Burst spinal cord stimulation (SCS) is a novel form of spinal cord stimulation introduced in 2010.Reference De Ridder, Vanneste, Plazier, van der Loo and Menovsky 1 In conventional, or “tonic,” SCS, pulses are delivered at a set constant frequency, amplitude, and pulsewidth. In contrast, burst SCS delivers stimulation in packets of high-frequency pulses. In tonic SCS, the area of pain suppression is delineated by the presence of paresthesias, which are an indicator that stimulation is occurring. It has been advocated that the advantage of burst SCS over tonic SCS is its potential to effect paresthesia-free pain suppression, as paresthesias are often a source of considerable discomfort for the patient.Reference De Ridder, Vanneste, Plazier, van der Loo and Menovsky 1 , Reference Hou, Kemp and Grabois 2 Because of this feature, various authors believe that burst SCS is superior to tonic SCS as a surgical management of pain.Reference De Ridder, Vanneste, Plazier, van der Loo and Menovsky 1 , Reference De Ridder, Plazier, Kamerling, Menovsky and Vanneste 3 - Reference Tjepkema-Cloostermans, de Vos, Wolters, Dijkstra-Scholten and Lenders 5 While the methodology behind such studies may be disputed, another shortcoming of these claims is a lack of discussion of the side effects and caveats of burst SCS.
At our institution, four patients who were initially implanted with Medtronic PrimeAdvanced stimulators for tonic SCS were switched to a St. Jude Medical Prodigy implantable pulse generator (IPG), currently the only neurostimulator capable of burst programming. For burst programming, the frequency and pulsewidth used were as described in De Ridder et al.Reference De Ridder, Vanneste, Plazier, van der Loo and Menovsky 1 Also as described, the amplitude was increased just to the point where the patient began to feel paresthesias over the covered area, then lowered so that the paresthesias disappeared. The higher stimulation threshold at which burst SCS caused paresthesias, compared to tonic SCS, allowed burst SCS to be used at higher amplitude levels without causing paresthesias. Electrode types, locations, configurations, and stimulation parameters are summarized in Table 1.
Table 1 Burst spinal cord stimulation electrode type, locations, configurations, and stimulation parameters of all patients
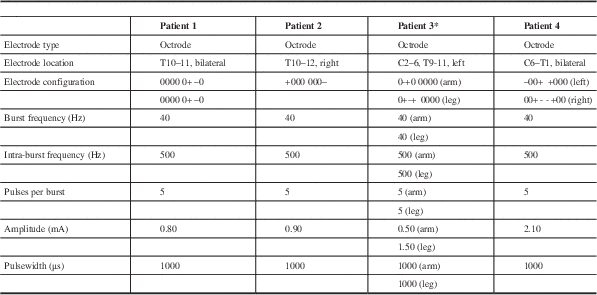
* Though two sets of parameters, one for the arm and the other for the leg, are listed, note that the patient was only able to use one of the two sets at any given time.
The first two patients were a 51-year-old male and a 33-year-old female. The former suffered from chronic back and leg pain due to heavy lifting, and while his tonic SCS provided pain relief, he was unable to tolerate the paresthesias. Hence the decision was made to switch him to burst SCS. The latter had chronic right back and right lower extremity pain after an ankle fracture and the birth of a baby. Her tonic SCS caused her jolting pain in the areas that it was meant to cover, leading her to switch to burst SCS. Both patients have tolerated their new burst SCS well.
Unfortunately, the third patient did not enjoy a positive outcome due to a programming limitation of the St. Jude Medical Prodigy IPG, as it allowed only one program for burst SCS that was unable to accommodate two different areas of pain, with different intensities, simultaneously. This was a 51-year-old gentleman who suffered from complex regional pain syndrome (CRPS) of his left arm and leg, after a motor vehicle accident at the age of 35, during which he sustained a neck injury that required an anterior cervical discectomy and fusion. The pain in his left leg was worse than that in his left arm. He initially received tonic SCS with one cervical lead and another thoracic lead, to cover both his left arm and leg. He was unable to tolerate the paresthesias, especially in his arm, despite some reduction in pain. As a result, he was switched over to burst SCS. Although the burst SCS was able to achieve a greater reduction in pain, it was limited in that it was able to run only one program for both the arm and the leg. (In contrast, tonic SCS could run two separate programs, one for the arm and another for the leg.) As his arm was less painful than the leg, the amplitude that could cover the arm without paresthesias was actually insufficient for the leg. Increasing the amplitude to cover the leg resulted in extremely unpleasant paresthesias in the arm. Consequently, his IPG was programmed with the option to use either tonic (arm: 30 Hz, 3.50 mA, 250 μs; leg: 30 Hz, 6.30 mA, 400 μs) or burst, and currently he uses tonic more frequently.
Our last patient was a 48-year-old woman who had just developed CRPS involving the left arm after having received left-sided sympathectomy for treatment of facial pain. She received initially tonic SCS. She experienced a 20% reduction in pain, but because of the severe intensity of her pain, it was decided that she should switch to burst SCS, which could be used at higher amplitude settings. Her Prodigy IPG allowed both tonic (70 Hz, 8.10 mA, 365 μs) and burst options. Although greater pain relief (30%) without paresthesias was achieved using burst, she actually used tonic more, as the paresthesias provided her psychological reassurance that she was being treated.
Our experience with four patients whose tonic spinal cord stimulation was switched to burst spinal cord stimulation illustrates caveats of burst SCS that are not found in other reports as of this date. While two of our patients tolerated their burst SCS better compared to tonic, the other two did not have a positive experience. The failure of one of them was completely due to a technical limitation of the St. Jude Medical Prodigy IPG. The other patient was interesting. Instead of enjoying the paresthesia-free pain suppression that burst SCS offered, she needed the paresthesias to convince her psychologically that stimulation was occurring. This is a beautiful reflection of the difficulty in conducting a randomized clinical trial for tonic SCS due to the placebo effect of paresthesias.
Burst SCS has the potential to eliminate such limitations due to its absence of paresthesias,Reference De Ridder, Vanneste, Plazier, van der Loo and Menovsky 1 but unfortunately there does not yet exist any methodologically sound comparisons between burst SCS and placebo. One such study was a crossover design by De Ridder et al.,Reference De Ridder, Plazier, Kamerling, Menovsky and Vanneste 3 but that study did not take into consideration the “period effect,” in which the order of interventions influences the outcomes of each intervention. In another study by Schu et al.,Reference Schu, Slotty, Bara, von Knop, Edgar and Vesper 4 randomization techniques were not convincing. But even when more concrete evidence for burst SCS becomes available, our last case suggests that patients who are switched from tonic to burst SCS may respond very differently from those who receive burst SCS as their first ever spinal cord stimulator. Our case series provides a glimpse into the complexity of treating pain syndromes surgically. Practitioners must consider the technical limitations of this new technology, as well as its potential impacts on the patient’s psychology, which is already complicated by the presence of chronic pain.
Statement of Authorship
Hao Li was responsible for review of the literature, analysis of cases, and writing of the manuscript. Karen Waterhouse was responsible for programming the stimulators and mining patient data. Aleksander Vitali was responsible for conception of the work and selection of cases.
Disclosures
Dr. Vitali reports one expert session (August of 2016) for the company producing the stimulator (St. Jude Medical). Hao Li and Karen Waterhouse do not have anything to disclose.



