Early life environment plays an important role for later susceptibility to chronic diseases. Inverse associations between birth weight and later risk for CVD and type 2 diabetes have been reported in several populations( Reference Forsdahl 1 – Reference Whincup, Kaye and Owen 3 ). However, subtle variations in environmental influences, such as nutritional factors, can probably produce a range of neonatal phenotypes which may affect the risk for adult disease, even in the absence of effects on birth weight( Reference Hanson and Gluckman 4 ).
Birth weight and neonatal body composition vary considerably across the world, and also between ethnic groups living in Europe. Neonates of mothers with origin from Asia and Africa have lower birth weight, smaller abdominal circumference and less fat-free mass, but similar amounts of fat mass, compared with neonates of European origin( Reference Sletner, Nakstad and Yajnik 5 – Reference Goedhart, van Eijsden and van der Wal 9 ). This phenotype seems to track throughout life and is associated with a higher risk for CVD and type 2 diabetes, as observed in several ethnic minority groups in Europe( Reference Sattar and Gill 10 ).
Vitamin D is essential for fetal and childhood skeletal development( Reference Javaid 11 ), and experimental animal studies support an active contribution of vitamin D to organ development( Reference Hart, Lucas and Walsh 12 , Reference Hewison and Adams 13 ). However, the effect of maternal vitamin D deficiency on birth weight and body composition is not clear. Several studies have reported a positive association between maternal vitamin D levels in pregnancy and offspring birth weight( Reference Harvey, Holroyd and Ntani 14 ), although results are inconsistent, both from observational( Reference Theodoratou, Tzoulaki and Zgaga 15 – Reference Thorne-Lyman and Fawzi 17 ) and randomised controlled studies (RCT)( Reference Theodoratou, Tzoulaki and Zgaga 15 , Reference Thorne-Lyman and Fawzi 17 – Reference De-Regil, Palacios and Lombardo 19 ). A Cochrane review from 2016, of RCT with vitamin D supplementation v. placebo, reported no significant effect on birth weight. Furthermore, although there was some indication that vitamin D supplementation increased infant length and head circumference at birth, the clinical significance of the observed effects of an increased vitamin D level is still unclear( Reference De-Regil, Palacios and Lombardo 19 ). In addition, few studies investigating the effect of vitamin D on neonatal size have included other anthropometric data than birth weight, many of these studies were small or only included women with moderate vitamin D deficiency. Just a few studies included ethnic minority women, with presented results stratified by ethnicity or adjusted for ethnicity( Reference Miliku, Vinkhuyzen and Blanken 20 – Reference Ong, Quah and Tint 23 ). This is particularly important, as the prevalence of severe vitamin D deficiency is high among pregnant ethnic minority women compared with ethnic Europeans( Reference Eggemoen, Falk and Knutsen 24 ). Whether low levels of vitamin D can contribute to low birth weight in ethnic minorities have been questioned. An independent association between maternal vitamin D levels and offspring birth weight or body composition would lend support to identifying these women early in pregnancy to offer vitamin D substitution.
Our aim was to explore associations between maternal 25-hydroxyvitamin D (25(OH)D) and neonatal birth weight and a wide range of anthropometric measurements to reflect body composition, before and after adjusting for other potentially explanatory factors, especially ethnicity.
Methods
Design, setting and study population
Data are from a population-based, prospective cohort of 823 presumably healthy women attending Maternal and Child Health Clinics (MCHC) for antenatal care in Groruddalen, Oslo, Norway, between May 2008 and March 2010, and their offspring (The STORK Groruddalen study)( Reference Jenum, Sletner and Voldner 25 ). The majority (75–85 %) of pregnant women residing in this area, situated at a latitude of 60°N, attend the Child Health Clinics for antenatal care. Antenatal care is free of charge in Norway and easily accessible. The study design has been described in detail elsewhere( Reference Jenum, Sletner and Voldner 25 ). In short, information material and questionnaires were translated to Arabic, English, Sorani, Somali, Tamil, Turkish, Urdu and Vietnamese and quality checked by bilingual health professionals. Women were eligible if they (1) lived in the district, (2) planned to give birth at one of the two study hospitals, (3) were in gestational week (GW) <20, (4) were not suffering from diseases necessitating intensive hospital follow-up during pregnancy, (5) could communicate in Norwegian or any of the specified languages and (6) were able to provide written consent to participate. In total, 59 % of the included women were of an ethnic minority background. The participation rate was 74 % (range: 64–83 % between ethnic groups), and the participating women were considered to be representative of the main ethnic groups attending MCHC( Reference Jenum, Sletner and Voldner 25 ). Maternal data were collected at 15 and 28 weeks of gestation, through interviews by study personnel (assisted by professional interpreters when needed). Clinical measurements for mothers and neonates were performed and blood samples collected according to the study protocol.
