Red meat produced from grass-fed animals is recognised as a dietary source of long-chain (LC) n-3 PUFA(Reference Leheska, Thompson and Howe1–Reference Nuernberg, Dannenberger and Nuernberg4). A ruminant diet of grass, compared with cereal-based concentrate feeds, is rich in α-linolenic acid (ALA) (18 : 3n-3), thereby allowing the elongation of LC n-3 PUFA and their incorporation into muscle tissue(Reference Dewhurst, Scollan and Lee5). A recent scientific opinion from the European Food Safety Authority (EFSA) panel indicates that 250 mg LC n-3 PUFA per d is an Adequate Intake for adults to reduce the risk of CVD(6–Reference Hu, Bronner and Willett8) as a result of the anti-thrombotic and anti-inflammatory effects induced by these fatty acids(Reference Calder9, Reference Ruxton, Reed and Simpson10).
Oily fish is the unsurpassed richest dietary source of LC n-3 PUFA, but oily fish is consumed by only one-third or less of the UK consumer population(Reference Henderson and Gregory11). In comparison, meat is consumed by the majority of the UK and Irish population and in larger quantities than fish (134 v. 35 g/d by Irish consumers)(Reference Henderson and Gregory11–13). Red meat, particularly that produced from grass-fed animals, is also a source of docosapentaenoic acid (DPA) (22 : 5n-3), which is not present in any significant amount in fish(Reference Williams14, Reference Howe, Meyer and Record15). Recent evidence shows that DPA can be interconverted to both EPA (20 : 5n-3) and DHA (22 : 6n-3) in rats(Reference Kaur, Begg and Barr16), suggesting that it may exert similar health benefits to EPA and DHA(Reference Akiba, Murata and Kitatani17, Reference Rissanen, Voutilainen and Nyyssönen18). Although red meat cannot be compared to oily fish in terms of its actual LC n-3 PUFA content and overall fatty acid profile, it is possible that red meat makes a greater contribution to total dietary intakes of LC n-3 PUFA than oily fish based on the present levels of consumption. This contention has been supported by Australian data, where the consumption of beef and lamb from predominantly grass-fed animals contributes 28 % of total LC n-3 PUFA intakes, compared with 48 % from oily fish(Reference Howe, Meyer and Record15).
Although lean red meat is known to be a bioavailable source of LC n-3 PUFA(Reference Sinclair, Johnson and O'Dea19), it is currently unknown to what extent the animal finishing diet has an impact on this bioavailability. The aim of the present study was to investigate the effects of regular moderate consumption of beef and lamb from grass-fed animals on LC n-3 PUFA status among free-living healthy adults. The secondary aim was to investigate the possible effects on serum cholesterol, TAG and blood pressure.
Methods
Subjects and study design
The 4-week study was a double-blind, randomised dietary intervention in forty healthy and free-living volunteers (twenty males and twenty females). All the volunteers were recruited from staff and students at the University of Ulster. Exclusion criteria included those with high cholesterol (>5·0 mmol/l)(Reference Wood, De Backer and Faergeman20), high blood pressure (systolic >140 and diastolic >90 mmHg)(Reference Ramsey, Williams and Johnson21), those on prescribed medication or taking dietary supplements containing PUFA, those who consumed oily fish or n-3 PUFA-enriched foodstuffs more than twice a month and those with a BMI < 18·5 or >30 kg/m2. A further exclusion criterion was with respect to volunteers who habitually consumed more than three portions of red meat per week. Participants were randomly allocated to one of two groups: to consume red meat from animals that had been offered a finishing diet of grass or to consume red meat from animals that had been offered a finishing diet of concentrate. During each week of the 4-week intervention, participants in each group were provided with, and required to consume in place of their habitual red meat intake, one portion of mince beef (250 g raw weight), one sirloin steak (200 g raw weight) and four small lamb medallion pieces (240 g raw weight). The weekly consumption of these meats, taking into account an approximate 32 % weight loss during cooking(Reference Matthews and Garrison22), did not exceed the limit of 500 g/week as recommended by the World Cancer Research Fund(23). Taking this weight loss into account, the weekly and daily intakes of red meat consumed by study participants were estimated to be 469 and 67 g, respectively. Participants were instructed not to consume any oily fish during the 4-week study period, but were otherwise encouraged to follow their normal dietary habits. Meat was kept at − 20°C until transferred to subjects in a cool bag, and the subjects were required to cook and prepare the supplied meat at home. Before the study, the participants were invited to attend a cookery demonstration by a trained home economist from the Livestock and Meat Commission. The aim of the demonstration was to make the study volunteers aware of how to handle red meat safely before consumption, as well as providing several recipe ideas. The present study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the University of Ulster Research Ethics Committee (REC-FCBMS-08-150). A written informed consent was obtained from all subjects.
