Non-alcoholic fatty liver disease (NAFLD) is one of the most common manifestations of chronic liver disorders worldwide(Reference Bellentani and Tiribelli1). NAFLD represents a spectrum of hepatic dysfunctions that occur in the absence of alcohol consumption in amounts generally considered to be harmful to the liver. It is characterised by histological changes consisting in an increased accumulation of lipids into small droplets inside the cytoplasm, in hepatocytes. This phenomenon occurs when the rate of hepatic fatty acid uptake from plasma and de novo fatty acid synthesis are greater than that of fatty acid oxidation and release of TAG within VLDL(Reference Fabrini, Sullivan and Klein2). NAFLD is closely associated with obesity and insulin resistance(Reference Marchesini, Bugianesi and Forlani3).
There are many models of NAFLD in animals that have been used in many studies. One of these models is the obese Zucker rat. This animal strain shows an earlier obesity, which is accompanied by many of the human metabolic syndrome features and liver steatosis. Peripheral insulin resistance in obese Zucker rats enhances the mobilisation of peripheral fat and the serum level of NEFA. However, the liver oxidation or utilisation of NEFA is inhibited. Thus, the liver in this rodent model synthesises excess TAG and oxidises a small amount of fatty acids, leading to fat infiltration of the hepatic parenchyma(Reference Argiles4).
Resveratrol (trans-3,5,4′-trihydroxystilbene) is a phytoalexin polyphenolic compound occurring naturally in various plants, including grapes, berries and peanuts, in response to stress and as a defence mechanism against fungal, viral, bacterial infections and damage from exposure to UV radiation(Reference Langcake and Pryce5, Reference Signorelli and Ghidoni6).
A remarkable range of biological functions have been ascribed to this molecule. For example, it acts as a cancer chemoprevention agent, as a powerful anti-inflammatory factor and as an antioxidant agent(Reference Frémont7, Reference Goswami and Das8). Cardiovascular properties of resveratrol, including inhibition of platelet aggregation and promotion of vasodilation by enhancing the production of NO, have also been described(Reference Cucciolla, Borriello and Oliva9). More recently, it has been demonstrated that resveratrol can modify TAG metabolism in adipose tissue and liver(Reference Baur, Pearson and Price10–Reference Szkudelska and Szkudelski18).
The aim of the present study was to assess the effect of two graded doses of resveratrol on liver fat accumulation in the fa/fa Zucker rat model of steatosis. To determine some of the mechanisms underlying this effect, we also analysed the influence of this polyphenol on the activity of enzymes involved in lipogenesis and fatty acid oxidation.
Materials and methods
Animals, diets and experimental design
The experiment was conducted on thirty male Zucker (fa/fa) rats aged 6 weeks (213 (sem 4) g) purchased from Harlan Ibérica (Barcelona, Spain) and took place in accordance with the institution's guide for the care and use of laboratory animals. The rats were individually housed in polycarbonate metabolic cages (Tecniplast Gazzada, Buguggiate, Italy) and placed in an air-conditioned room (22 ± 2°C) with a 12 h light–dark cycle. After a 6 d adaptation period, rats were randomly distributed in three experimental groups of ten animals each, and fed on a standard laboratory diet (Panlab, Barcelona, Spain). Rats in the resveratrol groups were orally administered resveratrol (15 mg/kg body weight per d in RSV15 group and 45 mg/kg body weight per d in RSV45 group) through an orogastric catheter for 6 weeks. Resveratrol was diluted in 1 ml ethanolic solution (20 %). Rats from control group (C group) received only the vehicle. All animals had free access to food and water. Food intake and body weight were measured daily.
At the end of the experimental period and after a fasting period of 6–8 h, animals were killed by cardiac exsanguination under anaesthesia by using an intraperitoneal injection of an overdose (45 mg/kg) of sodium pentobarbital. White adipose tissue from different anatomical locations (perirenal, epididymal, mesenteric and subcutaneous regions) and liver were dissected, weighed and immediately frozen.