Variables
Main outcome measures
Outcome variables were birth weight, crown–heel length, head circumference, abdominal circumference, sum of skinfolds, mid-upper arm circumference and ponderal index. Birth weight was routinely measured immediately after delivery by hospital staff. The other outcome variables were measured within 72 h after birth unless there were medical contraindications. Neonatal anthropometric measurements were performed by specially trained study personnel( Reference Sletner, Nakstad and Yajnik 5 ). Skinfold thickness was the sum of the triceps skinfold, the subscapular skinfold, the supra-iliac skinfold and the thigh skinfold, and named ‘sum of skinfolds’. All measurements, except length, were performed twice (circumferences and length to the nearest 0·1 cm, skinfolds to the nearest 0·1 mm) and calculated as the mean of two measurements. Ponderal index was calculated as birth weight (kg)/crown–heel length (m3)( Reference Sletner, Nakstad and Yajnik 5 , Reference Jenum, Sletner and Voldner 25 ). Small for gestational age (SGA) was defined as <10th percentile according to the Norwegian national references( Reference Skjaerven, Gjessing and Bakketeig 26 ).
Explanatory factors
At GW 15 and 28, maternal 25(OH)D was analysed by competitive RIA (DiaSorin) at the Hormone Laboratory, Oslo University Hospital, Aker. The method measures total 25(OH)D (both 25(OH)D2 and D3). The interassay CV gives information on the variation between measures in different batches; the lowest CV was 13 % and the highest CV was 16 % between 37 and 131 nmol/l for this method at the laboratory during the period from 2008 to 2010. The laboratory is accredited by the International Organization for Standardization and is part of the Vitamin D Quality Assessment Scheme. Concentrations of 25(OH)D <12 nmol/l were replaced with ‘11 nmol/l’ in the calculations (n 16), so as to not overestimate the effect of low vitamin D status. The laboratory’s reference range was 37–131 nmol/l based on the ethnic Norwegian population from the Oslo Health Study( Reference Meyer, Falch and Sogaard 27 ). Preplanned, and according to the protocol, women with 25(OH)D less than the laboratory’s lower reference range (<37 nmol/l) at GW 15 and 28 were provided written information about their 25(OH)D concentration, and recommended to consult their general practitioner for treatment( Reference Eggemoen, Falk and Knutsen 24 ).
Maternal and offspring ethnic origin was defined by the pregnant participant’s mother’s country of birth( Reference Bhopal 28 ). Ethnicity was further categorised into European (primarily from Norway and Sweden), Asian (primarily from Pakistan, Sri Lanka, Vietnam, India and the Philippines) and Middle Eastern/North African including the Horn of Africa (primarily from Somalia, Iraq, Turkey, Morocco and Afghanistan) (see footnote in Table 1). Parity was categorised as no (nulliparous), one (uniparous) or two or more (multiparous) previous pregnancies lasting >22 weeks. Education level was categorised as <10, 10–12 (high school education) and >12 (college/university education) years. Season of birth was categorised as summer (June, July, August), autumn (September, October, November), winter (December, January, February) and spring (March, April, May). Prepregnancy BMI (calculated from self-reported weight before pregnancy and height measured at inclusion), maternal age, GW at birth (derived from the 1st day of the woman’s last menstrual period and neonate sex were other variables of interest.
