Serum ferritin is the widely used marker for total body Fe stores in epidemiological studies, which accurately reflects differences in body Fe stores by sex and age( Reference Zacharski, Ornstein and Woloshin 1 ). Accumulating studies have reported serum ferritin concentrations are associated with insulin resistance (IR) measured by homoeostasis model assessment (HOMA-IR) and abnormal pancreatic β-cell function( Reference Pham, Nanri and Yi 2 – Reference Krisai, Leib and Aeschbacher 4 ). However, previous studies investigating this association have demonstrated conflicting results( Reference Pham, Nanri and Yi 2 – Reference Yeap, Divitini and Gunton 5 ). Bonfils et al.( Reference Bonfils, Ellervik and Friedrich 6 ) investigated 6392 individuals from the Danish general population and observed that elevated fasting serum ferritin levels were associated with surrogate measures of impaired β-cell function in both men and women, whereas the association with decreased insulin sensitivity was observed among men and older women but not among younger women. A cross-sectional study of 12 090 participants in Korea reported that increased serum ferritin concentrations were associated with IR in men, but not in women( Reference Kim, Kim and Bae 7 ), whereas the survey of 8235 participants in China reported that elevated serum ferritin levels were associated with HOMA-IR independent of several confounders in both men and women( Reference Zhan, Tang and Yu 8 ). Similarly, another study conducted in China with 2786 people (both sexes) with no previous history of metabolic disorders found a positive association between serum ferritin levels and HOMA-IR values (adjusted for age, BMI, TAG and systolic blood pressure (SBP))( Reference Chen, Li and Zhang 3 ). In contrast, serum ferritin was not independently associated with IR as assessed using HOMA-IR in men or women without diabetes in Western Australia( Reference Yeap, Divitini and Gunton 5 ). Therefore, the role played by ferritin in IR in men and women, especially among the Chinese population, remains controversial and poorly understood. Serum ferritin concentrations differ significantly according to sex and ethnicity( Reference Harris, McLaren and Reboussin 9 ). In addition, there may be inadequate consideration of potential confounding effects on the relationship. Although these studies do not agree, we can still consider that the serum ferritin concentrations could reflect the development of glucose homoeostasis and that there are differences between the sexes. Further studies are needed to clarify the sex differences and menopausal status among the Chinese population.
Thus, we investigated the association between body Fe as assessed by the serum ferritin concentration and glucose homoeostasis using HOMA of IR and β-cell function in the community-based population of Chinese middle-aged and elderly men and women.
Methods
Study population
This cross-sectional study used data obtained from the Changfeng study, a community-based study of chronic diseases among the middle-aged and elderly, which has been described elsewhere( Reference Gao, Hofman and Hu 10 ). From June 2009 to June 2012, 5375 participants were initially enrolled. Selection of the participants in the study was performed using the following exclusion criteria: lack of physical examination and laboratory assessments (n 54), use of drugs known to influence Fe metabolism including the use of post-menopausal hormone therapy, mineral supplements (including Fe), vitamins and glucocorticoid (n 491), prevalent CVD (myocardial infarction, stroke or peripheral arterial disease) (n 379), prevalent haemodialysis (n 5), having liver disease (e.g. serum liver enzyme (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) activities above two times of the upper normal limits), increased serum creatinine (>124μmol/l), tumour, chronic inflammatory disease (e.g. rheumatoid arthritis, Crohn’s disease) and/or a disorder of Fe metabolism (e.g. aplastic anaemia, β-thalassemia major and haemochromatosis) (n 403), diagnosed diabetes mellitus (DM) or the use of hypoglycaemic medications (n 366), diagnosed hypertension or the use of antihypertensive medications (n 1009), participants with laboratory evidences of inflammation or anaemia (leucocyte count >10 000/μl, platelet count >300 000/μl, haemoglobin <11 g/l) (n 149), having received a blood transfusion or donated blood in the past 3 months (n 1). Finally, 2518 participants were included in the analysis.
The study protocol, consent form, and participant-related materials were approved by the ethical committee of Zhongshan Hospital, Fudan University and all procedures were performed in accordance with the principles of the Declaration of Helsinki and later amendments. Informed consents were obtained from all individuals taking part in the current investigation. Interviews and physical examinations were performed at the Changfeng Community Health Service Center.