Red meat characteristics
Red meat was sourced from producers in Northern Ireland who offered diets to animals under experimental conditions. Eight beef cattle and forty-four lambs of a similar age, sex and breed were used in the study. Half of the animals were offered a finishing diet of fresh grass only, while the other half of the animals were offered a finishing diet of concentrate only for a minimum period of 6 weeks before slaughter. The typical composition of concentrate feeds offered in this region has been defined previously as a mixture of cereal, maize and soya with a vitamin/mineral mix(Reference French, Stanton and Lawless3). The compliance of the producers to strictly offer these diets to animals was monitored by the members of the research team throughout the 6 weeks. At the end of the pre-slaughter feeding regimen, animals were slaughtered according to routine practice at a commercial abattoir (Dunbia, Dungannon, Northern Ireland), after which strip loins were chilled for an ageing period of 16 d and lamb loins for 7 d. Beef topsides were used to prepare mince beef samples with the addition of a small amount of adipose tissue (5 %), to produce a fat content similar to that which is commercially available. After ageing, lamb loins were cut into small boneless medallions and sirloin steaks were cut from the strip loins. All the portions were vacuum sealed and stored at − 20°C until required for the human subject intervention study.
Lipid extraction
Additional samples (n 31) were taken from meat from each treatment group for confirmatory fatty acid analysis where possible. Meat samples were thawed and dissected to separate lean tissue, removing adipose tissue and discarding bone and connective tissue components. Total lipid was extracted from the lean tissue according to an adaptation of the Folch et al. (Reference Folch, Lees and Sloane Stanley24) method. Sub-samples of lean and adipose tissue were homogenised in a chloroform–methanol (2:1, v/v) mixture, antioxidant (butylated hydroxytoluene, 20 mg/ml) was added and homogenised samples were then filtered. Filtrate from lean and adipose tissue was mixed with 0·37 % KCl and allowed to settle overnight. The lower phase containing the lipid component was re-filtered and further evaporated under N2. Sub-samples were taken for total lipid estimation by oven drying at 40°C. Fatty acid methyl esters (FAME) were prepared using the transesterification method(25) by adding 5 % 2 m-KOH in anhydrous methanol.
Quantification of fatty acid methyl esters
FAME were quantified using a Varian CP 3800 GC (Varian Associates Limited, Walton-on-Thames, Surrey, UK) equipped with a temperature programmable injector operated in the split mode and a flame ionisation detector. Separation of the FAME was performed on a BPX70 capillary GC column (SGE Analytical Science, Milton Keynes, Bucks, UK) (length 120 m, internal diameter 0·25 mm and film thickness 0·25 μm), using He as the carrier gas at a flow rate of 1 ml/min. The samples were injected at a starting oven temperature of 50°C and the temperature was then ramped by 20°C/min to 120°C, then by 2°C/min to 180°C and finally by 4°C/min to 225°C, where it was held for 40 min. Fatty acids were identified by their retention time with reference to those of commercially available fatty acid standards (37 Supelco FAME mix; Sigma Aldrich Company Limited, Gillingham, Dorset, UK) and were quantified by use of internal standard C13 : 0 and C21 : 0 which were added before extraction (Sigma Aldrich Company Limited).
