Pilocytic astrocytoma (PA) is the most common primary brain tumor in the pediatric population but is less prevalent in adults.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1,Reference Stüer, Vilz, Majores, Becker, Schramm and Simon2 These slow-growing, well-circumscribed lesions are known to have heterogenous morphologies but usually have an excellent prognosis following surgery. As such, they are classified as WHO grade 1 glioma. The mainstay of therapy for PA is surgical resection, and adjuvant therapy is reserved for patients with inoperable tumors or those not amenable to gross total resection (GTR).Reference Louis, Perry and Wesseling3
While most PA follow a benign course, some studies report an increased tendency towards anaplastic transformation in adults.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1,Reference Stüer, Vilz, Majores, Becker, Schramm and Simon2,Reference Rodriguez, Brosnan-Cashman and Allen4 This subset, referred to as pilocytic astrocytoma with anaplasia (PA-A), has a worse median survival and progressionfree survival compared to those associated with PA.Reference Rodriguez, Brosnan-Cashman and Allen4 Anaplastic histological features including hypercellularity, moderate to severe cytologic atypia, and brisk mitotic activity have been observed at first presentation or in recurrent tumors.Reference Louis, Perry and Wesseling3 Furthermore, prior irradiation has been associated with de novo anaplastic transformation of PA.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1
Herein, we describe the anaplastic transformation of a cerebellar PA in an adult following surgery and radiotherapy. A comprehensive review of the literature yielded 15 similar cases. These cases will be described briefly, and their clinical features discussed.
A 34-year-old right-handed male presented with a one-year history of right-sided hearing loss, occipital headaches, and vertigo. MRI showed a right cerebellar intra-axial enhancing lesion with significant mass effect and was reported to be a glioma with high-grade features including a less pronounced cystic component and more diffuse in comparison to PA (Figure 1a). A midline suboccipital craniotomy was performed for tumor resection (Figure 1b). Due to the tumor’s proximity to the brainstem, GTR was not feasible. On neuropathological assessment, the specimen was diagnosed as a PA as there were characteristic features including eosinophilic granular bodies, rosenthal fibers, focal piloid morphology, strong diffuse GFAP immunoreactivity, and minimal proliferative activity (Figure 2).