Clinical measurements
Letters were sent to participants with instructions asking them not to alter their diet or level of physical activity for at least 3 d before the test. A questionnaire was administered by trained nurses to evaluate the medical history and lifestyle of each participant. All measurements and the sample collections were performed in the morning after overnight fasting. Body weight and height were assessed for the determination of BMI, which was calculated as the weight divided by the height squared (kg/m2). The waist:hip ratio (WHR) was calculated as the waist circumference divided by the hip circumference. The resting blood pressure was measured three times, and the mean value was used for the analysis. Serum was examined for glucose, total cholesterol (TC), HDL-cholesterol, TAG and liver enzymes using a model 7600 automated bio-analyser (Hitachi). The level of LDL-cholesterol was calculated using the Friedewald equation. The fasting blood glucose (FBG) and 2 h glucose levels following a 75-g oral glucose challenge (oral glucose tolerance test (OGTT) 2 h blood glucose (PPG)) for non-diabetics were measured using the glucose oxidase method. The serum samples, which had been stored at −80°C since the time of the baseline survey, were used for serum ferritin and insulin level measurements. Serum ferritin and insulin were measured using electrochemiluminescence immunoassay using an immunoassay analyzer (Cobas-6001; Roche; CV <4·0 and <5·0 %, respectively). The values of HOMA-IR index widely used for assessment of IR were calculated using the standard formula: HOMA-IR=(glucose×insulin)/22·5. The values of homeostatic model assessment β-cell function (HOMA-%B) were calculated using the formula: HOMA-%B=(20×insulin)/(glucose−23·5)( Reference Matthews, Hosker and Rudenski 11 ).
Hypertension was based on the Seventh Report of the Joint National Committee( Reference Chobanian, Bakris and Black 12 ). The diagnoses of impaired fasting glucose, impaired glucose tolerance and DM were determined using the American Diabetes Association 2010 criteria( 13 ). The diagnosis of CVD was according to self-reporting and confirmed using medical records.
Statistical analysis
The data were analysed using SPSS 16.0 for Windows (SPSS Inc.). The participants were categorised into serum ferritin tertiles as follows: (Q1, 8·1–164·0; Q2, 164·1–274·0; Q3, 274·1–1160·0 ng/ml in males; Q1, 5·5–49·6; Q2, 51·0–98·7; Q3, 102·3–476·0 ng/ml in pre-menopausal females; Q1, 8·2–120·0; Q2, 120·4–196·8; Q3, 197·5–1488·0 ng/ml in post-menopausal females). Skewed variables were logarithmically transformed to improve normality before analysis. The results were expressed as means and standard deviations or percentage and standard errors for quantitative variables. A weighted one-way ANOVA test or χ 2 test was used to compare the means of different variables between three categories in the field. Analysis of covariance and logistic regression, with adjustments for the potential confounding factors, were conducted to compare means and proportions, respectively, across the ferritin tertiles. Two-sided values of P value <0·05 were considered statistically significant.
Results
Population characteristics
A total of 1033 males and 1485 females were evaluated. The characteristics of the study participants are shown in Table 1. The mean value of ferritin was 200·8 (sd 148·5) ng/ml. The mean concentrations of serum ferritin were significantly lower in pre-menopausal women compared with post-menopausal women (94·6 (sd 82·0) v. 179·8 (sd 126·6) ng/ml, respectively) and still lower in post-menopausal women compared with men (179·8 (sd 126·6) v. 250·4 (sd 165·2) ng/ml, respectively). A total of 22·2 % of the participants were current smokers. The differences among men, pre-menopausal women and post-menopausal women in terms of age, BMI, WHR, SBP, diastolic blood pressure (DBP), ALT, AST, γ-glutamyl transpeptidase (GGT), leucocytes, TC, LDL-cholesterol, HDL-cholesterol, TAG, FBG, PPG, HOMA-IR, HOMA-%B, urine albumin:creatinine ratio (UACR) and the presence of current smokers were statistically significant.