Table 1 Maternal and infant characteristics stratified by geographic originFootnote * (Mean values and standard deviations; numbers and percentages)
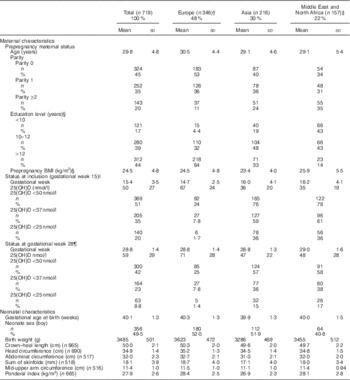
25(OH)D, 25-hydroxyvitamin D.
* Countries with ≥10 individuals are listed: 346 women from Europe, primarily from Norway (n 278) and Sweden (n 11); 216 women from Asia, primarily from Pakistan (n 111), Sri Lanka (n 55), Vietnam (n 16), India (n 12) and the Philippines (n 10); 157 women from the Middle East/North AfricaFootnote ‡, primarily from Somalia (n 35), Iraq (n 34), Turkey (n 26), Morocco (n 23) and Afghanistan (n 12).
† Including 3 women from USA and Canada.
‡ Including Horn of Africa.
§ Incomplete data on the variables because of missing values for 6–12 women.
|| Incomplete data on the variables because of missing values for 14 women.
¶ Incomplete data on the variables because of missing values for 36 women.
Ethics
The Regional Committee for Medical and Health Research Ethics for South-Eastern Norway (Ref. 2007/894) and the Norwegian Data Inspectorate approved the study protocol. Participation was based on informed written consent.
Statistical methods
Descriptive statistics are presented as frequencies, mean values and standard deviations and proportions. All continuous response variables were assessed for normality. Separate generalised linear models were performed to assess the relationship between the concentration of 25(OH)D and each of the following outcomes: birth weight, crown–heel length, head circumference, abdominal circumference, sum of skinfolds, mid-upper arm circumference and ponderal index. The study sample including preterm singleton (<37 weeks) neonates were used when analysing associations with SGA as the outcome (see flow chart, online Supplementary Fig. S1), and generalised logistic regression models were performed to assess the relationship between 25(OH)D and SGA. We accounted for the following potential explanatory factors: GW, neonate sex, season, maternal age, parity, education, prepregnancy BMI and ethnicity. Maternal 25(OH)D was first analysed as a continuous variable. We also categorised the 25(OH)D level during pregnancy as: consistently sufficient level (≥37nmol/l at GW 15 and 28), consistently deficient level (<37 nmol/l at GW 15 and 28), increasing level (<37 nmol/l at GW 15 and ≥37 nmol/l at GW 28) and decreasing level (≥37nmol/l at GW 15 and <37 nmol/l at GW 28). Variables with P<0·2 in the univariate analyses were included in the multiple regression analyses. Interactions between 25(OH)D and sex, between 25(OH)D and ethnicity and between ethnicity and season, were examined graphically and by adding interaction terms into the models. Results are presented as linear regression coefficients (β) and OR and 95 % CI. P<0·05 were considered statistically significant. Given the sample size, the actual CI are very small for the outcomes and thereby these results indicate precise estimates. SPSS software (version 22; IBM SPSS Statistics) and StataSE 14 were used for statistical analysis.
Results
Study sample
Of the 823 women included in the STORK Groruddalen project, twelve women from South or Central America and seventeen women from African countries other than North Africa including the Horn of Africa with low numbers of participating women were excluded because of low sample size. We also excluded thirty-six women with a multiple pregnancy, an abortion, a stillbirth, a neonatal death or with missing data at birth, leaving 758 (92 %) available for analyses of the outcome SGA. After excluding thirty-nine women with premature deliveries (<37 weeks), the main study sample consisted of 719 (87 %) live-born term neonates (flow chart, online Supplementary Fig. S1). Anthropometric data were obtained from 517 to 690 of these newborns.
Maternal 25-hydroxyvitamin D status
In early pregnancy, 51 % of the women had vitamin D deficiency, defined as 25(OH)D <50 nmol/l, ranging from 78 % among Middle Eastern/North African, 76 % among Asian and 24 % among European women. A high prevalence of severe deficiency (25(OH)D <25 nmol/l) was found among women from Asia (36 %) and the Middle East (36 %) compared with 1·7 % among Europeans. At inclusion, the mean 25(OH)D concentration values ranged from 35 to 67 nmol/l between the ethnic groups. However, ethnic differences were reduced at GW 28 after recommending vitamin D supplementation (Table 1). Maternal and infant characteristics related to 25(OH)D are presented in online Supplementary Table S1.
