Nutrition is one of the key modifiable risk factors that strongly influences the development of non-communicable chronic diseases( 1 , 2 ). The WHO estimated that about 20 % of deaths worldwide are attributable to inadequate diet or physical inactivity( 1 ). Most of the Western countries have developed public health nutrition programmes to tackle this issue, as in France, where the National Nutrition and Health Program (PNNS) has been implemented since the year 2000( Reference Hercberg, Chat-Yung and Chauliac 3 ). In this context, it has recently been proposed( Reference Hercberg 4 ) that putting a Five-Colour Nutrition Label (5-CNL) on the front-of-package of food products representing their overall nutritional quality might help consumers make healthier food choices. Nutritional quality of the product, and thus its colour, can be determined based on a nutrient profiling system (NPS). NPS aims at positioning individual foodstuffs based on their nutritional characteristics. It usually involves an algorithm that takes the nutritional content in various foodstuffs into account and gives a single-score output( Reference Azais-Braesco, Goffi and Labouze 5 – Reference Townsend 9 ). The NPS, developed in the UK by the Food Standards Agency (termed FSA-NPS), is one of the most scientifically validated systems in the European context( Reference Rayner, Scarborough and Lobstein 10 – Reference Rayner, Scarborough and Stockley 12 ). It has been developed and validated initially in the British food environment( Reference Arambepola, Scarborough and Rayner 13 ), but previous studies have demonstrated its applicability to the French context( Reference Julia, Touvier and Mejean 14 , Reference Julia, Kesse-Guyot and Touvier 15 ). Considering its simplicity of computation (the input variables being content of energy, total sugar, SFA, Na, fruits, vegetables, fibres and proteins), this profiling system has been proposed as a basis to determine the overall nutritional quality of foodstuffs in France (and thus the corresponding colour of the 5-CNL)( Reference Hercberg 4 ). It has been used in the UK for defining conditions of regulation of television advertisements and could also be used as a basis to implement food taxes or subsidies( Reference Nnoaham, Sacks and Rayner 16 ).
To evaluate the potential influence of such nutritional public health measures, it is important to assess whether subjects consuming a larger proportion of healthy food items (i.e. with lower FSA-NPS scores) develop less chronic pathologies such as cancer or CVD. In order to test this hypothesis, an individual dietary index (DI) based on the FSA-NPS has been previously defined and validated( Reference Arambepola, Scarborough and Rayner 13 , Reference Julia, Touvier and Mejean 14 ). This FSA-NPS DI corresponds to a weighted mean of all FSA-NPS scores of foods usually consumed by the individual, and thus reflects the nutritional quality of his/her diet. Cancer is one of the most incident non-communicable chronic diseases in developed countries. In France, it represents the first cause of death in men and the second in women( 17 ). As part of a series of aetiological analyses on FSA-NPS DI and health outcomes conducted by specialists of each disease, the aim of the present study was to investigate the association between the individual FSA-NPS DI and the risk of cancer in a large French prospective cohort (SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX)).
Methods
Study population
The SU.VI.MAX study is a population-based, double-blind, placebo-controlled, randomised trial (trial registration clinicaltrials.gov identifier: NCT00272428) initially designed to assess the effect of a daily antioxidant supplementation at nutritional doses on the incidence of CVD and cancer( Reference Hercberg, Galan and Preziosi 18 ). A total of 13 017 subjects were enroled in 1994–1995. The intervention study lasted 8 years, and observational follow-up of health events was maintained until September 2007. Subjects provided their written informed consent.
Ethical standards
The study was conducted according to the Declaration of Helsinki guidelines and was approved by the Ethics Committee for Studies with Human Subjects at the Paris-Cochin Hospital (CCPPRB n 706/ n 2364) and the ‘Commission Nationale de l’Informatique et des Libertés’ (CNIL n 334 641/ n 907 094).