Blood samples
Fasting blood samples were collected at baseline and post-intervention. Serum, plasma and platelets were extracted within 1 h. Plasma and serum aliquots were obtained by spinning whole blood at 2500 g for 15 min at 4°C. Platelets were extracted by centrifuging whole blood slowly at 150 g for 15 min and subsequently harvesting the top layer of platelet-rich plasma. The platelet-rich plasma was centrifuged at 2500 g for 15 min to obtain a pellet, which was washed with Tris–HCl (pH 7·6, 4°C) and re-suspended in 500 μl Tris–HCl. Prepared samples were then stored at − 80°C until subsequent analyses.
Biochemical analysis
Total lipid was extracted from plasma and platelet tissue using a method adapted from Folch et al. (Reference Folch, Lees and Sloane Stanley24). Internal standard, heptadecanoic acid (C17 : 0), was added to all the samples before extraction at a concentration of 1 mg/ml. The lipid extracts were esterified with boron trifluoride in methanol (Sigma Aldrich Company Limited). FAME were quantified using an Agilent 5975C GC MS (Agilent Technologies UK Limited, Stockport, UK) operated in split mode with a BPX70 capillary GC column (SGE Analytical Science) (length 100 m, internal diameter 0·25 mm and film thickness 0·25 μm) and using He as the carrier gas. The samples were injected at a temperature of 160°C and the temperature was ramped at 2°C/min to 208°C and held for 15 min and then at 1°C/min to 220°C, where it was held for 25 min. Fatty acids were identified by their retention time with reference to those of commercially available fatty acid standards (Sigma Aldrich Company Limited) and were quantified by use of an internal standard, heptadecanoic acid (C17 : 0) (Sigma Aldrich Company Limited).
Serum total cholesterol, HDL-cholesterol and TAG were analysed using the ILAB 650 Clinical Chemistry Analyser with ILAB test reagents (Instrumentation Laboratory, Warrington, Cheshire, UK). HDL-cholesterol was measured using the direct method. LDL-cholesterol was calculated using the Friedewald equation, formulated as(Reference Warnick, Knopp and Fitzpatrick26):
Dietary assessment
Dietary intake was assessed at baseline and at the 3-week point of the intervention using a 4 d food diary, which the subjects completed over two weekdays and two weekend days. The reported intake at 3 weeks was taken to represent the mean food and nutrient intakes during intervention. This assessment allowed mean daily macronutrient, micronutrient and fatty acid intake of each subject before and during the study period to be evaluated. Fatty acid measurements from meat consumed by the study participants were used to supplement the existing data for beef and lamb within food composition tables. Any meat consumed before the study and recorded in baseline food diaries was assumed to have a fatty acid composition comparable to that of meat from concentrate-fed animals. The prevalence of under-reporting (kcal/d) of energy intake was determined using the formula energy intake reported:BMR < 1·1, adapted from Goldberg et al. (Reference Goldberg, Black and Jebb27). Subjects completed an additional meat diary on days where they consumed any of the meat portions to aid compliance by recording details of any leftovers.
Anthropometric and blood pressure measures
Weight (kg) and height (m) of the participants were measured at baseline (with weight also measured at post-intervention) using calibrated scales and a stadiometer, respectively. BMI was calculated as weight (kg)/height2 (m2). Blood pressure was measured at both baseline and post-intervention using a blood pressure monitor (Omron, Milton Keynes, Bucks, UK).