Association between maternal 25-hydroxyvitamin D and birth weight
The mean birth weight was 3485 (sd 501) g, but differed by ethnic groups (P<0·01 for all): 3623 g among European, 3455 g among Middle Eastern and 3286 g among Asian neonates (Table 1 and online Supplementary Table S1). Maternal 25(OH)D at inclusion was positively associated with birth weight in univariate analysis, and also after adjusting for maternal age, parity, educational level, prepregnancy BMI, season, gestational age and neonate sex (P<0·01) (model 1, Table 2). However, after additional adjustment for ethnicity, 25(OH)D was no longer associated with birth weight (model 2, Table 2). Similar results were found for the association between 25(OH)D at GW 28 and birth weight (online Supplementary Table S3). This was also the case when using the categorised variable which reflected the 25(OH)D level throughout pregnancy (Table 3). Before adjusting for ethnicity, the mean birth weight was lower in neonates of women with consistently deficient (–116 g), or initially low, but increasing 25(OH)D (–105 g) compared with neonates of women with consistently sufficient 25(OH)D (reference) (model 1, Table 3). However, after additional adjustment for ethnicity, 25(OH)D levels during pregnancy were no longer associated with birth weight (compared with the reference group, neonates with consistently deficient mothers had 2·6 g higher birth weight and neonates with mothers having increasing 25(OH)D had 4·8 g lower birth weight (model 2, Table 3)). Similar results, where the association with 25(OH)D was not present after inclusion of ethnicity, were found from the logistic regression model of SGA as outcome (Table 4). Asian and Middle Eastern ethnic origins were associated with lower birth weight, independently of maternal 25(OH)D levels (not shown). Other factors independently associated with birth weight in the final model were parity, maternal BMI, offspring sex and duration of gestation.
Table 2 Associations between 25-hydroxyvitamin D (25(OH)D) (continuous) in early pregnancy and neonatal anthropometric measures (n 719)Footnote † (Regression coefficients and 95 % confidence intervals)
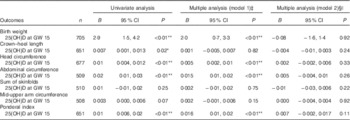
GW, gestational week; AIC, Akaike’s information criterion.
* P<0·05, ** P<0·01.
† Generalised linear regression analysis with birth weight, length, head circumference, abdominal circumference, sum of skinfolds, mid-upper arm circumference and ponderal index (at gestation age >37 weeks) as dependent variables.
‡ Adjusted model 1; multiple regression, additional adjustment for neonate sex, gestational age, season, maternal age, parity, educational level and prepregnancy BMI.
§ Adjusted model 2; as model 1, with additional adjustment for geographic origin.
|| Birth weight: n 693, AIC=10 376; crown–heel length: n 639, AIC=2608; head circumference: n 666, AIC=2151; abdominal circumference: n 502, AIC=213; sum of skinfolds: n 503, AIC=2768; mid-upper arm circumference: n 501, AIC=1364; ponderal index: n 639, AIC=2970.
Table 3 Associations between 25-hydroxyvitamin D (25(OH)D) (categorical) during two times in pregnancy and neonatal anthropometric measures (n 719)Footnote † (Regression coefficients and 95 % confidence intervals)
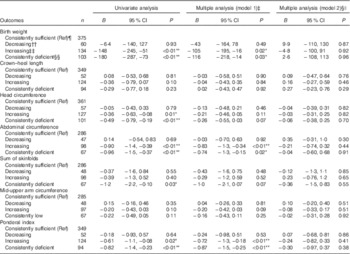
Ref, referent values; GW, gestational week; AIC, Akaike’s information criterion.
* P<0·05, ** P<0·01.
† Generalised linear regression analysis with birth weight, length, head circumference, abdominal circumference, sum of skinfolds, mid-upper arm circumference and ponderal index (at gestation age > 37 weeks) as dependent variable.