Dietary data collection
Participants were invited to complete a dietary record every 2 months between 1994 and 2002, in which they recalled all foods and beverages consumed during periods of 24 h. These dietary records were randomly distributed between weekdays and weekend days and over seasons to take into account intra-individual variability. Dietary records from the first 2 years of follow-up were used in the present study to comply with the prospective design. Completion was made through the Minitel Telematic Network, a French telephone-based terminal equivalent to an Internet prototype. Portion sizes were assessed, thanks to a validated picture booklet( Reference Le Moullec, Deheeger and Preziosi 19 ), and the amounts consumed from composite dishes were estimated using French recipes validated by food and nutrition professionals. Mean daily energy, alcohol and nutrient intakes were estimated using a published French food composition table( Reference Hercberg 20 ).
Data collection for covariates
At enrolment, self-administered questionnaires were filled in by participants about socio-demographic characteristics, smoking status, medication use, number of live births, family history of cancer and menopausal status. Baseline physical activity was self-evaluated by asking the participants whether they currently practiced a regular physical activity, and if yes whether it was equivalent to 1 h/d of walking or less. Anthropometric (height and weight) measurements were taken by study nurses and physicians during a baseline clinical examination; 35 ml fasting venous blood samples were collected in vacutainer tubes and were used to determine the baseline plasma concentrations of total prostate-specific antigen (PSA) measured by immunoassay (Roche Diagnostics).
Cases ascertainment
As described elsewhere( Reference Hercberg, Galan and Preziosi 18 ), health events occurring during the follow-up were self-reported by the participants. Medical data were then gathered through participants, physicians and/or hospitals and were reviewed by an independent physician expert committee. Pathological reports were used to validate the cases and to extract cancer characteristics. Cases were classified using the International Chronic Diseases Classification, 10th Revision, Clinical Modification (ICD-10) ( 21 ). All first incident primary cancers were considered as cases in this study.
Food Standards Agency Nutrient Profiling System Dietary Index computation
As described previously( Reference Rayner, Scarborough and Lobstein 10 , Reference Julia, Kesse-Guyot and Touvier 15 ), the FSA score for all foods (processed and unprocessed) and beverages was computed taking into account the nutrient content for 100 g. FSA-NPS scores for foods and beverages are based on a discrete continuous scale from −15 (most healthy) to +40 (less healthy) (see online Supplementary material 1). FSA score allocates points (0–10) for content in energy (kJ), total sugar (g), SFA (g) and Na (mg). Points (0–5) are subtracted from the previous sum according to content in fruits, vegetables, fibres and proteins. An increase of this score, therefore, reflects decreasing nutritional quality of the food or beverage item.
In a second step, the FSA-NPS DI was computed at the individual level using arithmetic energy-weighted means with the following equation( Reference Julia, Touvier and Mejean 14 ), in which FS i represents the food (or beverage) score and E i represents energy intake from that particular food or beverage:
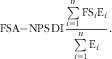 $${\rm FSA{\minus}NPS}\,{\rm DI}{\equals}{{\mathop{\sum}\limits_{i{=}{\rm 1}}^n {{\rm FS}_{i} {\rm E}_{i} } } \over {\mathop{\sum}\limits_{i{=}{\rm 1}}^n {{\rm E}_{i} } }}.$$
$${\rm FSA{\minus}NPS}\,{\rm DI}{\equals}{{\mathop{\sum}\limits_{i{=}{\rm 1}}^n {{\rm FS}_{i} {\rm E}_{i} } } \over {\mathop{\sum}\limits_{i{=}{\rm 1}}^n {{\rm E}_{i} } }}.$$
This energy-weighted aggregation system was selected rather than other systems based on weighting by food quantity in grams for instance, as the latter gives excessive and disproportionate weight to water, beverages and foods with high water content, as tested previously( Reference Scarborough, Arambepola and Kaur 22 ). Increasing FSA-NPS DI reflects decreasing quality in foods consumed.