Figure 1: Post-gadolinium axial MRI (A) preoperative, (B) immediately postoperative (C) following radiation, (D) prior to 4th cycle of bevacizumab, (E) prior to 7th cycle of bevacizumab, (F) prior to 8th cycle of bevacizumab and 2nd cycle of etoposide.
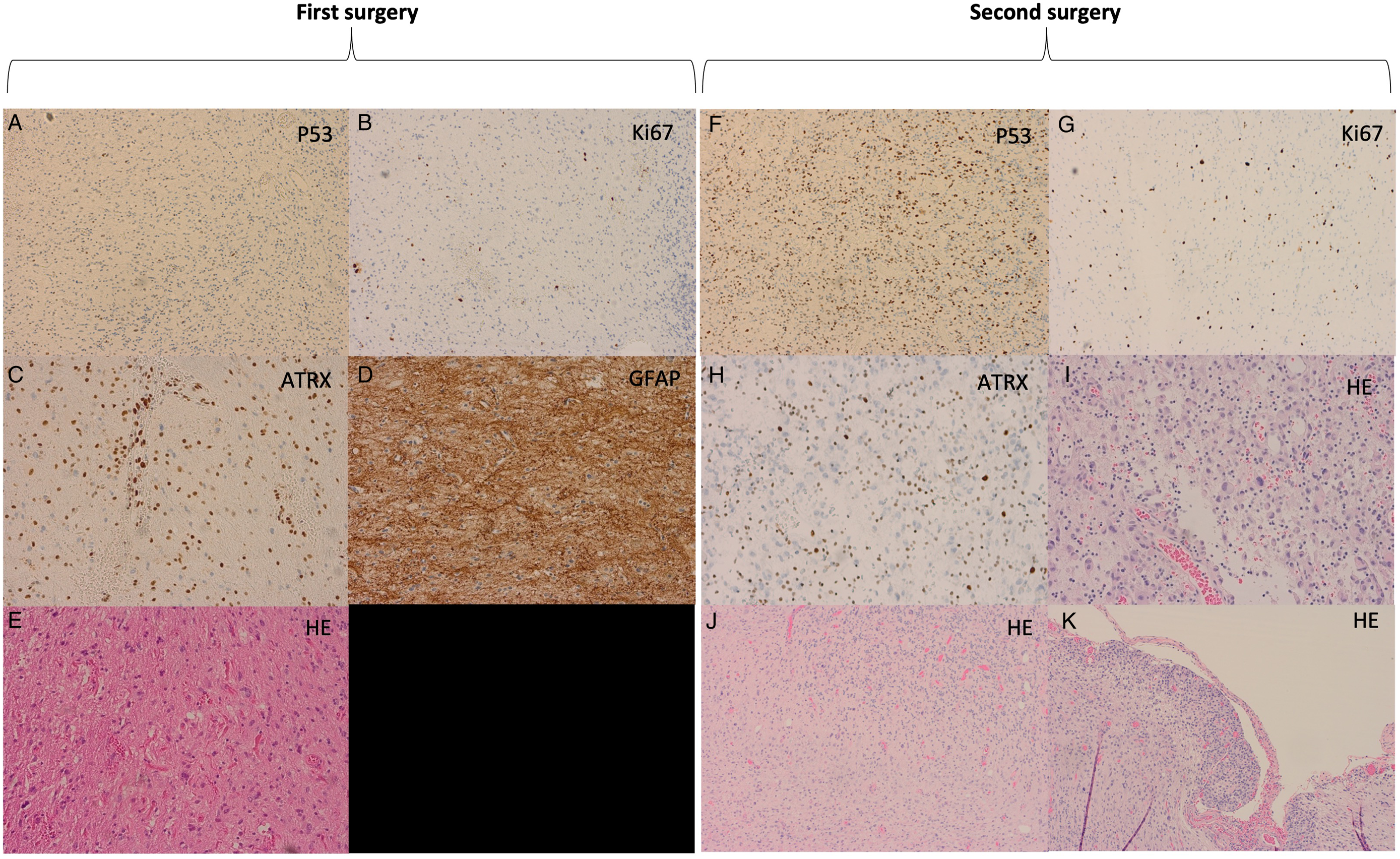
Figure 2: (A-E) Histopathology from the first surgical specimen diagnosed as pilocytic astrocytoma. (F-K) Histopathology from the second surgical specimen diagnosed as pilocytic astrocytoma with anaplastic transformation.
Post-resection imaging suggested disease progression, and the patient underwent adjuvant radiation (54-Gy in 30 fractions) 18 months after surgery. 39 months from initial resection, a repeat MRI once again demonstrated progression of the disease in the cerebellum with leptomeningeal enhancement of the cerebellar hemispheres. These findings were concordant with his symptoms of right upper extremity dysmetria and dysdiadochokinesis. The patient underwent repeat resection to obtain tissue and decompression of the 4th ventricle (Figure 1c).
Histopathological evaluation of the recurrent mass demonstrated pilocytic features as seen previously, in addition to anaplastic features including an increased mitotic rate up to five mitotic figures per 10 HPF, increased cellularity, and stark pleomorphism. Ki-67 demonstrated a modest proliferative index estimated at 5%. Immunohistochemistry was strongly and diffusely positive for GFAP and negative for BRAF V600E, IDH1 R132H, and H3F3A K27M. In addition, there was loss of nuclear ATRX immunoreactivity, and P53 immunohistochemistry demonstrated numerous strongly immunoreactive nuclei suggestive of a TP53 missense mutation (Figure 2). Lastly, molecular analysis revealed a KIAA1549::BRAF fusion rearrangement and MGMT promoter hypermethylation. These findings were suggestive of a recurrent PA with anaplastic transformation to PA-A.
Three months after repeat resection, MRI demonstrated progressive enhancing disease and the patient was started on bevacizumab. Prior to his 7th cycle of bevacizumab, imaging showed radiographic progression warranting the addition of a second chemotherapeutic agent, etoposide (Figure 1e). To date, 10 months since his repeat resection, the patient has completed eight cycles of bevacizumab and two cycles of etoposide with minimal progression on MRI (Figure 1f).