Statistical analysis
Sample size was determined using power calculations based on a similar study(Reference Sinclair, Johnson and O'Dea19) where the consumption of beef significantly increased LC n-3 PUFA concentrations within plasma phospholipids of healthy subjects, with a difference between means of 0·59/100 g and with a standard deviation of 0·37. It was predicted that a sample of thirty subjects (n 15) would be required to find significant differences at a level of 5 % and with a power of 80 %. Therefore, it was decided that a sample size of forty (n 20) would allow for potential dropouts during the study.
The SPSS version 11.5 (Chicago, IL, USA) statistical package was used for all data analysis. Data were initially tested for normality. Comparisons of baseline characteristics, platelet fatty acid status and serum lipids between groups were analysed by one-way ANOVA. Where there were significant differences between values at baseline, differences in plasma fatty acid status, nutrient intake and meat fatty acid composition between groups at post-intervention were analysed by ANCOVA, adjusting for appropriate covariates with Bonferroni correction. All the data are presented as means and standard deviations or means with their standard errors where appropriate and all the data were considered significant at P < 0·05.
Results
Of the forty volunteers who were recruited, two withdrew as a result of being unable to commit to the study requirements. Therefore, eighteen subjects in the group who consumed meat from grass-fed animals and twenty subjects in the group who consumed meat from concentrate-fed animals successfully completed the study by consuming all portions of provided beef and lamb per week for 4 weeks. None of the subjects reported difficulty with compliance, and meat intakes during the intervention were not significantly different from baseline. No subjects reported in their food diaries of having consumed either oily fish or any n-3 PUFA-enriched foodstuffs during the week before the study commenced or during the 4-week study period. Results of the meat diaries showed subjects reported consuming almost all provided portions of mince (97 %), steak (99 %) and lamb (98 %) throughout the 4 weeks. No subjects reported difficulty in consuming any of the meat provided. Dietary data were available for thirty-seven of the thirty-eight subjects.
Anthropometry
Mean values for the subjects' age, height, weight, BMI, blood pressure and lipid profiles at baseline are presented in Table 1. There were no significant differences with respect to any subject characteristics between the two groups at baseline.
Table 1 Basal characteristics of the study participants (n 38)
(Mean values with their standard deviations, except for age (Mean (range))
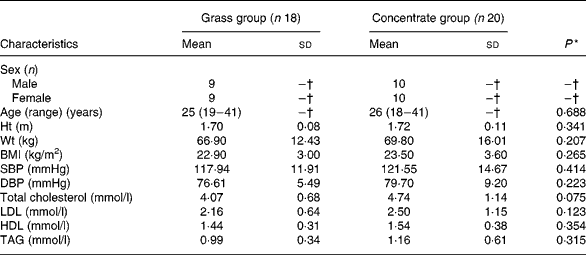
SBP, systolic blood pressure; DBP, diastolic blood pressure.
* Significance in mean values between groups at baseline in one-way ANOVA (P < 0·05).
† Not calculated.
Plasma and platelet fatty acids
Table 2 shows plasma fatty acid composition at baseline and post-intervention for each study group (expressed as % total fatty acids). There were significant differences between groups at baseline for EPA (P = 0·04) and DHA (P = 0·04). In response to the intervention, stearic acid (18 : 0), ALA, DHA, LC n-3 PUFA and total n-3 PUFA were significantly increased (P < 0·05), and the n-6:n-3 ratio was significantly decreased (P < 0·01) within the group that consumed meat from grass-fed animals compared with the group that consumed meat from concentrate-fed animals.
Table 2 Fatty acid composition of plasma at baseline and post-intervention according to study group (% of total fatty acids)
(Adjusted mean values with their standard errors)

LA, linoleic acid; ALA, α-linolenic acid; TVA, trans-vaccenic acid; CLA, conjugated linoleic acid; AA, arachidonic acid; DPA, docosapentaenoic acid.
* Significance in mean values between groups at baseline.
† Significance in mean values between groups at post-intervention with baseline value as covariate in ANCOVA (P < 0·05).
‡ SFA: sum of 14 : 0, 16 : 0 and 18 : 0.