Table 1 Characteristics of the study participants (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))
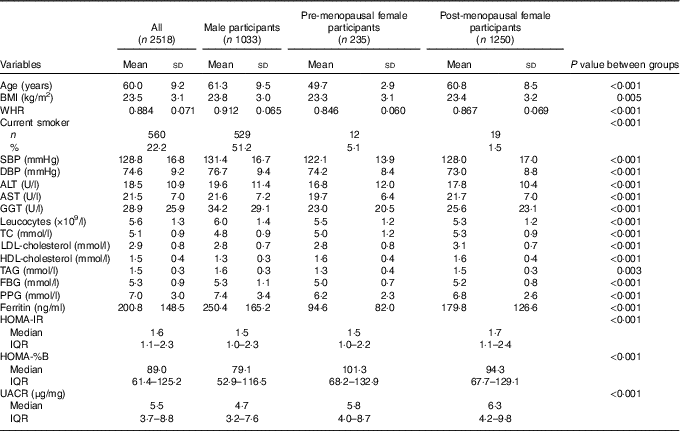
WHR, waist:hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; TC, total cholesterol; FBG, fasting blood glucose; PPG, oral glucose tolerance test 2 h blood glucose; HOMA-IR, homoeostasis model assessment index for insulin resistance; HOMA-%B, homoeostasis model assessment index for β-cell function; UACR, urine albumin:creatinine ratio.
Characteristics of the participants according to the ferritin tertiles
Participant characteristics across the tertiles of serum ferritin concentrations in men and women are summarised in Table 2. In males, when the traditional risk factors were examined, age, BMI, WHR, SBP, DBP, ALT, AST, GGT, leucocytes, TC, LDL-cholesterol, HDL-cholesterol, TAG, FBG, PPG, HOMA-IR, HOMA-%B and UACR were significantly associated with the ferritin tertiles. In pre-menopausal females, GGT, LDL-cholesterol, HDL-cholesterol and TAG were significantly associated with the tertiles groups. In post-menopausal females, age, BMI, WHR, ALT, AST, GGT, TC, LDL-cholesterol, HDL-cholesterol, TAG, FBG, PPG, HOMA-IR and HOMA-%B were significantly associated with the ferritin tertiles. Other parameters were not significantly different among the tertile groups.
Table 2 Characteristics of the participants according to tertile groups for ferritin in male, pre-menopausal and post-menopausal female participants (Mean values and standard deviations; numbers and percentages; medians and interquartile ranges (IQR))
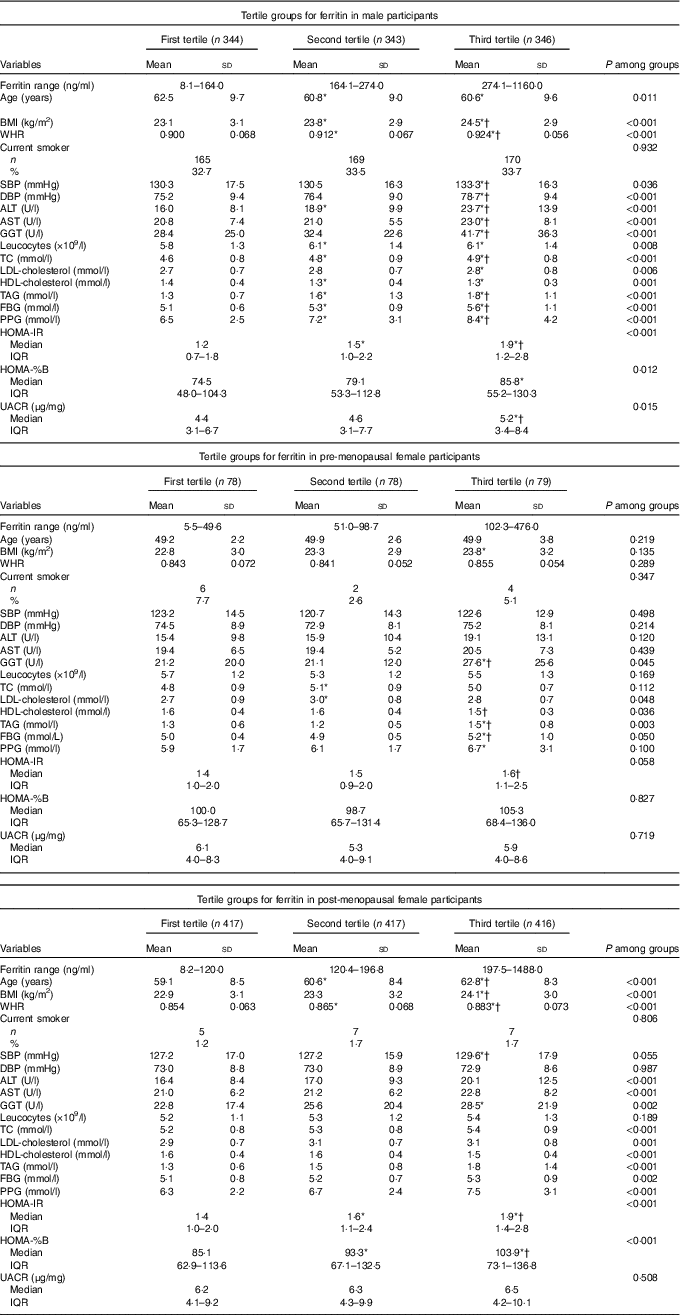
WHR, waist:hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; TC, total cholesterol; FBG, fasting blood glucose; PPG, oral glucose tolerance test 2 h blood glucose; HOMA-IR, homoeostasis model assessment index for insulin resistance; HOMA-%B, homoeostasis model assessment index for β-cell function; UACR, urine albumin:creatinine ratio; LSD, least significant difference.
