We report an example of imaging features of neuromyelitis optica spectrum disorder (NMOSD) that overlapped those of multiple sclerosis (MS), a coexistence that raises questions about how disease-specific these features may actually be and implications for treatment. Although distinctive imaging findings can often help to differentiate NMOSD from MS,Reference Kim, Paul and Lana-Peixoto 1 the overlap between both disorders remains an open area of study. Furthermore, when the diagnostic criteria for NMOSD were revised in 2015, the International Panel for NMO Diagnosis agreed that non–organ-specific systemic autoimmunity may not only coexist with NMO but support its diagnosis.Reference Wingerchuk, Banwell and Bennett 2 Our case also highlights this concept because our patient demonstrated several laboratory features of autoimmunity.
A 57-year-old, right-handed, African-American woman with history of hypertension and recently diagnosed nondiabetic gastroparesis (8% emptying over 90 minutes) was admitted to our hospital with acute left-sided weakness and tingling. Additional history included intractable nausea, vomiting, and 60-lb weight loss over a 3-month period. She also described far-gaze binocular diplopia, bilateral lower extremity pain, and tingling sensation in extremities with neck movement. Family history was significant for breast and lung cancer in several first- and second-degree relatives.
Examination revealed left lateral gaze palsy, horizontal nystagmus, left-sided hyperalgesia within the trigeminal distribution of V1-V3, left-sided weakness that was most prominent in hip flexors, diffuse hyperreflexia, ankle clonus, and left-sided Hoffman’s but otherwise absent Babinski signs. Sensory examination showed marked loss of vibration in distal joints of all extremities, loss of proprioception in bilateral toes, and patchy (nonlocalizing) areas of either decreased or increased perception for light touch and pinprick. She required bilateral assistance to stand up from sitting and walk.
Diagnostic workup of serum and cerebrospinal fluid revealed additional abnormalities in immunological tests. Findings included positive antinuclear antibody screen with speckled pattern (titer 1:80), elevated sedimentation rate (71 mm/hour), and elevated C-reactive protein (36.4 mg/l). Specific serum antibodies were elevated: neuronal voltage-gated potassium channel antibodies, 0.41 (0.0-0.02); Anti-Sjögren’s-syndrome-related antigen A (SSA), 2.27 (0.0-0.9); Anti-Sjögren’s-syndrome-related antigen B (SSB), 1.61 (0.0-0.9); anti Jo-1, 1.36 (0.0-0.9); and positive anti-aquaporin 4 antibodies (AQP-4). Tests for anti-neutrophil cytoplasmic antibodies, double-stranded DNA, scleroderma 70 kD, anti-smith antibodies, antimyeloperoxidase antibodies, beta-2 glycoprotein, and cryoglobulin were negative. Cerebrospinal fluids showed a predominantly lymphocytic pleocytosis (89 cells/mm3); protein, 28 mg/dl; glucose, 61 mg/dl (serum, 98 mg/dl); negative gram stain and culture; immunoglobulin G index elevated to 1.1; and two matched oligoclonal bands were identified. Cytology and other viral studies were negative.
Brain magnetic resonance imaging (MRI) showed areas of increased signal intensity perpendicular to the lateral ventricles (Dawson’s fingers) and within the area postrema (Figure 1). No abnormal findings were seen on MRI of the thoracic spine or contrast-enhanced computed tomography scans of the chest, abdomen, and pelvis. Clinical rheumatological evaluation failed to confirm a clinical diagnosis of lupus or Sjögren’s syndrome. The patient remained stable and was scheduled to start rituximab infusions.
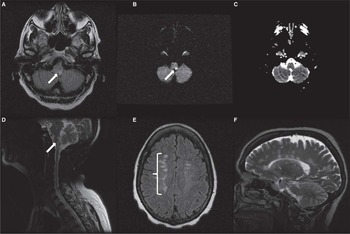
Figure 1 MRI showing atypical combination of area postrema involvement and Dawson’s fingers. Fluid-attenuated inversion recovery (FLAIR) hyperintensity in posterior medulla (arrow) (A). Corresponding area of diffusion restriction (B), but absent signal reduction on apparent diffusion coefficient imaging (C). T2-weighted image shows extensive area of signal change within area postrema in the sagittal view (D). Dawson’s fingers in axial FLAIR showing areas hyperintensities superior to the lateral ventricles, mainly on the right side (brace) (E). T2-weighted imaging showing extensive involvement of the corpus callosum in a perpendicular distribution consistent with Dawson’s fingers (F).