‡ Adjusted model 1; multiple regression, additional adjustment for neonate sex, gestational age, season, maternal age, parity, educational level and prepregnancy BMI.
§ Adjusted model 2; as model 1, with additional adjustment for geographic origin.
|| Birth weight: n 661, AIC=9898; crown–heel length: n 608, AIC=2479; head circumference: n 636, AIC=2054; abdominal circumference: n 491, AIC=2099; sum of skinfolds: n 492, AIC=2709; mid-upper arm circumference: n 490, AIC=1341; ponderal index: n 608, AIC=2844.
¶ Consistently sufficient: 25(OH)D≥37 nmol/l at GW 15 and 28.
†† Decreasing: 25(OH)D≥37 nmol/l at GW 15 and <37 at GW 28.
‡‡ Increasing: 25(OH)D<37 nmol/l at GW 15 and ≥37 at GW 28.
§§ Consistently deficient: 25(OH)D<37 nmol/l at GW 15 and 28.
Table 4 Associations between 25-hydroxyvitamin D (25(OH)D) (continuous and categorically) in pregnancy and small for gestational age (SGA) (n 758)Footnote * (Odds ratios and 95 % confidence intervals)
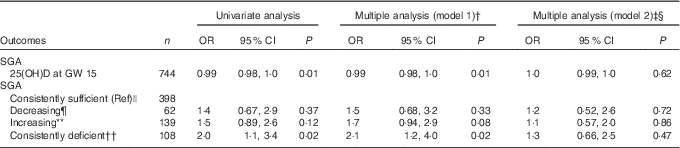
GW, gestational week; Ref, referent values; AIC, Akaike’s information criterion.
* Generalised logistic regression analysis with SGA (birth weight <10th percentile according to Norwegian national references) as a dependent variable. Preterm included (n 39)
† Adjusted model 1; multiple regression, additional adjustment for neonate sex, gestational age, season, maternal age, parity, educational level and prepregnancy BMI.
‡ Adjusted model 2; as model 1, with additional adjustment for geographic origin.
§ Pregnancy: n 729, AIC=562; SGA: n 693, AIC=535.
|| Consistently sufficient: 25(OH)D≥37 nmol/l at GW 15 and 28.
¶ Decreasing: 25(OH)D≥37 nmol/l at GW 15 and <37 at GW 28.
** Increasing: 25(OH)D<37 nmol/l at GW 15 and ≥37 at GW 28.
†† Consistently deficient: 25(OH)D<37 nmol/l at GW 15 and 28.
Associations between maternal 25-hydroxyvitamin D and other neonatal anthropometric measures
Maternal 25(OH)D at inclusion was associated with crown–heel length, head circumference, abdominal circumference and ponderal index in univariate analyses and in models adjusted for maternal age, parity, educational level, prepregnancy BMI, season, gestational age and neonate sex. However, none of these outcomes was associated with maternal 25(OH)D when ethnicity was included in the models (Table 2). Results were similar when using levels of 25(OH)D during pregnancy (categories) or in GW 28 (Table 3 and online Supplementary Table S3). Asian origin was associated with lower crown–heel length, head circumference, abdominal circumference, sum of skinfold and ponderal index, and this association was not influenced by maternal 25(OH)D levels (not shown).
Interactions between neonate sex and 25-hydroxyvitamin D
We also tested for effect modifications, and we found no significant interactions or non-significant trends indicating that the effect of 25(OH)D on neonatal anthropometry differed by ethnicity (data not shown). However, we found a significant interaction between neonate sex and 25(OH)D at GW 15 for abdominal circumference (P 0·01) and sum of skinfolds (P 0·02). Nevertheless, these interaction terms were not entered into the final models, as they only marginally affected the effect estimates. When we stratified by neonate sex in the adjusted model including ethnicity, we found that for each unit increase in 25(OH)D, the estimated abdominal circumference increased (0·01 cm (P 0·04)) in girls but not in boys. Similarly for each unit’s increase in 25(OH)D, the estimated sum of skinfolds decreased (0·03 mm (P 0·02)) for boys only.