Statistical analyses
From the 13 017 participants of the SU.VI.MAX study, 161 were excluded because they reported a diagnosis of cancer before the start of follow-up. We also excluded participants in whom an incident cancer was diagnosed within the first 3 years of follow-up (n 106) in order to avoid pre-clinical bias (i.e. influence of the pre-cancerous condition on dietary behaviour) and to guarantee a prospective design. Among the remaining participants, 6584 provided at least six 24 h dietary records within the first 2 years of follow-up, among whom 6435 were normo-energy reporters, and were thus included in the analyses. Indeed, as usually performed in the SU.VI.MAX cohort( Reference Kesse-Guyot, Touvier and Henegar 23 ), 24 h records that reported <418·4 kJ/d (<100 kcal/d) or 25 104 kJ/d (>6000 kcal/d) were dropped, and men reporting 3347·2 kJ/d (<800 kcal/d) and women reporting 2092 kJ/d (<500 kcal/d) across ≥60 % of their dietary records were excluded (n 149). There was no missing value for all covariates, except for smoking status, physical activity and BMI, for which <5 % of values were missing and were replaced by the mode.
In order to check for dietary consistency over time, we computed the Pearson’s correlation coefficient between the FSA-NPS DI of each subject calculated: (1) in 1994–1995 (i.e. first 2 years of follow-up used in this study to comply with a prospective design) and (2) during the whole dietary assessment period of the SU.VI.MAX study (8·5 years, 1994–2002). Baseline characteristics of participants were compared across sex-specific quintiles of FSA-NPS DI using ordinal polytomous logistic regressions. Hazard ratios (HR) and 95 % CI obtained from Cox proportional hazards models with age as the primary time variable were used to characterise the association between FSA-NPS DI (coded as a continuous variable and as sex-specific quintiles) and overall cancer risk, as well as prostate and breast cancer risks (i.e. most incident cancer sites in France). Participants contributed person-time to the Cox models until the date of diagnosis of the studied cancer type (overall, breast or prostate), the date of last completed questionnaire (if subjects were lost to follow-up before September 2007), the date of death or September 2007, whichever occurred first. For cancer site-specific analyses, participants who reported other cancer types than the ones studied during the study period were included and censored at the date of diagnosis (except those with basal cell skin carcinoma, which was not considered as cancer). We confirmed that the assumptions of proportionality were satisfied through examination of the log–log (survival) v. log–time plots. Tests for linear trends were performed using the ordinal score on quintiles of FSA-NPS DI. Multivariate models for overall cancer risk were adjusted for age (time-scale in the Cox model), sex, intervention group of the initial SU.VI.MAX trial (antioxidant/placebo), number of dietary records (continuous), baseline BMI (<25/25 to <30/≥30 kg/m2), physical activity (irregular, < or ≥1 h/d walking or equivalent), smoking status (never/former-current), educational level (primary/secondary/university), family history of cancer in first-degree relatives (yes/no) and alcohol intake (continuous). Breast cancer models were further adjusted for baseline menopausal status (menopausal/non menopausal), use of hormonal treatment for menopause at baseline (current, yes/no), number of live births (continuous), height (continuous) and family history of breast cancer (yes/no). Prostate cancer models were further adjusted for baseline PSA concentration (continuous), height (continuous) and family history of prostate cancer (yes/no).
Analyses stratified by the sex-specific median of energy intake without alcohol were also carried out, as it has been suggested that overall nutritional quality of the diet may more thoroughly impact disease risk in people with moderate energy intake and less in people with high energy intake( Reference Cottet, Touvier and Fournier 24 ). Breast cancer models were also computed separately for post-menopausal women only (women contributed to the post-menopausal model from their age of menopause). All the tests were two-sided, and P<0·05 was considered statistically significant. SAS version 9.3 (SAS Institute) was used for the analyses.
Results
The median follow-up time was 12·6 years; 42·4 % of the participants were males, and 5·2 % of the subjects were lost to follow-up. A total of 453 incident cancer cases were diagnosed during follow-up: 125 breast (thirty-one pre-menopausal and ninety-four post-menopausal), 112 prostate, thirty-five skin, twenty-four colorectal, eleven lung, eleven ovary and 135 other cancers. Mean age at diagnosis was 60·0 years, 57·4 for breast cancer and 63·5 for prostate cancer. Among breast cancers, 59 % were oestrogen receptor positive and 43 % were progesterone receptor positive; 62 % were ductal, 15 % were lobular and 23 % were derived from other types. Among prostate cancer cases, 5 % had a Gleason score between 2 and 4, 87 % between 5 and 7 and 8 % between 8 and 10.