§ MUFA: sum of 16 : 1c and 18 : 1c.
∥ Total n-6 PUFA: sum of LA and AA.
¶ LC n-3 PUFA: sum of EPA, DPA and DHA.
** Total n-3 PUFA: sum of ALA, EPA, DPA and DHA.
†† n-6:n-3: total n-6/total n-3.
Table 3 shows platelet fatty acid composition at baseline and post-intervention for each study group (% total fatty acids). There were no significant differences in fatty acid data between groups at baseline. In response to the intervention, EPA, DPA, DHA, LC n-3 PUFA and total n-3 PUFA were significantly increased (P < 0·05) and the n-6:n-3 ratio was significantly decreased (P < 0·001) within the group that consumed meat from grass-fed animals compared with the group that consumed meat from concentrate-fed animals.
Table 3 Fatty acid composition of platelets at baseline and post-intervention according to study group (% of total fatty acids)
(Observed mean values and standard deviations)

LA, linoleic acid; ALA, α-linolenic acid; TVA, trans-vaccenic acid; CLA, conjugated linoleic acid; AA, arachidonic acid; DPA, docosapentaenoic acid.
* Significance in mean values between groups at baseline.
† Significance in mean values between groups at post-intervention in one-way ANOVA (P < 0·05).
‡ SFA: sum of 14 : 0, 16 : 0 and 18 : 0.
§ MUFA: sum of 16 : 1c and 18 : 1c.
∥ Total n-6 PUFA: sum of LA and AA.
¶ LC n-3 PUFA: sum of EPA, DPA and DHA.
** Total n-3 PUFA: sum of ALA, EPA, DPA and DHA.
†† n-6:n-3: total n-6/total n-3.
Serum lipids and lipoproteins
In response to the intervention, there were no significant differences in serum lipids, lipoproteins, TAG or blood pressure between the study groups (data not shown).
Nutrient intakes
Under-reporting was identified in fourteen of the seventy-four completed food diaries, using the equation energy intake: BMR < 1·1, adapted from Goldberg et al. (Reference Goldberg, Black and Jebb27). Removing these diaries did not result in any notable changes to group intakes of energy, macronutrients or fatty acids; therefore, it was decided not to exclude them from the analysis. At baseline, arachidonic acid (20 : 4n-6) and DPA intakes (P = 0·04) were significantly greater within the group consuming meat from grass-fed animals than those consuming meat from concentrate-fed animals (Table 4); therefore, baseline intakes of each fatty acid were adjusted for in subsequent analyses between groups. During the intervention, arachidonic acid (20 : 4n-6) (P = 0·01) and DPA intakes (P < 0·001) were significantly increased in the group consuming meat from grass-fed animals compared with intakes in the group consuming meat from concentrate-fed animals. There were no other significant differences between groups in response to the intervention. The mean total daily intake of LC n-3 PUFA in subjects in the group consuming meat from grass-fed animals during the intervention was 65 mg/d compared with 44 mg/d in the group consuming meat from concentrate-fed animals. Dietary analysis showed that red meat and other meats were responsible for contributing 94 and 6 % of total LC n-3 PUFA during the intervention within the group consuming meat from grass-fed animals and 87 and 13 % within the group consuming meat from concentrate-fed animals.
Table 4 Fat and fatty acid intakes at baseline and post-intervention according to study group (mg/d)
(Adjusted mean values with their standard errors)

LA, linoleic acid; ALA, α-linolenic acid; AA, arachidonic acid; DPA, docosapentaenoic acid.
* Significance in mean values between groups at baseline.
† Significance in mean values between groups at post-intervention with baseline value as covariate in ANCOVA (P < 0·05).