* ANOVA with LSD post hoc test or χ 2 statistical analysis: P<0·05 v. first tertile.
† ANOVA with LSD post hoc test or χ 2 statistical analysis: P<0·05 v. second tertile.
Association between ferritin and glucose homoeostasis
Table 3 presents the IR measured by HOMA-IR and HOMA-%B of the participants according to the ferritin tertiles and sexes. Higher concentrations of serum ferritin were significantly associated with higher values for IR after adjusting for age, current smoking, BMI, WHR, SBP, DBP, TAG, HDL-cholesterol, LDL-cholesterol, logUACR and leucocytes (model 2) in men. Further adjustment for ALT, AST and GGT (model 3) did not materially attenuate the association of serum ferritin with HOMA-IR (P for trend=0·001), although the association with HOMA-%B remained statistically non-significant (P for trend=0·432). In fully adjusted models (model 3), serum ferritin concentrations were significantly associated with IR and β-cell function in post-menopausal females, and the null association in pre-menopausal females remained in all models.
Table 3 Homoeostasis model assessment index for insulin resistance (HOMA-IR) and homoeostasis model assessment index for β-cell function (HOMA-%B) in the participants according to tertile groups for ferritin in male, pre-menopausal and post-menopausal female participants (Mean values with their standard errors)
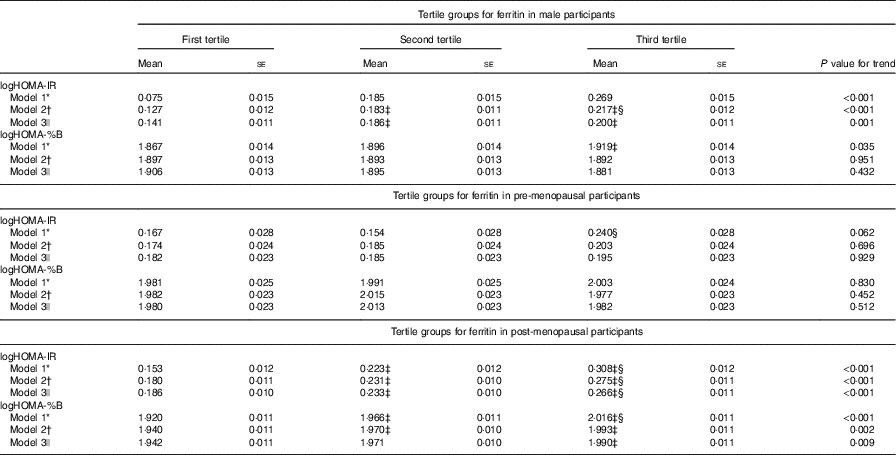
WHR, waist:hip ratio; SBP, systolic blood pressure; DBP, diastolic blood pressure; UACR, urine albumin:creatinine ratio; ALT, alanine aminotransferase; AST, aspartate aminotransferase; GGT, γ-glutamyl transpeptidase; LSD, least significant difference.
* Model 1: adjusted for age.
† Model 2: adjusted for the factor in model 1 plus current smoking (yes or no), BMI, WHR, SBP, DBP, TAG, HDL-cholesterol, LDL-cholesterol, logUACR and leucocytes.
‡ ANOVA with LSD post hoc test: P<0·05 v. first tertile.
§ ANOVA with LSD post hoc test: P<0·05 v. second tertile.
|| Model 3: adjusted for factors in model 2 as well as ALT, AST and GGT.
