The reported prevalence of neurological symptoms in immunocompetent patients diagnosed with infectious mononucleosis due to Epstein–Barr virus (EBV) ranges between 0.37% and 7.3%. Reference Silverstein, Steinberg and Nathanson1 We here report a case of acute infectious myelitis in an immunocompetent adult and emphasize the importance of considering EBV in the differential diagnosis of patients presenting with transverse myelitis.
A previously healthy 52-year-old woman presented with a 1-week history of bilateral lower extremity paresthesias. She reported pelvic girdle and hip joint numbness with descending paresthesias from her upper thighs to her toes. She reported 1 week history of asthenia, diffuse myalgias, chills, and left paravertebral lumbar pain. She denied any weakness, sphincter disturbance, weight loss, or infectious symptoms. There was no history of hiking or camping nor infectious contacts nor recent vaccinations.
General medical examination was unremarkable, and no meningeal signs were found. Cranial nerve examination was normal. Tone and strength were both normal in upper and lower extremities. Deep tendon reflexes were brisk except for patellar hyporeflexia bilaterally. Both plantars were flexor. Abdominal reflexes were absent bilaterally. Sensory examination revealed impairment of light touch, pain, and temperature sensation with a well-defined sensory level at T11/12. At the time of presentation, these modalities were intact below L2. The numbness progressed along the rostrocaudal axis over 3 days following the patient’s admission. Vibration and joint position sensations were preserved. There were no cerebellar signs.
Laboratory examinations revealed a slightly decreased lymphocyte count and increased C-reactive protein. A computed tomography urogram was performed to rule out kidney stones and revealed multiple enlarged retroperitoneal lymph nodes. Further extensive workup including serum vitamin B12, human immunodeficiency virus, syphilis enzyme immunoassay, antinuclear antigen, rheumatoid factor, anti-cyclic citrullinated peptide, and serum protein electrophoresis were normal. Positron emission tomography scan showed generalized lymphadenopathy in the abdomen with increased fluorodeoxyglucose uptake, suggesting either a lymphoma or a reactive lymphadenopathy.
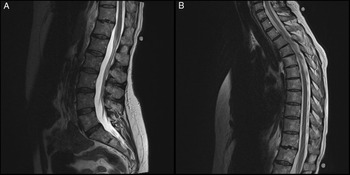
Magnetic resonance imaging (MRI) without contrast of the thoracic and dorsolumbar spine showed no abnormalities (Figure 1). A lumbar puncture revealed increased proteins (1.31 g/L) and white blood cell count (27 per mm3) with 99% lymphocytes. Despite the MRI study, the clinical findings and the result of the cerebrospinal fluid (CSF) examination were consistent with a presumptive diagnosis of either parainfectious or infectious myelitis, with a presumed lesion involving the anterior aspect of the lower thoracic spinal cord, affecting the decussation of sensory fibers en route to the spinothalamic tracts at the level of the lesion, explaining the bilateral suspended sensory loss of light touch, pain, and temperature.

Figure 1: (A) Sagittal view of the lumbosacral spine (T2 sequence, unenhanced). (B) Sagittal view of the thoracolumbar spine (T2 sequence, unenhanced).
Further serological studies were negative for cytomegalovirus (CMV), Lyme disease, hepatitis B and C and West Nile virus. Although a first monospot test was negative, a repeat test a week later was positive. The presence of immunoglobulin M and immunoglobulin G (IgG) antibodies for the viral capsid antigen and the absence of Epstein–Barr nuclear antigen IgG antibodies confirmed acute EBV mononucleosis. Finally, an EBV-polymerase chain reaction performed on the CSF was positive with more than 700 copies/mL and negative for CMV, varicella zoster virus, HSV, and enterovirus. The patient was diagnosed with partial myelitis as manifestation of an acute EBV infection.
Since there were only sensory symptoms, the decision was made not to start a specific treatment. At follow-up, the sensory examination was normal with no impairment of the light touch, pain, and temperature sensation. Both neurological and systemic symptoms completely resolved without any residual deficits.
Myelitis is an acute inflammatory disease of the central nervous system that can present with sensory alteration, weakness, and autonomic dysfunction. Reference Silverstein, Steinberg and Nathanson1 Symptoms usually develop over a short period of time (hours or days) and worsen over days to weeks. Inflammation, usually bilateral and restricted to a few segments, produces variable weakness and sensory impairment below the lesion. The neurological deficits recapitulate relevant neuroanatomy. In the case presented, we infer that the inflammation selectively involved the anterior white commissure where the spinothalamic second-order neurons decussate, explaining the bilateral suspended sensory loss of light touch, pain, and temperature, the sensory level on examination typically one or two spinal segments below the spinal cord lesion due to a majority of sensory fibres ascending one or two segments in the cord before decussating. Proprioceptive fibers are spared, being posteriorly located.
MRI with gadolinium contrast is the imaging modality of choice. Generally, it shows T2 hyperintensity involving a long segment of the cord. Previously described cases of EBV myelitis. Reference Poorthuis, Battjes, Dorigo-Zetsma and de Kruijk2–Reference Caldas, Bernicker, Nogare and Luby4 almost consistently report this finding on MRI. The fact that our patient had a negative MRI does not exclude the diagnosis of an acute myelitis as the diagnostic criteria established by the Transverse Myelitis Consortium Working Group in 2002 state that spinal cord inflammation must be “demonstrated by CSF pleocytosis or elevated IgG or gadolinium enhancement.” 5 In fact, data show that the involved segment of the cord is swollen in half of the cases and gadolinium enhancement would be present in 37–74% of cases. Reference West6
EBV can be the cause of a number of neurological disorders including mono- and polyneuropathies, anterior horn syndrome, cranial nerve palsies, meningitis, encephalitis, and myelitis, Reference Connelly and DeWitt7 which is the rarest neurological complication of EBV and happens more frequently in children than in adults. Reference Caldas, Bernicker, Nogare and Luby4 Stigmata of infectious mononucleosis such as fever, lymphadenopathy, or splenomegaly may or may not be present. Reference Caldas, Bernicker, Nogare and Luby4 EBV-deoxyribonucleic acid was detected in our patient’s CSF, supporting the etiological role of the virus in her neurological symptoms.
There are currently no evidence-based recommendations regarding the treatment for EBV-related neurological disorders and even less data concerning the prognosis of these patients. Corticosteroids could be considered if there is swelling of the spinal cord on imaging, which was not the case for our patient. Corticosteroids are also usually started when patients present with debilitating symptoms and should be considered if there is any weakness or sphincter disturbance. Reference Caldas, Bernicker, Nogare and Luby4 It is, however, unclear if patient improvement is spontaneous or results of steroid administration. Furthermore, there are no evidence-based recommendations regarding the administration of antiviral drugs to patients with neurological symptoms. Reference Poorthuis, Battjes, Dorigo-Zetsma and de Kruijk2
In conclusion, although EBV rarely causes neurological complications, it must be part of the differential diagnosis of acute myelitis in immunocompetent patients of all ages considering the high prevalence of EBV infection. Treatment should be individualized according to the patient’s symptoms as there are currently no evidence-based recommendations.
Funding
None.
Disclosures
None relevant to the case.
Statement of Authorship
This short case report was written by AD under the supervision of YE and AL.