Discussion
Main findings
In our multiethnic population with high prevalence of vitamin D deficiency in early pregnancy, we found strong associations between maternal 25(OH)D and birth weight and measures of neonatal body composition, when adjusted for gestational age, neonate sex, season, maternal age, parity, education and prepregnancy BMI. However, after including ethnicity in the models, maternal 25(OH)D in pregnancy was no longer associated with birth weight or any of the offspring anthropometric measures reflecting body composition at birth, whereas the strong association between ethnicity and the neonatal outcomes persisted. These results were consistent, irrespective of whether 25(OH)D was measured in GW 15 or 28, or whether changes in 25(OH)D levels were observed between these time points. Our results, however, may suggest sex-specific associations for some outcomes like abdominal circumference and sum of skinfolds.
Strengths and limitations
The strengths of the present study include its population-based longitudinal design, the high attendance rate, minor loss to follow-up and the relatively large sample size in a multiethnic European context. The questionnaires in nine different languages, available professional interpreters and data collection methods facilitated inclusion of ethnic minorities, even illiterate women. Not least, we have a representative sample of neonates with standardised detailed neonatal anthropometric measurements( Reference Sletner, Nakstad and Yajnik 5 ). Relevant explanatory factors were included, and 25(OH)D was measured at two time points in pregnancy. Further, in accordance with recent studies( Reference Aiken and Ozanne 29 ), we have specifically explored interactions with infant sex. Blood samples were collected and analysed with standardised methods at the same high-quality laboratory, and 25(OH)D levels ranged from highly sufficient to severely deficient, with 51 %<50 nmol/l. Method-related differences in measurement of 25(OH)D are widespread, and as a consequence the Vitamin D Standardization Program developed protocols for standardising 25(OH)D worldwide; the ‘gold standard’ of measuring 25(OH)D is standardised liquid chromatography-tandem MS (LC-MS/MS). On reanalysing 25(OH)D data from four Nordic population samples by LC-MS/MS and comparing results from the immunoassay methods with the ‘gold standard’ method, the results from the Hormone Laboratory, Oslo University Hospital, were found to be only modestly changed, indicating that the analyses were reliable( Reference Cashman, Dowling and Skrabakova 30 ).
The main limitation of this study is that we have merged ethnic groups to represent large geographical regions based on country of birth. Consequently, we cannot rule out that differences within the Asian and Middle Eastern groups may exist. Statistical power may also be a limitation. However, CI of the effect estimates for the association between 25(OH)D levels and neonatal size were very small, indicating a relatively precise estimate. Hence, we would need a very large sample size to find significant differences, and the clinical relevance of such small effects, although statistically significant, could be questioned. Furthermore, the categorisation of 25(OH)D may not be optimal as we do not separate women with a large increase in 25(OH)D from those with only a small increase from just below to above 37 nmol/l. However, in a sensitivity analysis, we did not identify a trend suggesting that an increase in 25(OH)D of >20 nmol/l, from <25 nmol/l in early pregnancy, was associated with any of the neonatal anthropometric measures, when compared with neonates of women with consistently sufficient levels. In addition, eligible neonates without study-specific measurements were missed at random because of logistic reasons, for example, holidays and temps not familiar with the study and hence not reporting the birth to the study midwives( Reference Sletner, Nakstad and Yajnik 5 ).
Interpretation
First, before including ethnicity in the analyses, we found a strong association between maternal 25(OH)D and birth weight. This is in line with several other observational studies, although results are inconsistent. Four systematic reviews (meta-analyses) have found a positive association between maternal 25(OH)D status or vitamin D supplementation and birth weight( Reference Harvey, Holroyd and Ntani 14 – Reference Thorne-Lyman and Fawzi 17 ). In line with our study, seven cohort studies found no association between maternal 25(OH)D and birth weight( Reference Morley, Carlin and Pasco 31 – Reference Prentice, Jarjou and Goldberg 35 ), whereas three found a positive association( Reference Miliku, Vinkhuyzen and Blanken 20 – Reference Bowyer, Catling-Paull and Diamond 22 ). A recent Cochrane review( Reference De-Regil, Palacios and Lombardo 19 ) and previous meta-analysis of RCT( Reference Harvey, Holroyd and Ntani 14 – Reference Pérez-López, Pasupuleti and Mezones-Holguin 18 , Reference Wei, Qi and Luo 36 – Reference Hollis, Johnson and Hulsey 40 ), show inconclusive results regarding the effect of birth weight.