Steatosis assessment
Total lipids were extracted from liver following the method described by Folch et al. (Reference Folch, Lees and Sloane Stanley19). The lipid extract was dissolved in isopropanol. TAG and cholesterol contents were measured by using commercial kits (Spinreact, Barcelona, Spain). Moreover, a histological study was performed on the livers. Just after killing, a piece of liver was placed in 10 % buffered formalin and subsequently embedded in paraffin. Liver sections were stained with haematoxylin and eosin using standard techniques. Sections were viewed without knowing the treatment group to which each animal belonged. Biopsies were classified into four grades depending on fat accumulation using Brunt et al. (Reference Brunt, Janney and Di Bisceglie20) classification, assigning grade 0 when no fat was found in the liver, grade 1 when fat vacuoles were seen in less than 33 % of hepatocytes, grade 2 when 33–66 % of hepatocytes were affected by fat vacuoles and grade 3 when fat vacuoles were found in more than 66 % of hepatocytes. Two experienced pathologists who were masked to the experiment evaluated all samples, and reached an agreement.
Enzyme activities
For lipogenic enzyme analysis, samples of liver (500 mg) were homogenised in 5 ml buffer (pH 7·6) containing 150 mm-KCl, 1 mm-MgCl2, 10 mm-N-acetylcysteine and 0·5 mm-dithiothreitol. After centrifugation at 100 000 g for 40 min at 4°C, the supernatant fraction was used for quantitation of the enzyme activities. Fatty acid synthase (FAS, EC 2.3.1.85), glucose-6-phosphate dehydrogenase (G6PDH, EC 1.1.1.49), malic enzyme (EC 1.1.1.40) and acetyl-CoA carboxylase (ACC, EC 6.4.1.2) activities were measured as previously described(Reference Zabala, Churruca and Macarulla21). Enzyme activities were expressed either as nmol NADPH consumed (FAS), nmol NADPH produced (G6PDH and malic enzyme) or nmol HCO3− incorporated (ACC), per min, per mg of protein.
Carnitine palmitoyltransferase-Ia (CPT-Ia) and ACO activities were assessed in the mitochondrial/peroxisomal fraction. Liver samples (500 mg) were homogenised in 3 volumes (wt/vol) of buffer (pH 7·4) containing 0·25 mol/l sucrose, 1 mm-EDTA and 10 mm-Tris–HCl. Homogenates were centrifuged (700 g for 10 min at 4°C) and supernatant fluid was again centrifuged (12 000 g for 15 min at 4°C). Pellets were resuspended in 70 mm-sucrose, 220 mm-mannitol, 1 mm-EDTA, 2 mm-HEPES buffer (pH 7·4). CPT-I activity was assayed by using Bieber et al. (Reference Bieber, Abraham and Helmrath22) method and ACO activity was assayed following the Lazarow method(Reference Lazarow23). The pellet protein content was determined according to the Bradford method(Reference Bradford24). CPT-I activity was expressed as nmol CoA formed/min per mg protein and ACO activity as nmol NADH formed/min per mg protein.
Oxidative stress parameters
Lipid peroxidation was determined by measuring the formation of thiobarbituric acid reactive substrates TBARS (TBARS Assay Kit; Cayman Chemical Company, Ann Arbor, MI, USA) in liver homogenates. Concentrations were measured against a standard curve obtained with malonaldehyde. TBARS values were expressed as μM malonaldehyde/mg protein.
GSH and GSSG levels were determined spectrophotometrically by using a commercial kit (Glutathione Detection Kit; Assay Designs, Plymouth Meeting, PA, USA). The amounts of GSH and GSSG were calculated from a standard curve and were expressed in μm/mg protein. The redox index was calculated by dividing the concentration of GSH by that of GSSG.
For superoxide dismutase (SOD) assessment a commercial kit was also used (Superoxide Dismutase Activity Assay Kit; BioVision Research Products, Mountain View, CA, USA).
Serum parameters
For the evaluation of serum parameters commercial kits were used: NEFA (Roche Diagnostics GmbH, Mannheim, Germany); TAG; total cholesterol and HDL-cholesterol (BioSystems, Barcelona, Spain), glycerol (BioVision Research Products); ketone bodies (Roche Diagnostics GmbH); glucose (BioSystems); insulin (EZRMI 13K; Linco, St Charles, MO, USA); aspartate aminotransferase (AST/GOT); alanine aminotransferase (ALT/GPT); alkaline phosphatase (Spinreact) activities, and adiponectin (catalogue no. EZRADP-62K; Millipore Iberica, Madrid, Spain). Cholesterol, TAG, AST, ALT and alkaline phosphatase levels were determined in fresh samples and other parameters were assessed in frozen samples.