The FSA-NPS DI value was highly consistent over time (Pearson’s correlation coefficient=0·92 between the first 2 years of follow-up and the whole dietary assessment period).
Characteristics of the participants are described in Table 1. Participants with a lower FSA-NPS DI (i.e. with a healthier diet) tended to be older, never-smokers, more physically active, with lower alcohol and energy intakes, have lower educational level and higher BMI.
Table 1 Baseline characteristics of the study population, SUpplémentation en VItamines et Minéraux AntioXydants Cohort, France, 1994–2007 (Mean values and standard deviations for quantitative variables; numbers and percentages for qualitative variables)
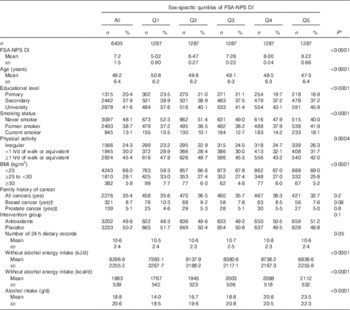
FSA-NPS DI Food Standards Agency Nutrient Profiling System Dietary Index.
* P value for the comparison between quintiles of FSA-NPS DI, by ordinal polytomous logistic regressions.
† In women only.
‡ In men only.
Table 2 presents associations between quintiles of FSA-NPS DI and overall, breast and prostate cancer risks. FSA-NPS DI was associated with increased overall cancer risk (HRfor an increment of 1 point of the score=1·08 (95 % CI 1·01, 1·15), P trend=0·02, HRQ5 v. Q1=1·34 (95 % CI 1·00, 1·81), P trend=0·03). Similar results were obtained when the analyses were restricted to invasive cancer cases (n 416, HRfor an increment of 1 point of the score=1·08 (95 % CI 1·01, 1·16), P trend=0·02, data not tabulated). Associations with prostate (P trend, continuous score=0·3) and breast (P trend, continuous score=0·9) cancers were not statistically significant. Breast cancer results were unchanged when focusing on post-menopausal women only (P trend=0·4 for the continuous score and 0·8 for quintiles, data not tabulated).
Table 2 Associations between quintiles (Q)Footnote * of Food Standards Agency Nutrient Profiling System Dietary Index (FSA-NPS DI) and overall, prostate and breast cancer risk from multivariate Cox proportional hazards model, SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) cohort, France, 1994–2007 (Hazard ratios (HR) and 95 % confidence intervals)

* Sex-specific cut-offs for quintiles of FSA-NPS DI were 6·12/6·97/7·66/8·41 for men and 5·88/6·82/7·59/8·43 for women.
† Adjusted for age, sex, intervention group of the initial SU.VI.MAX trial, number of dietary records, smoking status, educational level, physical activity, BMI, family history of overall cancer and alcohol intake.
‡ Same adjustment+baseline prostate-specific antigen level, height and family history of prostate (instead of overall) cancer.
§ Same adjustment+menopausal status at baseline, use of hormonal treatment for menopause at baseline, number of live births, height and family history of breast (instead of overall) cancer.
Similar results were obtained when adjusting all the models for daily energy intake as a continuous variable (HRfor an increment of 1 point of the score=1·08 (95 % CI 1·01, 1·15), P trend=0·03 for overall cancer risk; P trend, continuous score=0·3 for prostate cancer risk and P trend, continuous score=0·9 for breast cancer risk, data not tabulated).
Association between FSA-NPS DI and overall cancer risk stratified by the median of energy intake without alcohol is shown in Table 3. Although the interaction between FSA-NPS DI and energy intake was not statistically significant (P=0·27), results tended to differ across energy strata. FSA-NPS DI was directly associated with overall cancer risk in subjects with low-to-moderate energy intake (HRfor an increment of 1 point of the score=1·10 (95 % CI 1·01, 1·20), P trend=0·03), but not in subjects with higher energy intake (P trend=0·3), despite similar statistical power in both energy strata.