Furthermore, we found that the association between maternal 25(OH)D and birth weight disappeared after adjusting for ethnicity. The effect of ethnicity as a confounder may, however, represent other aspects than ethnicity per se. Skin colour affects the production of 25(OH)D in the skin as a response to sunlight, but could also be considered a proxy measure of socioeconomic status( Reference Chen, Chimeh and Lu 41 , Reference Clemens, Adams and Henderson 42 ). Some other studies indicate that lower birth weight in ethnic minority groups seems to be linked to maternal early life factors among others, and may be transmitted over generations( Reference Yajnik, Fall and Coyaji 8 , Reference Sattar and Gill 10 , Reference Sletner, Jenum and Morkrid 43 ). In general, few studies have explored these relationships in a multiethnic population, and few have included the same ethnicities as in our study( Reference Bowyer, Catling-Paull and Diamond 22 , Reference Ong, Quah and Tint 23 ). In one of these studies, an association was found with 25(OH)D <30 nmol/l( Reference Leffelaar, Vrijkotte and van Eijsden 21 ). Another study adjusted for few confounders, and ethnicity was poorly defined, making comparison for specific ethnic groups difficult( Reference Bowyer, Catling-Paull and Diamond 22 ). Recently, a large study from the Netherlands found a positive association between maternal 25(OH)D measured once in pregnancy, and fetal growth, birth weight, length and head circumference at birth( Reference Miliku, Vinkhuyzen and Blanken 20 ), indicating a difference in birth weight of approximately 80 g between those with low levels compared with optimal levels of 25(OH)D. However, only estimates for differences in z score – adjusted for a large number of maternal factors – were reported, making comparison difficult. We can only speculate on the reasons for the discrepancy between our study and the two studies from the Netherlands( Reference Miliku, Vinkhuyzen and Blanken 20 , Reference Leffelaar, Vrijkotte and van Eijsden 21 ). We cannot rule out that the biological effect of 25(OH)D could differ between the populations. One potential factor could be genotypes coding for the vitamin D receptor, vitamin D binding protein and regulatory enzymes; which may all differ by ethnicity and be potential effect modifiers( Reference Bodnar, Catov and Zmuda 44 , Reference Wang, Zhang and Richards 45 ). In addition, the ethnic and socioeconomic composition of our sample differed somewhat from the two Dutch studies. The relation between these sociodemographic factors and 25(OH)D, and also birth weight, may differ between populations. Hence, it is more likely that this difference in results between studies is a result of residual confounding.
As in many other studies, we examined the association between 25(OH)D and birth size as a continuous outcome. However, others have argued that this may be problematic, as 25(OH)D may be more strongly related to pathological fetal growth, such as SGA, than in accounting for variation in normal fetal growth( Reference Bodnar, Catov and Zmuda 44 ). Furthermore, the relationship may not be linear, but U-shaped, although this was not found in our study. Moreover, optimal fetal growth may differ between ethnic groups, and actually remains to be defined. The mean birth weight of Asian neonates is several hundred grams lower than that of European neonates( Reference Yajnik, Fall and Coyaji 8 , Reference Urquia, Sorbye and Wanigaratne 46 ). Hence, applying a universal standard for SGA would lead to more Asians being diagnosed as SGA than Europeans, with the Asian group probably including a higher proportion of constitutionally small babies.