In addition to the multiple lesions shown (Figure 1), other brain areas involved by the disease included parts of the left pons, right cerebellum, and left thalamus. The multifocal nature of the disease could potentially explain the wide range of clinical manifestations observed (e.g. cranial nerves palsies, motor and sensory disturbances).
NMOSD was the most likely diagnosis given the specificity of anti AQP-4 antibodies and area postrema involvement. Despite extensive evaluation and close follow-up of our patient, no clinical evidence for rheumatological disease was found. Although the presence of concurrent autoimmunity in NMOSD has been previously describedReference Pittock, Lennon and de Seze 3 and occasionally thought to be part of a more generalized paraneoplastic process,Reference Cai, He and Chu 4 the clinical significance of such serological findings is unclear. It remains an open question whether coexistent non–organ-specific autoimmunity in NMOSD is part of the syndrome or is a separate finding indicative of increased predisposition to autoimmune disorders or, alternatively, a false-positive laboratory finding.
Contrasting with the classic NMO presentation of recurrent optic neuritis or longitudinal spinal cord lesion,Reference Wingerchuk, Banwell and Bennett 2 our patient’s severe nausea and vomiting were most likely a manifestation of area postrema involvement, confirming earlier observations and the importance of considering NMOSD in cases of intractable vomiting.Reference Popescu, Lennon and Parisi 5 The presence of antibodies against voltage-gated potassium channels possibly hindered peristalsis and manifested as gastroparesis.Reference Sandhiya and Dkhar 6
Our case illustrates an example of classic imaging features of NMO overlapping those of MS. Area postrema hyperintensity on brain MRI, considered highly suggestive of NMOSD,Reference Wingerchuk, Banwell and Bennett 2 is atypical in MS. In contrast, corpus callosal lesions perpendicular to the lateral ventricle (i.e. Dawson’s fingers) are highly suggestive radiographic findings of MSReference Matthews, Marasco and Jenkinson 7 , Reference Liao, Chang and Lyu 8 and maybe considered a red flag for diagnosis of NMOSD (except for longitudinal callosal lesions).Reference Wingerchuk, Banwell and Bennett 2 , Reference Matthews, Marasco and Jenkinson 7
The coexistence of these MRI signal characteristics is in line with earlier reports that such patterns may not be as disease-specific as currently believed.Reference Liao, Chang and Lyu 8 In a study of Taiwanese patients with MS or NMOSD,Reference Liao, Chang and Lyu 8 imaging patterns considered to be specificReference Liao, Chang and Lyu 8 failed to distinguish the two conditions: 11 (44%) of 25 NMOSD patients and 16 (55%) of 29 MS patients showed findings consistent with Dawson’s fingers.Reference Liao, Chang and Lyu 8 In striking contrast, Matthews et al reported Dawson’s fingers in none of the 26 patients with NMOSD versus 41 (82%) of 50 patients with MS.Reference Matthews, Marasco and Jenkinson 7 Such discrepancies in observations may suggest that these imaging patterns are indicative of ethnic variations or simply the diversity of idiosyncratic response patterns; however, we found no reports of unique imaging for MS or NMOSD in populations of African descent. Because of the involvement of area postrema in our patient, we tested for NMOSD rather than assume a diagnosis of MS. This is important in terms of outcome because some disease-modifying therapies for MS (e.g. beta interferons, fingolimod, natalizumab) have been shown to worsen NMOSD.Reference Wingerchuk, Banwell and Bennett 2
Finally, the overlap of clinical and radiographic features between MS and NMOSD is not uncommon. Regardless of imaging findings, we recommend broad testing by screening for AQP-4 and other autoantibodies, especially for MS patients who do not respond well to traditional disease-modifying agents and suffer continued relapses. Further studies aiming to determine the prevalence and the clinical significance of observed coexistent serological autoimmunity in patients with NMOSD are warranted.
Acknowledgments
The authors thank Mary Kemper for medical editing.
Statement of Authorship
AO collected data, designed the study, and prepared the first manuscript draft. AO, CJ, and AZ edited, revised, and approved the final draft for scientific content. AZ mentored the study.
Disclosures
AO and CJ have no disclosures to report. AZ has received honoraria and served on speakers bureaus for ACORDA, Biogen, and Novartis, and received honoraria, served on a speakers bureau, and served as a consultant for Genzyme/Sanofi.