Non-HDL-cholesterol was calculated by the difference between total cholesterol and HDL-cholesterol.
Statistical analysis
Results are presented as means with their standard errors. Statistical analysis was performed using SPSS 17.0 (SPSS, Inc., Chicago, IL, USA). Data were analysed by ANOVA I test and Newman–Keuls as post hoc test. Statistical significance was set up at the P < 0·05 level.
Results
Body weight, food intake and white adipose tissue weights
Rats treated with resveratrol showed significantly reduced final body weight (P < 0·05), without significant changes in food intake. The reduction in body weight was in part accounted for by a reduction in adipose tissue. More specifically, a significant reduction was observed in internal depots (perirenal+epididymal+mesenteric; P < 0·01). No significant differences were found between both resveratrol doses (Table 1).
Table 1 Final body weight, body weight increase, food intake and adipose tissue weights of obese Zucker rats treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 and 45 mg/g body weight per d in RSV45) for 6 weeks
(Mean values with their standard errors, n 10)
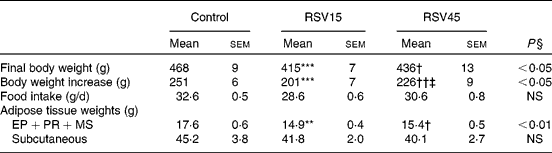
EP, epididymal; PR, perirenal; MS, mesenteric.
Values were significantly different (Newman–Keuls test): control v. RSV15 (** P < 0·01; *** P < 0·001); control v. RSV45 († P < 0·05; †† P < 0·01); RSV15 v. RSV45 (‡ P < 0·05).
§ Values were significantly different among groups (ANOVA).
Liver weight and fat content
Resveratrol significantly reduced liver weight (P < 0·001; Fig. 1(A)). Hepatic index, obtained by dividing liver weight by body weight × 100, was also lower in rats supplemented with resveratrol (P < 0·05; Fig. 1(B)). TAG content was significantly reduced by resveratrol when expressed either as mg/g tissue (P < 0·001) or as total amount (g) (P < 0·001; Fig. 2(A) and (B)) but no differences were found between both doses. No significant changes were induced by resveratrol in cholesterol content (6·74 (sem 0·44) mg/g in the control group, 6·73 (sem 0·46) mg/g in the RSV 15 group and 5·82 (sem 0·27) mg/g in the RSV45 group).
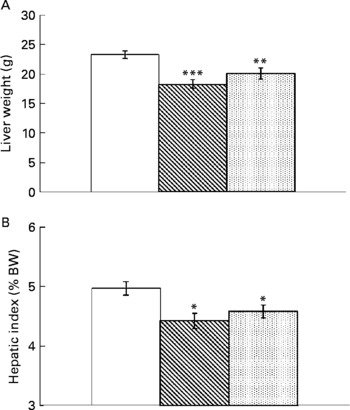
Fig. 1 (A) Liver weight and (B) hepatic index (expressed as percentage of body weight (BW)) in obese Zucker rats either treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 (![]() ) and 45 mg/kg body weight per d in RSV45 (
) and 45 mg/kg body weight per d in RSV45 (![]() )) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: * P < 0·05, ** P < 0·01, *** P < 0·001. □, Control.
)) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: * P < 0·05, ** P < 0·01, *** P < 0·001. □, Control.
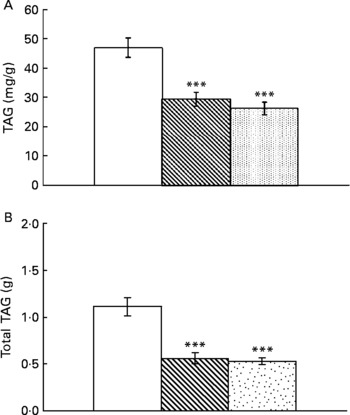
Fig. 2 (A) Hepatic TAG content expressed as mg/g tissue or (B) total amount in obese Zucker rats either treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 (![]() ) and 45 mg/kg body weight per d in RSV45 (
) and 45 mg/kg body weight per d in RSV45 (![]() )) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: *** P < 0·001. □, Control.
)) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: *** P < 0·001. □, Control.
Mean fat infiltration in the non-treated rats was 3. Fat depot in this group was classified as macrovesicular. Mean fat infiltration in the RSV15 group was 1, and fat depot was mixed. Finally, fat infiltration in the RSV45 group was 2 (Fig. 3). Inter-observer agreement was 0·84 and intra-observer agreement was 0·79.

Fig. 3 Histological study in liver from obese Zucker rats either treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 and 45 mg/kg body weight per d in RSV45) for 6 weeks. Haematoxylin and eosin staining of liver tissue × 40.
Enzyme activities
No significant differences in the activity of lipogenic enzymes were found among the three experimental groups (Fig. 4). On the contrary, resveratrol significantly increased CPT-Ia activity (P < 0·05) and ACO activity (P < 0·05) in both RSV groups (Fig. 5).

Fig. 4 Glucose-6P-dehydrogenase (G6PDH), malic enzyme (ME), fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) activities in liver from obese Zucker rats either treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 (![]() ) and 45 mg/kg body weight per d in RSV45 (
) and 45 mg/kg body weight per d in RSV45 (![]() )) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. □, Control.
)) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. □, Control.

Fig. 5 Acyl-coenzyme A oxidase (ACO) and carnitine palmitoyltransferase-Ia (CPT-Ia) activities in liver from obese Zucker rats either treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 (![]() ) and 45 mg/kg body weight per d in RSV45 (
) and 45 mg/kg body weight per d in RSV45 (![]() ) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: * P < 0·05, ** P < 0·01. □, Control.
) for 6 weeks. Values are means, with their standard errors represented by vertical bars, n 10. Mean values were significantly different: * P < 0·05, ** P < 0·01. □, Control.
Serum parameters
Resveratrol significantly reduced concentration of NEFA (P < 0·001) and total cholesterol (P < 0·01). In the RSV15 group levels of both HDL-cholesterol and non-HDL-cholesterol were significantly decreased (P < 0·01), but in the RSV45 group only the HDL fraction was reduced (P < 0·01). Although levels of serum TAG were not significantly modified, a trend (P = 0·07) towards reduced values was observed in the RSV15 group. Concentrations of glucose, glycerol and ketone bodies remained unchanged. Regarding parameters related to liver damage, values of AST/GOT, ALT/GPT and alkaline phosphatase were significantly reduced by the lowest dose (P < 0·05). A trend towards lower values was observed with the high dose (P = 0·07) in transaminases. Although statistical significance was not reached in insulin concentrations, a clear tendency (P = 0·057) towards increased values was found in the resveratrol-treated groups. No changes were observed in adiponectin (Table 2).
Table 2 Serum parameters of obese Zucker rats treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 and 45 mg/g body weight per d in RSV45) for 6 weeks
(Mean values with their standard errors, n 10)

AcAc, acetoacetate; β-HB, β-hydroxybutyrate; AST/GOT, aspartate aminotransferase; ALT/GPT, alanine aminotransferase; ALP, alkaline phosphatase.
Values were significantly different between groups (Newman-Keuls test): control v. RSV15 (* P < 0·05; ** P < 0·01; *** P < 0·001); control v. RSV45 († P < 0·05; †† P < 0·01; ††† P < 0·001); RSV15 v. RSV45 (‡ P < 0·05; ‡‡ P < 0·01).
§ Values were significantly different among groups (ANOVA).
Oxidative stress
Resveratrol significantly decreased hepatic TBARS formation, indicating an antioxidant effect and protection from the oxidative stress induced by obesity and steatosis in Zucker rats (Table 3). Also, the high dose was able to diminish the amount of GSSG as well as to increase the GSH:GSSG ratio, a sensitive and reliable measure of the overall level of oxidative stress (Table 3). These results suggest that the glutathione redox state has become less pro-oxidising due to supplementation with resveratrol. However, the reactive oxygen species scavenging enzyme superoxide dismutase seems not to be involved in the resveratrol-induced reduction of oxidative stress.