Fatty acid composition of meat portions
Table 5 shows the concentrations of fatty acids in meat portions from grass-fed and concentrate-fed animals (mg/100 g muscle). Focus has been given to the fatty acid composition of muscle, as it is common to remove adipose tissue before consumption; therefore, the fatty acid composition of muscle should have a greater impact on status(Reference Nute, Francombe and Dransfield28). Results show that the total fat content was significantly increased in all meat portions from concentrate-fed animals than that from grass-fed animals (P < 0·01). Beef steaks from grass-fed animals had significantly higher concentrations of ALA, EPA, LC n-3 PUFA and total n-3 PUFA (P < 0·05) than steaks from concentrate-fed animals. Mince beef from grass-fed animals had significantly lower concentrations of linoleic acid (18 : 2n-6), arachidonic acid and total n-6 PUFA, with significantly higher ALA, EPA, LC n-3 PUFA and total n-3 PUFA than mince from concentrate-fed animals (P < 0·01). Lamb from grass-fed animals had significantly lower concentrations of total SFA, linoleic acid and arachidonic acid (P < 0·001) and significantly higher conjugated linoleic acid (18 : 2c9, t11), DPA, LC n-3 PUFA and total n-3 PUFA than lamb from concentrate-fed animals (P < 0·05). Ratios of n-6:n-3 were significantly lower in all meat portions from animals offered grass compared with those offered concentrate (P < 0·001). The sum of (total n-6, total n-3):SFA ratio was significantly higher in lamb from grass-fed animals (P < 0·001). DHA was not detected in the beef mince samples in meat from either grass-fed or concentrate-fed animals, and it was not significantly increased within steaks or lamb from grass-fed animals possibly owing to limited elongation of this LC n-3 PUFA(Reference Wood, Enser and Fisher29). Steaks, mince and lamb from grass-fed animals contained 25·97, 28·38 and 36·94 mg of LC n-3 PUFA per 100 g muscle, respectively, compared with 18·69, 16·86 and 28·94 mg per 100 g muscle from concentrate-fed animals.
Table 5 Fat content and fatty acid composition of meat portions from animals fed a diet of grass or concentrate (mg/100 g muscle)
(Adjusted mean values with their standard errors)

TVA, trans-vaccenic acid; CLA, conjugated linoleic acid; LA, linoleic acid; AA, arachidonic acid; ALA, α-linolenic acid; DPA, docosapentaenoic acid; ND, not detected.
* Significance in mean values between treatment groups with total fat content as covariate in ANCOVA (P < 0·05).
† SFA: sum of 10 : 0,12 : 0,14 : 0,15 : 0,16 : 0,17 : 0,18 : 0.
‡ MUFA: sum of 14 : 1c,15 : 1c,16 : 1c,16 : 1t,17 : 1c,18 : 1c9,18 : 1c11.
§ Total n-6 PUFA: sum of LA and AA.
∥ LC n-3 PUFA: sum of EPA, DPA and DHA.
¶ Total n-3 PUFA: sum of ALA, EPA, DPA and DHA.
** n-6:n-3: total n-6/total n-3.
†† P:S: sum of (total n-6, total n-3):SFA.
Discussion
The present study demonstrates that moderate consumption of red meat from grass-fed animals can contribute to increased plasma and platelet LC n-3 PUFA concentrations among healthy individuals. Sinclair et al. (Reference Sinclair, Johnson and O'Dea19) previously reported that 500 g/d of lean beef could increase plasma concentrations of LC n-3 PUFA compared with an intake of 30–100 g/d of beef. In the present study, the approximate daily intake of red meat (67 g) is similar to the quantity which 88 % of the Irish population are presently consuming(Reference Cosgrove, Flynn and Kiely12), suggesting that it may be possible to modify total LC n-3 PUFA intakes in this population without changing dietary habits. Furthermore, this intake is below the upper limit of red meat consumption advised by the World Cancer Research Fund(23), and, as such, is not thought to cause any negative effect to health.