Several groups have found higher rates of recurrence and anaplastic transformation in adult PA compared to pediatric cases.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1,Reference Stüer, Vilz, Majores, Becker, Schramm and Simon2,Reference Rodriguez, Brosnan-Cashman and Allen4 The current WHO criteria for diagnosis of pilocytic astrocytoma with anaplasia remain unclear; however, common features include pseudopalisading necrosis, high cellularity, cytologic anaplasia, endothelial proliferation, and multiple mitoses per HPF.Reference Cyrine, Sonia and Mounir5 These features tend to overlap with other high-grade gliomas; thus, clinical behavior cannot be predicted reliably based on histology alone.Reference Reinhardt, Stichel and Schrimpf6
A review of the literature yielded four studies.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1,Reference Rodriguez, Brosnan-Cashman and Allen4,Reference Cyrine, Sonia and Mounir5,Reference Otero-Rodríguez, Sarabia-Herrero, García-Tejeiro and Zamora-Martínez7 In total, 15 sporadic precursor PA was identified that recurred following therapy and demonstrated secondary anaplastic transformation (Table 1). 11 of 15 cases received radiotherapy; however, details regarding radiation type, dose, and temporal data were not uniformly available. Previous studies have suggested a relationship between radiation and progression towards anaplasia.Reference Ellis, Waziri, Balmaceda, Canoll, Bruce and Sisti1 Anaplastic transformation has been observed after SRS which receives a therapeutic margin dose of 12–25 Gy. It has been established that radiation exposure in the 1–10-Gy range can cause sublethal DNA damage that can induce oncogenic mutations.Reference Ron, Modan and Boice8
Table 1: Reported adult cases of PA-A transformation from initial primary PA

GTR = gross total resection, STR = subtotal resection, NR = not reported, N/A = Not applicable.
Within the category of histologically defined PA-A, several disparate molecular alterations have been described including ATRX loss, CDKN2A/B homozygous deletions, H3F3A K27M mutations, and alternative lengthening of telomeres (ALT), among others.Reference Rodriguez, Brosnan-Cashman and Allen4,Reference Reinhardt, Stichel and Schrimpf6 Clinically relevant molecular PA-A subtypes have yet to be definitively isolated; however, some molecular alterations including ATRX loss and ALT have been associated with worse clinical outcomes.Reference Rodriguez, Brosnan-Cashman and Allen4 Methylation profiling of PA-A and high-grade astrocytomas has led to the isolation of high-grade astrocytoma with piloid features as a distinct tumor classification in the 2021 CNS WHO.Reference Louis, Perry and Wesseling3 Interestingly, Rodriguez et al. identified precursor PA with later PA-A transformation to be associated with worse clinical outcomes.Reference Rodriguez, Brosnan-Cashman and Allen4 Further molecular characterization will help to differentiate between morphologically similar gliomas.
In addition to presenting a rare and interesting diagnosis, this case poses questions regarding current tumor grading and management of PA. It remains unclear if the initial PA was inherently aggressive or whether radiation might have played a role in its transformation as the preoperative MRI scan demonstrated high-grade features despite histological diagnosis of PA. Our patient’s tumor retrogression following bevacizumab suggests that chemotherapy may provide a role as adjuvant or salvage therapy in aggressive adult PA cases. Prospective studies examining the therapeutic benefit of chemotherapy in this population are needed.
In summary, the findings of the present study suggest that a subset of PA in adults do not necessarily follow a benign course. Further studies exploring relevant subtypes and tumor grading may prove beneficial for prognostication and guidance of therapy. Lastly, the association between irradiation and anaplastic transformation of PA needs to be carefully investigated before conclusions can be drawn.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosures
The authors declare no conflicts of interest.
Statement of Authorship
VK assisted in data extraction and manuscript synthesis
MH assisted in manuscript synthesis and editing
BJ assisted with manuscript synthesis and histopathology analysis
PG assisted in manuscript editing and provided expertise in case management