Table 3 Oxidative stress parameters in liver of obese Zucker rats treated or not treated with resveratrol (15 mg/kg body weight per d in RSV15 and 45 mg/g body weight per d in RSV45) for 6 weeks
(Mean values with their standard errors, n 10)
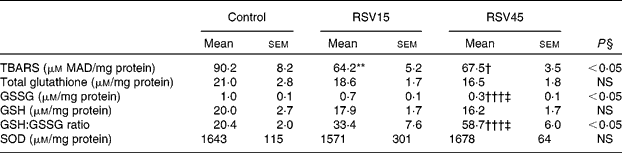
MAD, malonaldehyde; SOD, superoxide dismutase.
Values were significantly different between groups (Newman–Keuls test): control v. RSV15 (** P < 0·01); control v. RSV45 († P < 0·05; ††† P < 0·001); RSV15 v. RSV45 (‡ P < 0·05).
§ Values were significantly different among groups (ANOVA).
Discussion
Resveratrol has been reported to have significant effects on lipid metabolism. In this context, several studies have shown reduced fat accumulation either in isolated adipocytes or in adipose tissue(Reference Lagouge, Argmann and Gerhart-Hines11, Reference Ahn, Cho and Kim13) and liver(Reference Shang, Chen and Xiao14, Reference Rivera, Morón and Zarzuelo16, Reference Bujanda, Hijona and Larzabal25), increased fatty acid oxidation in skeletal muscle(Reference Baur, Pearson and Price10, Reference Lagouge, Argmann and Gerhart-Hines11) and reduced serum lipids(Reference Rivera, Morón and Zarzuelo16) induced by this polyphenol. All these effects support the hypothesis that resveratrol could be a useful molecule for the prevention of obesity, steatosis and dyslipidaemia.
In the present study, the effects of resveratrol on serum lipids, liver lipids and body fat accumulation have been assessed in fa/fa Zucker rats, an animal model that shows obesity, liver steatosis and dyslipidaemia. Nevertheless, as described in the beginning, the main purpose of the study was to analyse the effects of this polyphenol on liver TAG metabolism.
Liver in obese Zucker rat shows an increased lipogenesis, potentiated by a huge glycolytic flux that leads to the formation of reduction potential in the form of NADPH, and a strongly reduced fatty acid oxidation. Plasma concentrations of glycerol and NEFA are high, which represents an increased flux of these metabolic substrates to the liver for TAG synthesis. Taken together, these alterations lead to steatosis(Reference Argiles4).
In the present study, resveratrol treatment resulted in a significant reduction in liver weight. This was not just a consequence of the generalised reduction in body weight, but a direct effect on the organ because significant differences were also observed in the hepatic index (percentage of liver weight with regard to final body weight).
The decrease in liver weight was partly due to a reduction in TAG, as revealed by the assessment of the histological analysis. This is a semi-quantitative system referred to as ‘grading’ of steatosis(Reference Kleiner, Brunt and Natta26). Quantifying fat biochemically is a more objective and accurate method of determining the fat content in liver tissue. Thus, we also analysed the amount of liver TAG by using a spectrophotometric method. The results confirmed the de-lipidating effect of resveratrol. This effect is in good accordance with other published studies performed either in mice(Reference Baur, Pearson and Price10, Reference Zang, Xu and Maitland-Toolan12, Reference Ahn, Cho and Kim13) or in rats(Reference Shang, Chen and Xiao14, Reference Rivera, Morón and Zarzuelo16, Reference Bujanda, Hijona and Larzabal25).
Taking into account that lipogenesis and fatty acid oxidation are two key metabolic pathways in the control of hepatic TAG metabolism and hence in fat accumulation in the liver, and considering that both of them are altered in obese Zucker rats, the effects of resveratrol on the activity of several enzymes involved in these pathways were investigated to assess the mechanisms by which this polyphenol decreased liver TAG content. To the best of our knowledge, this is the first paper that analyses the effects of resveratrol on the balance between fatty acid oxidation and activities of lipogenesis enzyme in the liver, to explain its beneficial effects on hepatic steatosis.