Table 3 Associations between quintiles (Q)Footnote * of Food Standards Agency Nutrient Profiling System Dietary Index (FSA-NPS DI) and overall cancer risk from multivariate Cox proportional hazards modelsFootnote † stratified by daily energy intake, SUpplémentation en VItamines et Minéraux AntioXydants (SU.VI.MAX) cohort, France, 1994–2007 (Hazard ratios (HR) and 95 % confidence intervals)
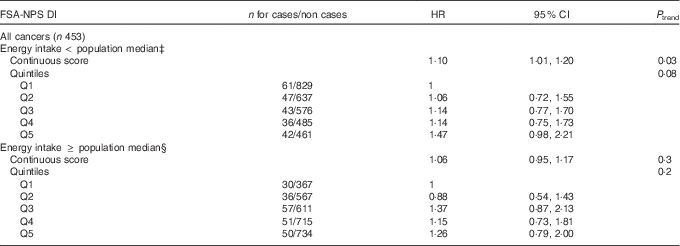
* In both energy strata, sex-specific cut-offs for quintiles of FSA-NPS DI were 6·12/6·97/7·66/8·41 for men and 5·88/6·82/7·59/8·43 for women.
† Adjusted for age, sex, intervention group of the initial SU.VI.MAX trial, number of dietary records, smoking status, educational level, physical activity, BMI, family history of overall cancer and alcohol intake.
‡ Below the population median of without alcohol daily energy intake, that is, 9380·5 kJ/d (2242 kcal/d) for men and 7296·9 kJ/d (1744 kcal/d) for women.
§ Equal to or above the population median of without alcohol daily energy intake.
Discussion
For the first time, this prospective study investigated the association between the FSA-NPS individual DI and cancer risk. The results showed that participants with higher FSA-NPS DI values (corresponding to food choices of lower nutritional quality) were at higher risk of developing overall cancer.
Other existing a priori scores that have been specifically designed based on current nutritional recommendations for cancer prevention (e.g. the World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR) score, which is more cancer-specific)( Reference Romaguera, Vergnaud and Peeters 25 , Reference McCullough, Patel and Kushi 26 ) probably have stronger associations with cancer risk because they include additional items that are relevant regarding this pathology (food-related items such as red/processed meat or alcohol and other nutrition-related items such as weight status or physical activity). Increasing the degree of complexity of the score with the introduction of additional nutritional compounds (e.g. some vitamins or minerals) associated with cancer risk would probably increase its pertinence regarding cancer risk prediction. However, the aim of the present study was to test specifically the association between FSA-NPS DI and cancer risk, as FSA-NPS (or a modified form of this score) is envisioned to serve as a basis for food labelling in the framework of public health policies in several countries such as France and Australia. Indeed, FSA-NPS presents several key advantages in this context. Notably, it has been designed in a perspective of prevention of a large range of chronic diseases (not only cancers) and is easy to compute for industrials and public health stakeholders( Reference Reedy and Kirkpatrick 27 – Reference Jakicic 29 ).
Previous prospective studies investigated the relationship between individual scores of overall nutritional quality of the diet and cancer incidence or cancer mortality, and showed either inverse (better nutritional quality associated with lower risk)( Reference Romaguera, Vergnaud and Peeters 25 , Reference McCullough, Patel and Kushi 26 , Reference Waijers, Feskens and Ocke 30 – Reference Kvaavik, Batty and Ursin 37 ) or null( Reference Kesse-Guyot, Touvier and Henegar 23 , Reference Waijers, Feskens and Ocke 30 , Reference Chiuve, Sampson and Willett 38 – Reference McCullough, Feskanich and Stampfer 40 ) associations. In particular, Chiuve et al. ( Reference Chiuve, Sampson and Willett 38 ) investigated the association between the Overall Nutritional Quality Index (ONQI) and the risk of chronic diseases within the Nurses’ Health Study and the Health Professional Follow-up Study and observed no association with cancer risk. Despite a large sample, their null result (v. a positive association in our cohort) may be explained by (1) the use of an aggregated FFQ (135–138 items) providing less-precise estimates of dietary intakes than 24 h dietary records and (2) the computation of the score involving thirty nutrients, many of them having no consistent association with cancer risk, which may have diluted the relevance of this score regarding the cancer outcome. To our knowledge, this study( Reference Chiuve, Sampson and Willett 38 ) was the only one that investigated score/classification of foods eaten in relationship with individual health outcomes, and no previous longitudinal study has specifically provided such information for FSA-NPS DI.