Discussion
In the present cross-sectional study, we found that a higher serum ferritin concentration was significantly associated with IR in male and post-menopausal participants, independently of possible risk factors. Furthermore, an increased β-cell function associated with higher ferritin level was observed in post-menopausal participants, but not in male participants.
Recent studies showed conflicting results with positive, negative or no associations between ferritin and IR in various populations with and without comorbidities( Reference Pham, Nanri and Yi 2 – Reference Yeap, Divitini and Gunton 5 ). We identified differences by sex and menopausal status in the relationship of ferritin with IR as significant associations were found in men and post-menopausal women, but not in pre-menopausal women, in our study. Our findings correspond well with the previous study by Lee et al. ( Reference Lee, Kim and Kim 14 ). Lee et al conducted a cross-sectional study of 6311 Korean adults older than 20 years who participated in the 2008 Korean National Health and Nutrition Examination Survey and found an association between serum ferritin and HOMA-IR in men and post-menopausal women but not in pre-menopausal women. Consistent with our findings, the previous studies( Reference Jehn, Clark and Guallar 15 ) showed no association between circulating ferritin concentrations and HOMA-IR in pre-menopausal women. The mean concentrations of serum ferritin in the pre-menopausal women in our study were nearly 2·5-fold lower than those in men, and the ferritin levels were about 2-fold lower in pre-menopausal women than those in post-menopausal women. This is likely attributable to menstrual Fe loss in pre-menopausal women. As serum ferritin differs significantly between men and women, it is thought that ferritin plays a different role in IR in each sex( Reference Mateo-Gallego, Calmarza and Jarauta 16 ). Theoretically, in men and post-menopausal women, the relationship between ferritin and IR might be more obvious because of higher Fe accumulation than that in pre-menopausal women. Moreover, changes in oestrogen levels may influence the association. The pre-menopausal women have a more insulin-sensitive environment than post-menopausal women due to different oestrogen levels( Reference Geer and Shen 17 ), which may contribute to the different associations with the ferritin levels in pre-menopausal and post-menopausal women. The reduction in oestrogen levels seen at menopause has also been shown to increase levels of oxidative stress( Reference Doshi and Agarwal 18 ), probably reinforcing the association between ferritin and IR. It has been reported that serum ferritin levels were lower in post-menopausal women using hormone therapy compared with those not using hormone therapy( Reference Penckofer and Schwertz 19 ). However, the relationship between ferritin and IR appeared to be statistically significant when comparing the second and third tertiles of ferrtin with the lowest tertile (the first tertile) in the whole group of study participants after adjusting for age, sex, current smoking, BMI, WHR, SBP, DBP, logUACR, leucocytes, lipid profiles and liver enzymes (data not shown). The null association between ferritin and IR in the pre-menopausal women in our study may be due to the limited sample size and a relatively small proportion compared with men and post-menopausal women.
However, some studies have shown a lack of consistency in the association between circulating ferritin and IR according to sex and menopausal status( Reference Pham, Nanri and Yi 2 – Reference Krisai, Leib and Aeschbacher 4 ). A recent Chinese study showed that elevated serum ferritin levels were independently associated with IR regardless of sex( Reference Chen, Li and Zhang 3 ). Chen et al.’s( Reference Chen, Li and Zhang 3 ) study excluded participants with a known history of diabetes, liver disease, chronic or acute inflammatory disease or atherosclerosis, similarly as ours. It should be noted, the BMI, blood pressure and blood glucose of the female participants in our study were relatively improved in comparison with those in Chen et al.’s, which may contribute to the insignificant correlation in pre-menopausal women. The median values of HOMA-IR were markedly lower in our study compared with that in Chen et al.’s (1·5 and 1·7 in pre-menopausal and post-menopausal women v. 1·83). The fact that no association was observed for pre-menopausal women in our study may be due to the aforementioned different results. Pham et al. ( Reference Pham, Nanri and Yi 2 ) reported that serum ferritin levels were positively associated with HOMA-IR in men but not in women, even among post-menopausal women in a Japanese working population aged 20–68 years old. The mean values of ferritin were significantly lower in the Japanese population, compared with those in our study (23·4 and 75·4 ng/ml in pre-menopausal and post-menopausal women in Japanese, respectively v. 94·6 and 179·8 ng/ml in ours). Ferritin measurement methods generally vary widely among measurement methods( Reference Kamei and Akiba 20 ). The earlier two studies used different ferritin measurement methods. If there is a positive association between ferritin and IR markers, the association would not be discernible in participants who have lower concentrations of serum ferritin. These may explain the discrepancy found in the link between ferritin levels and IR in the women. Moreover, statistical power could also explain the discrepant findings, because most of the studies describing associations had larger sample sizes for pre-menopausal women than those with no association( Reference Yoo, Ko and Park 21 – Reference Cho, Shin and Yi 23 ).
