Health, Labour and Welfare, Japan; JSPS KAKENHI grant numbers 26253043, 17H04121, 18K10097 and 21H03194; and Japan FULLHAPP.
S. A., K. Y., K. M. and H. Iso. substantially contributed to the conception or design of the work. All the authors contributed to the acquisition, analysis or interpretation of data for the work. S. A. drafted the manuscript, and K. Y., K. M., A. I., M. N., H. N., M. U., M. H.-T., I. M., C. O., M. T., R. K., T. K., M. T., Y. S., T. O., H. Imano, T. S., T. O., T. T., A. K., M. K. and H. Iso. revised the manuscript critically for important intellectual content.
There are no conflicts of interest.
Individual consent was not necessarily required since the present study used secondary data obtained for public health practice aimed at prevention of CVD in the local community at that time. Instead, we obtained informed consent from community representatives. However, considering the relevant guidelines and regulations, we offered the participants the opportunity to withdraw their data from analysis retrospectively. The study was approved by the institutional review boards of the relevant institutions (Osaka Center of Cancer and Cardiovascular Disease Prevention, University of Tsukuba, and Osaka University).
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711452400014X