Our finding is consistent with the knowledge regarding the relationships between components of the score and cancer risk. Indeed, based on systematic reviews, meta-analyses and collective expertise of epidemiological and mechanistic literature, the WCRF/AICR( 41 – 45 ) determined that dietary fibre intake is associated with decreased colorectal cancer risk (evidence qualified as ‘convincing’) and fruit and vegetable intake is associated with lower mouth, pharynx, larynx, oesophagus, gastric and lung (fruit only) cancer risk (evidence qualified as ‘probable’). Conversely, the WCRF/AICR also showed that salt intake is associated with increased gastric cancer risk (‘probable’) and overweight/obesity (favoured by high energy-dense food intake) is associated with higher risk of oesophagus, pancreatic, colorectal, breast (post-menopausal), endometrial, kidney (‘convincing’), ovary and gall-bladder (‘probable’) cancers. The WCRF/AICR also concluded that intake of foods with high glycaemic load may increase the risk of endometrial cancer (evidence qualified as ‘probable’) and foods containing sugars may be associated with higher colorectal cancer risk (evidence qualified as ‘suggested’).
The fact that no statistically significant findings were observed in this study regarding breast and prostate cancers is not surprising, as no strong association between most components of the FSA-NPS DI score and these two cancer locations has been demonstrated( 46 , 47 ).This score probably has a greater impact on other cancer sites (such as digestive cancers) more strongly associated with these nutritional components, but these cancer locations could not be investigated separately in the present study due to limited number of cases.
As energy density of food items was one of the key components of the score, our main results were not adjusted for daily energy intake. However, we tested this further adjustment in the sensitivity analyses. Results remained statistically significant, although less pronounced, supporting the idea that the energy component of the score plays a role but does not entirely explain the observed associations.
Interestingly, in the stratified analyses, the direct association between FSA-NPS DI and cancer risk tended to be more specifically observed in subjects with energy intake below the population median but not in those with higher energy intake. This result may suggest that nutritional quality of the diet could play a key preventive role in people with moderate energy intakes, whereas this beneficial effect could be offset by high energy intake. This result is in line with findings from a previous study showing that a healthy/Mediterranean pattern was associated with a reduced risk of breast cancer only when energy intake remained within recommendations( Reference Cottet, Touvier and Fournier 24 ). However, as the P value for interaction was not statistically significant in the present study, this finding should be considered with caution and confirmed in future studies.
A lower FSA-NPS DI was associated with higher BMI and lower educational level in the cross-sectional crude analyses presented in Table 1. Regarding BMI, this result is consistent with the findings observed in other cross-sectional investigations on BMI and diet quality( Reference Alkerwi, Sauvageot and Nau 48 ) and is probably due to the fact that overweight/obese people are more likely to be aware of nutritional recommendations or to be dieting( Reference Julia, Peneau and Andreeva 49 ). Regarding educational level, the association with FSA-NPS DI was not statistically significant in a multivariate model including all the descriptors of Table 1 (P=0·2, data not shown).