Our study provided an insight into the finding of intriguing relationships between ferritin and increased β-cell function in post-menopausal participants. However, most studies reported that serum ferritin levels were associated with decreased β-cell function( Reference Vari, Balkau and Kettaneh 24 ). As high serum ferritin levels is a risk marker for the development of IR in post-menopausal women, enhanced insulin secretion by β cells in response to the increasing IR is a reasonable compensatory mechanism, and subsequently, the compensatory ability possibly starts to fail, resulting in the decreased β-cell function. Similarly, in a cross-sectional study, a weak and direct association was observed between ferritin quartiles and β-cell function using HOMA-%B in normoglycaemia, which was direct in the case of pre-diabetes and inverse in DM( Reference Aregbesola, Virtanen and Voutilainen 25 ).
The mechanisms for the effect of ferritin on IR remain unknown, although several explanations have been suggested. First, Fe is a major catalyst in the production of highly reactive hydroxyl radicals and excess body Fe may be directly involved in insulin signalling( Reference Messner, Rhieu and Kowdley 26 ). Second, Fe causes the peroxidation of lipids, especially free fatty acids, leading to accelerated production of free radicals; thereafter, the increased free fatty acids oxidation causes decreased glucose uptake in the muscles, which stimulates gluconeogenesis in the liver and results in increased IR( Reference Aregbesola, Virtanen and Voutilainen 27 ). Third, Fe negatively regulates adiponectin transcription via forkhead box protein O1 (FOXO1)-mediated repression. Serum ferritin level was increased and adiponectin level was decreased in type 2 DM and in obese diabetic participants( Reference Gabrielsen, Gao and Simcox 28 ). Lower plasma adiponectin concentrations in men than women contribute to sex differences in insulin sensitivity( Reference Nishizawa, Shimomura and Kishida 29 ). Finally, Fe was closely related to inflammation, which imposed a higher risk on IR. However, in our study after adjusting for leucocytes and UACR, the associations between serum ferritin and IR were still significant, which might be suggestive of other pathways of the effects of serum ferritin on IR. Another possible explanation is that Fe deposition in the liver can result in hepatic IR and increase hepatic glucose production( Reference Forouhi, Harding and Allison 30 ). Furthermore, other studies have suggested a link between serum ferritin and nonalcoholic fatty liver disease, currently regarded as one of the independent risk factors for IR( Reference Kowdley, Belt and Wilson 31 ). We did observe a positive relationship between the ferritin tertile groups and liver enzymes in male and post-menopausal participants (i.e. ALT, AST and GGT; Table 2).
IR plays an important role in the development of diabetes, hypertension, the metabolic syndrome and atherosclerotic diseases( Reference Cho, Park and Choi 32 , Reference Han, Wang and Li 33 ). The incidence of IR is increasing and early management has become important to prevent patients from developing more severe diseases. Decreased Fe stores resulting from frequent blood donation or bloodletting have been associated with improved IR( Reference Houschyar, Ludtke and Dobos 34 , Reference Fernández-Real, López-Bermejo and Ricart 35 ), supporting the notion that serum ferritin may become a useful biomarker to predict IR. Our findings underscore the public health importance of monitoring ferritin level, and also suggested that Fe metabolism may underlie the aetiology of glucose homoeostasis.
Furthermore, the association between serum ferritin levels and DM has been broadly concerned in China. To date, several case–control studies and prospective studies have investigated the association; however, their results were inconsistent( Reference Guo, Zhou and An 36 – Reference Chen, Li and Zhang 40 ). Possible reasons for the differences among the aforementioned studies include the study population, the length of the study and the definition of diabetes.