As far as lipogenic enzymes are concerned, no changes were observed in the activities of G6PDH and malic enzyme, the enzymes involved in the production of NADPH, or in ACC and FAS. No data concerning the effects of resveratrol on the activity of G6PDH, malic enzyme and FAS in liver have been reported in the literature. On the contrary, the effect of resveratrol of FAS mRNA levels has been addressed. Shang et al. (Reference Shang, Chen and Xiao14) reported that levels of FAS mRNA were significantly reduced in rats treated with resveratrol (100 mg/kg body weight) for 16 weeks. The results in the present study are not in good accordance with those reported by Shang et al. (Reference Shang, Chen and Xiao14). The discrepancy between the lack of effect on FAS activity in the present study and the reduction in the gene expression in the reported study can be due to important differences in experimental design. Whereas in the study reported by Shang et al. (Reference Shang, Chen and Xiao14) the dose of resveratrol was 100 mg/kg body weight and the length of the treatment was 16 weeks, in the present study lower doses (15 and 45 mg/kg body weight) and a shorter experimental period (6 weeks) were used. It can be hypothesised that FAS needs high doses of resveratrol and/or longer treatments to be modified. Moreover, whereas in the study reported by Shang et al. (Reference Shang, Chen and Xiao14) resveratrol was administered 4 h before killing, in the present study the last resveratrol administration took place the day before killing. Taking into account the short half-life of this polyphenol(Reference Bertelli, Giovannini and Stradi27), this is an important difference between both studies. Ahn et al. (Reference Ahn, Cho and Kim13) found the same effects in the liver of a mice who was fed a diet enriched with resveratrol (0·0125 %) for 8 weeks. Once again this experimental period is longer than the one used in the present study. Moreover, mice are the most sensitive species with regard to the effects of some functional ingredients. This has been demonstrated, for instance, in the case of conjugated linoleic acid(Reference Zabala, Churruca and Macarulla28).
It should be also pointed out that the reduction in FAS mRNA levels observed by Shang et al. (Reference Shang, Chen and Xiao14) was probably related to the decrease in serum insulin induced by resveratrol that they observed. In the present study, serum insulin concentrations in resveratrol-treated groups showed a clear tendency towards increased values. The effect of resveratrol on insulin levels is unclear. Thus, while several authors have reported decreased values of serum insulin in resveratrol-treated animals, others authors have found increased values(Reference Szkudelska and Szkudelski29). Nevertheless, and despite all the mentioned differences, it should be emphasised that while Shan et al. (Reference Shang, Chen and Xiao14) and Ahn et al. (Reference Ahn, Cho and Kim13) evaluated FAS expression, the present study measured FAS activity. It is well known that although one of the most important mechanisms of FAS regulation takes place at the transcriptional level, other post-transcriptional mechanisms also contributes to enzyme activity regulation(Reference Katsurada, Iritani and Fukuda30, Reference Kim, Park and Kim31).
With regard to ACC, it has been proposed that resveratrol can phosphorylate this enzyme by AMP-activated protein kinase, thus leading to a decrease in its activity. This effect was observed by Rivera et al. (Reference Rivera, Morón and Zarzuelo16) in obese Zucker rats treated with resveratrol for 8 weeks and also by Shang et al. (Reference Shang, Chen and Xiao14) in the afore-mentioned paper. In the present study, instead of measuring the phosphorylation of ACC to estimate the activation/deactivation process, we directly measured the activity of the enzyme by radiometry. Interestingly, this activity was not reduced in the rats treated with resveratrol.
To understand these results it is important to remember that the short-term control of ACC activity is achieved not only by phosphorylation/dephosphorylation, but also by allosteric regulation. Citrate and fatty acid concentrations, which can perturb the equilibrium of ACC polymerisation process, are the main molecules responsible for allosteric regulation(Reference Munday32). Citrate activates ACC by causing its polymerisation. Contrastingly, long-chain fatty acyl CoA esters are potent inhibitors of ACC because they promote the dissociation of the polymer into protomers(Reference Tong33).
In the present study, the concentrations of these molecules were not measured but, as stated further on in this discussion, fatty acid availability seems to decrease in the liver of rats treated with resveratrol. Thus, a compensation between ACC inhibition, due to an increased phosphorylation, and the enzyme activation, due to the reduction of the physiological inhibitor (fatty acyl-CoA), probably led to the lack of change when the activity of this enzyme was directly measured.