When it comes to associations between 25(OH)D and other anthropometric measures, studies are fewer( Reference Harvey, Holroyd and Ntani 14 ). We found a strong association between maternal 25(OH)D and head circumference, abdominal circumference and sum of skinfolds. Also for these outcomes the association disappeared after adjusting for ethnicity. In a recent Cochrane review( Reference De-Regil, Palacios and Lombardo 19 ) there was some indication that maternal treatment during pregnancy may increase infant length and head circumference at birth. In some observational studies no associations were found between 25(OH)D and birth length and head circumference after adjusting for confounders( Reference Ong, Quah and Tint 23 , Reference Morley, Carlin and Pasco 31 – Reference Prentice, Jarjou and Goldberg 35 , Reference Rodriguez, Garcia-Esteban and Basterretxea 47 ), whereas other studies found an association( Reference Harvey, Holroyd and Ntani 14 , Reference Miliku, Vinkhuyzen and Blanken 20 ). We identified only four observational cohort studies of the association between 25(OH)D and skinfold thickness and circumferences( Reference Harvey, Holroyd and Ntani 14 , Reference Ong, Quah and Tint 23 ). Two studies found an association( Reference Morley, Carlin and Pasco 31 , Reference Farrant, Krishnaveni and Hill 33 ) whereas two did not, in line with our study( Reference Ong, Quah and Tint 23 , Reference Gale, Robinson and Harvey 32 ). A study measuring body composition by dual energy X-ray absorptiometry found a positive association between 25(OH)D and offspring fat mass at birth( Reference Crozier, Harvey and Inskip 48 ). Recently, a multiethnic study of an Asian population (Chinese, Malay or Indian) from Singapore reported several anthropometric measures and found no associations between maternal 25(OH)D and any of the birth outcomes, including skinfolds and abdominal circumference, in line with our study( Reference Ong, Quah and Tint 23 ). This study adjusted for ethnicity and approximately the same explanatory factors as we did. This study sample had a low prevalence (1·6 %) of severe maternal deficiency. Hence, our study with high prevalence of severe deficiency may complement their findings.
However, we observed an interaction with sex, indicating that increased maternal 25(OH)D during pregnancy was associated with less subcutaneous fat in boys only. Interestingly, we also found an interaction between 25(OH)D and sex for the outcome abdominal circumference, indicating that there may be a differential effect of 25(OH)D depending on whether the fetus is a boy or a girl. To our knowledge, this has not been reported from previous studies, but is in accordance with studies suggesting sex-specific responses to an adverse fetal environment( Reference Aiken and Ozanne 29 ). Nevertheless, the clinical implications of these results are unknown.
Implications for practice/research
From a public health perspective and according to the Cochrane review there is still a need for high-quality RCT studies in vitamin D-deficient pregnant populations on the effect of vitamin D supplementation on clinically important neonatal outcomes other than bone health. In a European context, a vitamin D-deficient population will mostly consist of women of non-Western origin. Further, there is a need to study subtle differences in phenotypes and possible sex differences in the effect for some outcomes.
Conclusion
To conclude, our study did not identify any associations between levels of vitamin D during pregnancy and a wide range of anthropometric measures of the newborn. The possibility of a sex-specific response to low levels of vitamin D during pregnancy warrants further studies. However, our study cannot rule out possible detrimental effects on the newborn not reflected in anthropometric data.
Acknowledgements
The authors would like to thank the midwives and research staff at Grorud, Bjerke and Stovner Child Health Clinics and the women who participated in the STORK Groruddalen study. The authors would also like to thank Kåre I Birkeland at Oslo University Hospital for initiating the study in close collaboration with A. K. J. The authors would also like to thank the Hormone Laboratory at Oslo University Hospital for analysis and interpretation of 25(OH)D in serum.
The Norwegian Research Fund for General Practice has funded the PhD fellowship for Å. R. E. Data collection was supported by the Norwegian Research Council, the South-Eastern Norway Regional Health Authority, the Norwegian Directorate of Health and collaborative partners in the city of Oslo, Stovner, Grorud and Bjerke administrative districts.
A. K. J. initiated the STORK Groruddalen study. L. S. participated in data collection. Å. R. E., K. V. K. and A. K. J. designed the sub-study. Å. R. E. prepared the first version of the manuscript. A. K. J., I. M., K. V. K., L. S. and P. L. contributed to the discussion and results. Å. R. E. and I. M. performed the statistical analyses. All authors have revised the manuscript and approved the final version.
The authors declare that there are no conflicts of interest.
Supplementary material
For supplementary material/s referred to in this article, please visit https://doi.org/10.1017/S000711451700068X