Animals were offered grass for a 6-week period before slaughter. The LC n-3 PUFA concentrations found within meat from grass-fed animals compared well with those reported by others for beef(Reference Realini, Duckett and Brito30, Reference Nurnberg, Nurnberg and Ender31) and lamb(Reference Aurousseau, Bauchart and Calichon2). Intake of LC n-3 PUFA when red meat from grass-fed animals was included in the diet was estimated at 65 mg/d, compared to 44 mg/d when red meat from concentrate-fed animals was consumed. The difference in LC n-3 PUFA intake between groups attributed to the red meat consumed was estimated at 18 mg/d, an acknowledgeable low intake which was nonetheless shown to contribute to increased plasma and platelet LC n-3 PUFA status. Fish consumption can make it difficult to isolate and measure the effect of meat consumption on n-3 PUFA status(Reference Li, Zhang and Hsu-Hage32). In the present study, however, the subjects omitted fish from their diet for the 4-week study duration and were infrequent consumers of n-3 PUFA-enriched foodstuffs. The dietary data suggest that red meat was the primary component responsible for the rise in blood concentrations of LC n-3 PUFA within the group that consumed meat from grass-fed animals compared with the group that consumed meat from concentrate-fed animals. Limitations associated with dietary analysis and food composition tables must be considered in the interpretation of dietary intake data, where LC n-3 PUFA data for many foodstuffs are lacking.
In the present study, an increase in DHA status occurred within the consumers of meat from grass-fed animals. The synthesis of DHA from ALA and EPA is known to be relatively poor(Reference Arterburn, Bailey Hall and Oken33); however, it is probable that DPA could be used to synthesise some DHA in consumers of red meat. The rate of this synthesis has been proposed to be 37 % in humans and was recently described in an animal study where DPA supplementation increased DHA status(Reference Kaur, Begg and Barr16, Reference Pawlosky, Hibbeln and Novotny34). As DHA synthesis occurs in a peroxisomal reaction, it is also possible that this step may be independently regulated from the typical LC n-3 PUFA elongation pathway(Reference Pawlosky, Hibbeln and Novotny34). While acknowledging the complexity of DHA metabolism, it is possible that the observed increase in DHA status within the consumers of meat from grass-fed animals is a result of increased DPA intakes during the intervention, which were significantly greater than intakes within the consumers of meat from concentrate-fed animals.
In the group that consumed meat from grass-fed animals, the increase in LC n-3 PUFA concentrations in platelets was more pronounced than in plasma. As plasma is an effective short-term marker of LC n-3 PUFA status(Reference Fekete, Marosvolgyi and Jakobik35), it is possible that some wash-out of LC n-3 PUFA had occurred between the time of the last meal of meat from grass-fed animals and blood collection at the end of the intervention. In comparison, platelets are a better reflection of long-term LC n-3 PUFA status(Reference Fekete, Marosvolgyi and Jakobik35), and the significant increases observed in both plasma and platelet measures confirm the bioavailability of LC n-3 PUFA from red meat from grass-fed animals. Plasma fatty acid values measured in the present study compare well to those of similar studies, albeit where plasma phospholipids were measured(Reference Sinclair, Johnson and O'Dea19, Reference Li, Zhang and Hsu-Hage32, Reference Vidgren, Agren and Schwab36, Reference Hodge, Simpson and Gibson37).
It was not surprising to observe no significant differences in serum concentrations of cholesterol, TAG or blood pressure between groups. Firstly, there is inconsistent evidence that low doses of LC n-3 PUFA can reduce total or LDL-cholesterol serum concentrations(Reference Gorjao, Azevedo-Martins and Rodrigues38–Reference Balk, Lichtenstein and Chung40). Generally, the LC n-3 PUFA are recognised for their ability to decrease TAG concentrations and this potential has been shown predominantly with LC n-3 PUFA doses >450 mg/d(Reference Geleijnse, Giltay and Grobbee41). Therefore, it is probable that a combined effect of the low dose of LC n-3 PUFA received through the meat from grass-fed animals, the short study duration and the absence of hyperlipidaemia in subjects resulted in a lack of effect on TAG concentrations in the present study. In addition, there is a lack of evidence to show that LC n-3 PUFA can reduce blood pressure at low doses or in non-hypertensive individuals(Reference Hodgson, Wards and Burke42). Nonetheless, it is important to acknowledge the aspect that red meat consumption had no effect on serum cholesterol, TAG or blood pressure in the present study, as it concurs with other studies showing moderate red meat consumption has no negative effects to health(Reference Flynn, Naumann and Nolph43, Reference O'Dea, Traianedes and Chisholm44).