Our study has several strengths. Serum ferritin is considered to be the best indicator of body Fe stores, despite being shown to be affected by inflammation status. As mentioned in previous studies( Reference Eftekhari, Mozaffari-Khosravi and Shidfar 41 ), ferritin can be elevated during inflammation, as well as in the case of cancer and liver disease. Serum ferritin concentration can also be increased in some conditions like obesity and non-alcoholic fatty liver disease, which are associated risk factors for IR( Reference Kitade, Chen and Ni 42 ). We controlled for possible confounding in the study by several ways. All participants had no confounding co-morbidities such as CVD, anaemia, renal disease, liver diseases and chronic inflammatory diseases( Reference Houschyar, Ludtke and Dobos 34 ). We restricted the study population to those without overt liver disease, defined as no more than two times of the upper normal limits in liver enzymes. Therefore, it provided a unique opportunity to study the association between the markers of Fe metabolism and IR in the middle-aged and elderly population. Moreover, the association remained robust even after adjustment for multiple confounding factors including inflammation controlling for leucocytes and UACR levels, and ALT, AST and GGT for liver diseases in the multivariate models, which have seldom been accounted for in the previous studies.
The present study had several limitations that should be acknowledged. First, standard measurements of IR and β-cell dysfunction (hyperinsulinaemic–euglycaemic and hyperglycaemic clamps) were not used in our study. However, HOMA-IR and HOMA-%B are the most employed tools in epidemiological studies. Second, the cross-sectional design does not allow us to determine a causal relationship among the evaluated variables. Third, we could not adjust for dietary factors due to lack of data on dietary haem Fe intake. Fourth, socio-economic factors are associated with lifestyles and behavioural risk factors. However, we do not have data on the socio-economic factors and lifestyle factors including physical activity and energy intake. Notably, recent studies found that, among the behavioural risk factors considered, BMI and waist circumference may serve as a lifetime proxy for diet quality and level of physical activity of health behaviours( Reference Yang, Hall and Piccolo 43 , Reference Stringhini, Sabia and Shipley 44 ). We have already fully adjusted for BMI and waist circumference in the study. Fifth, our participants were middle-aged and elderly participants, and so, the results cannot be applied to younger participants. Moreover, we cannot rule out the residual confounding effects due to a failure to adjust for C-reactive protein, as serum ferritin is an acute-phase reactant and may be artificially elevated in the presence of inflammation( Reference Gabay and Kushner 45 ).
Fe element is an inorganic constituent needed in minute quantities but considered an essential nutrient for human health. Therein, it is worth noting that deficiency or the excessive accumulation of Fe element could be detrimental. In the context of human health, screening based on ferritin would enable clinicians to provide early interventions. The individuals with increased or decreased ferritin levels might benefit from more aggressive lifestyle modifications and dietary regimen. The supply of three macro-nutrients (carbohydrate, protein and lipid) was sufficient in mainland China( Reference Guo, Zhao and He 46 ). However, it was a common issue that there was deficient intake of micronutrients such as Fe and Zn, most of them being among women and children living in rural areas( Reference Guo, Zhao and He 46 ). Fe supplementation and fortification can be used as short-term interventions, while dietary diversification and biofortification could be long-term interventions( Reference Ma, Jin and Li 47 ).
In conclusion, we evaluated ferritin and glucose homoeostasis obtained from OGTT in 2518 middle-aged and elderly Chinese individuals and observed that elevated fasting serum ferritin levels were associated with surrogate measures of increased IR in both men and post-menopausal women, but not among pre-menopausal women, whereas the association with increased β-cell function was present in post-menopausal women. These findings suggest that ferritin may contribute to the development of IR. Therefore, our study added further insight into the public health importance of monitoring ferritin level.
Acknowledgements
The Shanghai Changfeng Study has also received great support from Changfeng Health Center, the Health Bureau of Putuo District and the committees of all the sub-communities of Changfeng. The contributions of all the working staffs and inhabitants are greatly acknowledged.
This work was supported by grants from the Shanghai Science and Technology Committee (STCSM) Foundation (16411954800 to X. G.), and the Three-year Action Plan on Public Health, Phase IV, Shanghai, China (grant no. 15GWZK0801 to H. L. and X. G.).
H. M., H. L., X. L., Y. H., X. J., J. G., N. Z., B. P. and X. G. conceived and designed the experiments. H. M., H. L., X. L., W. H., J. G. performed the experiments. H. M., N. Z., J. G. analysed the data. H. M., X. G. wrote the paper. All authors read and approved the final manuscript.
The authors declare that there are no conflicts of interest.