Altogether these results show that, under the present experimental conditions, a reduction in de novo lipogenesis did not contribute to the reduction in liver TAG induced by resveratrol.
The effect of resveratrol on the activities of CPT-Ia, a key enzyme in mitochondrial fatty acid oxidation, and ACO, a key enzyme in peroxisome fatty acid oxidation, was also assessed in the present study. Resveratrol treatment resulted in increased activity of both enzymes. It has been proposed that resveratrol, by activation of sirtuins(Reference Howitz, Bittrman and Cohen34, Reference Borra, Smith and Denu35), deacetylates and thus activates the peroxisome proliferator-activated receptor-γ coactivator-1(Reference Szkudelska and Szkudelski18), leading to increased mitochondrial activity and function(Reference Baur, Pearson and Price10, Reference Szkudelska and Szkudelski18, Reference Medina-Gómez, Gray and Vidal-Puig36). The increased CPT-Ia activity observed in the present study supports this hypothesis. The lack of changes in serum ketone bodies is noteworthy. Perhaps the increase in enzyme activity was not high enough to enhance this parameter. A similar situation has been reported by other authors. Stefanovic-Racic et al. (Reference Stefanovic-Racic, Perdomo and Mantell37) observed that 60 % over-expression of CPT-Ia in liver resulted in a significant increase in the rate of fatty acid oxidation (45 %) and a significant decrease in TAG content (70 %), without changes in plasma ketone bodies.
Adiponectin appears to have a pivotal role in improving fatty acid oxidation and decreasing fatty acid synthesis(Reference Xu, Wang and Keshaw38). The liver has adiponectin receptors, and their stimulation leads to increased fatty acid β-oxidation and thereby decreased hepatic TAG content. In the present study, despite the significant reduction induced by resveratrol in fat depot weights, no changes were observed in serum adiponectin concentrations among the three experimental groups, meaning that the effect of resveratrol on liver fatty acid oxidation was not mediated by this adipokine.
As stated in the beginning, plasma NEFA levels are high in obese Zucker rats because of increased adipose tissue mass and peripheral insulin resistance. These lipids can enter the hepatocyte and there they play an important role in stimulating hepatic TAG production(Reference Raz, Eldor and Cernea39). Inside the hepatocytes NEFA can be either oxidised or esterified. In obese Zucker rats, enhanced esterification and reduced oxidation leads to ectopic deposition of TAG in the liver. In the present study resveratrol significantly reduced serum NEFA. This effect was probably related to the observed reduction in adipose tissue mass ( − 15·3 % and − 12·5 % in internal depots for the RSV15 and RSV45 groups, respectively, and − 7·5 and − 11·3 % in the subcutaneous depot for the RSV15 and RSV45 groups, respectively). Nevertheless, the involvement of a potential reduction in the ex vivo lipoprotein lipase-dependent VLDL-TAG hydrolysis cannot be discarded(Reference Pruneta, Autran and Ponsin40) because in the present experimental design blood collected was not treated with anti-lipolytic agents to block this process. Although NEFA flux into the liver was not directly assessed in the present study, it could be hypothesised that it may be reduced.
Collectively, these results demonstrate that resveratrol reduces the availability of NEFA, an important substrate for TAG synthesis in the liver, by increasing their oxidation and probably by reducing their flux from plasma, thus resulting in a decrease in fat accumulation.
Both mitochondrial and peroxisomal fatty acid oxidation are capable of producing hepatotoxic free oxygen radicals that contribute to the development of oxidative stress(Reference Duvnjak, Lerotic and Barsic41), an imbalance between oxidants and antioxidants systems in favour of the former. Moreover, it has been demonstrated that oxidative stress can, at least to some extent, be responsible for further progression from steatosis to steatohepatitis and fibrosis(Reference Spolarics and Meyenhofer42, Reference Albano, Mottaran and Vidali43). In the present study, fatty acid oxidation was likely to increase as a consequence of enhanced CPT-Ia and ACO activities. In this context, the levels of oxidative stress are a matter of concern. With regard to this issue, it should be pointed out that resveratrol has been reported to show antioxidant properties, but it is important not to forget that resveratrol, as well as other antioxidants, can become pro-oxidant at high doses(Reference Van der Spuy and Pretorius17, Reference Faine, Rodrigues and Galhardi44, Reference Valdecantos, Pérez-Matute and Martínez45).