Other means of increasing LC n-3 PUFA content of meat include addition of oilseeds or fish oil in the animal diet(Reference Scollan, Hocquette and Nuernberg45–Reference Ponnampalam, Sinclair and Egan47). For example, Medeiros et al. (Reference Medeiros, Hampton and Kurtzer46) showed reduced concentrations of vascular cell adhesion molecule-1 in rats consuming beef from cattle offered a flaxseed-supplemented diet compared to a typical diet of maize. Moreover, another study showed human consumption of linseed-enriched animal products to cause an increase in plasma concentrations of LC n-3 PUFA(Reference Weill, Schmitt and Chesneau48). However, the advantages of meat from grass-fed animals are that the content of total fat, SFA or trans-fatty acids in the meat are not simultaneously increased(Reference Scollan, Dhanoa and Choi49), the palatability is not affected as natural levels of α-tocopherol in the grass reduce susceptibility to lipid peroxidation(Reference Wood, Enser and Fisher29, Reference Moloney, Mooney and Kerry50) and offering animals a grass diet would be more cost-effective to the producer and more sustainable with respect to the environment than feeding concentrates to the animals. However, future studies should consider increasing the length of the finishing period to allow greater increments in LC n-3 PUFA concentrations in meat tissue of grass-fed animals to occur.
Overall, the present study has shown that an animal diet of grass before slaughter can help to increase the LC n-3 PUFA content of red meat. Furthermore, increases in plasma and platelet concentrations of LC n-3 PUFA were observed among consumers of this meat. This observation may have implications for the red meat industry, where increased production of red meat from grass-fed animals would have greater appeal to the consumer, adding marketable value to the product. Furthermore, the consumption of red meat from grass-fed animals may contribute to raising the overall LC n-3 PUFA intake closer to the recommended intake of 450 mg/d(6) without a change being made to dietary habits, which in turn would be beneficial for cardiovascular health.
Conclusions
The present study is novel in the sense that an animal diet of grass before slaughter has been shown to significantly have an impact on LC n-3 PUFA status in free-living healthy consumers of red meat and at a level of consumption similar to the present intakes among the Irish population. Overall, the results of the present study suggest that consumption of red meat from grass-fed animals may provide valuable amounts of LC n-3 PUFA to the consumer and increased production of red meat from grass-fed animals may thereby help to increase LC n-3 PUFA intakes of consumers.
Acknowledgements
The present study was funded by the Department of Employment and Learning Co-operative Award in Science and Technology, AgriSearch and the Livestock and Meat Commission for Northern Ireland. The authors declare that there are no conflicts of interest. All authors have contributed to the paper and agree with the present version of the paper. E. M. Mc. S., J. M. W. W., J. J. S. and G. J. C. helped to design the study. A. J. Mc. A. contributed to volunteer recruitment, collection of data, laboratory work, data interpretation and writing of the paper. J. A. M. B. contributed to analysis of fatty acids in meat by GC technology. G. J. C., A. M. F. and B. W. M. assisted with the sourcing of meat and the preparation of meat portions. J. M. W. W. and E. M. Mc. S. contributed to data interpretation and writing of the paper. G. J. C., A. M. F., B. W. M., M. P. B. and J. J. S. also contributed to writing of the paper.