The SU.VI.MAX cohort is based on a relatively long follow-up period (13 years). However, the latencies of several cancers are longer than this period. Thus, the associations observed in this study may be due to the conjunction of two phenomena. First, as this is usually hypothesised in nutritional epidemiology( Reference Willett 50 ), mean daily dietary intake measured during the first 2 years of follow-up reflects the general eating behaviour throughout adult life (and thus several years before the beginning of the study). In this case, dietary protective and risk factors may have played a role in the first stages of carcinogenesis. No information was available regarding dietary intake of the subjects before their inclusion in the cohort. However, we verified that, in this middle-aged population, diet was quite stable over time with a high consistency in subjects’ FSA-NPS DI when calculated at baseline (first 2 years of follow-up) and later on (taking into account the whole dietary follow-up, i.e. 8·5 years).
Second, eating behaviour may affect not only initiation of malignant transformation but also survival of a deviating cell population with latent (pre-clinical) tumours. In this case, no assumption about previous eating behaviour is necessary.
Strengths of this study include its prospective design and an accurate assessment of dietary data by repeated 24 h dietary records, accounting for intra-individual day-to-day and seasonal variability. Some limitations should be acknowledged. First, caution is needed when extrapolating our results to the entire adult French population. Indeed, participants were volunteers involved in a long-term nutrition study. This is probably associated with a more health-conscious behaviour and with overall healthier food choices. Thus, unhealthy dietary behaviours may have been under-represented in this study, which may have weakened the observed associations by reducing the range of represented dietary behaviours and the contrast between compared groups. In addition, as in most cohorts, better educated subjects are over-represented in this population study compared with the general population. Next, residual confounding cannot be ruled out. However, a large range of confounding factors has been taken into account, limiting this potential bias. Finally, as mentioned earlier, statistical power did not allow us to perform site-specific analyses for cancers other than breast and prostate cancers.
In conclusion, to our knowledge, this study was the first to investigate the prospective association between the FSA-NPS individual score and cancer risk. These results suggest that unhealthy food choices (as represented by higher FSA-NPS DI values) may be associated with a 34 % increase in overall cancer risk (when comparing quintiles 5 to 1 of the score). In further studies, the associations between FSA-NPS DI and the risk for specific cancers potentially strongly influenced by nutrition (such as colorectal or gastric cancers) should be specifically investigated. Future work in the SU.VI.MAX cohort will also bring new insights on the relationships between this score and other major health outcomes such as CVD, weight gain, metabolic syndrome and cognition. This study supports the potential interest of developing front-of-pack nutrition labels based on the FSA-NPS, such as the proposed 5-CNL. Such public health measure would (1) provide the consumers with simplified information about the nutritional content at a glance, in order to help them make healthier food choices at the time of purchase, and (2) stimulate manufacturers to positively impact the nutritional value of their food products through re-formulations. If future studies demonstrate a positive impact of food labelling on individual food choices, both aspects could strongly contribute to decrease the FSA-NPS DI at the individual level.
Acknowledgements
The authors thank Younes Esseddik, Paul Flanzy, Mohand Ait Oufella, Yasmina Chelghoum and Van Than Duong (computer scientists) and Anne-Sophie Chhim, Nathalie Arnault, Véronique Gourlet, Fabien Szabo, Laurent Bourhis and Stephen Besseau (statisticians) for their technical contribution to the SU.VI.MAX study.
This work was supported by the French Ministry of Health (DGS) and the National Institute for Prevention and Health Education (INPES). Mathilde Donnenfeld was funded by a grant from the French Ministry of Research and Higher Education. Mélanie Deschasaux was funded by a PhD grant from the ‘Cancéropôle Ile-de-France’ (public funding from the Paris region). The funders had no role in the design, analysis or writing of this article.
The authors’ responsibilities were as follows: M. Do and M. T. designed the research; S. H., M. T., C. J. and E. K. G. conducted the research; M. Do performed the statistical analyses; M. Do and M. T. wrote the paper; C. J., E. K. G., C. M., P. D., S. P., M. D., P. L. M., L. F. and S. H. contributed to the data interpretation and revised each draft for important intellectual content; and M. T. had the primary responsibility for the final content. All the authors read and approved the final version of the manuscript.
There are no conflicts of interest to declare.
Supplementary material
For supplementary material/s referred to in this article, please visit http://dx.doi.org/doi:10.1017/S0007114515003384