To shed light onto this situation, we analysed several markers of hepatic oxidative stress. The biological antioxidant defence system is an integrated array of enzymes and antioxidants(Reference Yu46). TBARS levels are one of the most extensively used markers of lipid peroxidation and oxidative damage. Moreover, the glutathione antioxidant system is considered very efficient at decreasing the presence of free radicals. GSH is required to maintain the normal reduced state and to counteract the deleterious effects of oxidative stress. It is commonly recognised that reduction in GSH:GSSG ratio denotes the presence of oxidative stress. Superoxide dismutase is the enzyme that catalyses the destruction of O2− by dismutation and H2O2 formation.
The present results show that resveratrol administration was able to reduce the oxidative damage in liver, measured by TBARS and the amount of GSSG, although not by superoxide dismutase. With this mechanism, resveratrol can limit the progression of liver steatosis. As previously stated in this section, resveratrol, as well as other antioxidants, can become pro-oxidant at high doses. The present results show that in the range of 15–45 mg/kg body weight, this polyphenol maintains its antioxidant properties.
The results reported here show that resveratrol has a beneficial effect on liver in obese Zucker rats because it attenuates steatosis and decreases oxidative stress. The suspicion of NAFLD is usually prompted by abnormal serum liver biochemical findings. Usually the levels of ALT, AST or both are increased mildly to moderately. In the present study, control animals showed high levels of both transaminases, in comparison with published values in normal rats not showing steatosis(Reference Kleiner, Brunt and Natta26, Reference Thong-Ngam, Samuhasaneeto and Kulaputana47), in good accordance with the presence of fatty liver in these animals. Resveratrol induced a reduction in AST, ALT and alkaline phosphatase levels that reached statistical significance in RSV15 group and remained as a tendency in RSV45 group. Both cholesterol and TAG contribute to liver damage, but in the present study changes in transaminases were only related to the decrease in TAG content because cholesterol content remained unchanged.
While liver cholesterol was not modified, serum total cholesterol was significantly reduced in resveratrol-treated rats. Both HDL-cholesterol and non-HDL-cholesterol were decreased in the RSV15 group and HDL-cholesterol in the RSV45 group. Several mechanisms could be involved in these effects: (1) reduced liver cholesterogenesis, a metabolic process that is over-stimulated in obese Zucker rats(Reference Argiles4), according to the reduction in the expression of hydroxymethylglutaryl CoA reductase reported by Cho et al. (Reference Cho, Ahn and Kim48); (2) decreased endogenous cholesterol re-absorption(Reference Sbarra, Ristorcelli and Le Pétit-Thévenin49); (3) increased excretion of bile acids into faeces(Reference Miura, Miura and Yagasaki50) and (4) decreased HDL stability, as reported by Noll et al. (Reference Noll, Hamalet and Ducros51).
In general terms, a dose–response pattern was not found, and only punctual differences were found between both resveratrol doses in several parameters. This suggests that a ‘plateau’ is apparently reached when the beneficial effects of this polyphenol on fatty liver are considered, and thus that the high dose provides no additional benefit over the lowest dose.
In conclusion, this study demonstrates that resveratrol can protect the liver from NAFLD by reducing fatty acid availability. Moreover, it protects this organ from oxidative stress.
Acknowledgements
This study was supported by grants from the Ministerio de Ciencia e Innovación (AGL2008-1005-ALI), Instituto de Salud Carlos III (RETIC PREDIMED and CIBERehd) and Government of País Vasco (IT-386-10; CTP09/R5). S. G. Z. is a recipient of a doctoral fellowship from the University of País Vasco. S. G. Z., A. F. Q. and M. T. M. measured enzyme activities and plasma metabolites; L. A. and F. M. carried out oxidative stress measurements; E. H. and L. B. treated the rats and analysed histological preparations; M. T. M., J. A. M. and M. P. P. designed the research; M. P. P. wrote the paper and had the responsibility for the final content. All authors read and approved the final manuscript. No conflicts of interest are reported by any of the authors.










