Lambert-Eaton myasthenic syndrome (LEMS) is an autoimmune, paraneoplastic, or non-tumor-related neuromuscular junction (NMJ) disorder characterized by reduced presynaptic release of acetylcholine. First described by Lambert, Eaton, and Rooke in 1956, clinical presentation typically includes subacute onset, symmetrical proximal limb weakness with lower extremity predominance, and autonomic dysfunction with male predominance. Reference Eaton and Lambert1 LEMS occurs secondary to IgG antibodies directed at the presynaptic voltage-gated calcium channels (VGCC) in the NMJ. Reference Benatar, Blaes and Johnston2 Typical electrophysiology shows diffusely low or borderline compound muscle action potential (CMAP) amplitudes, with normal sensory nerve action potential (SNAP), latencies, and conduction velocities. Decrement is seen with slow-rate (2–5 Hz) repetitive nerve stimulation (RNS), and an incremental response (at least 60%, but often greater than 100%) is seen with either high-rate (30–50 Hz) RNS or after brief exercise (10 s of maximal voluntary exercise). The above triad of clinical presentation, antibody positivity, and electrodiagnostic (Edx) findings support the diagnosis of LEMS. Reference Briggs, Gozzard and Talbot3
A 76-year-old Caucasian female presented to the emergency department with acute onset dyspnea, diarrhea, and productive cough superimposed on a 3-month history of progressive fatigue and generalized weakness. Her past medical history included chronic obstructive pulmonary disease (COPD) and hypothyroidism. She was admitted to Internal Medicine and failed treatment for an acute exacerbation of COPD. She rapidly progressed into hypercapnic respiratory failure, prompting intubation and transfer to the intensive care unit. Neurologic assessment revealed bilateral horizontal gaze reversing nystagmus, mild left eye ptosis with no Horner’s (syndrome of ptosis, miosis, enophthalmos, and anhidrosis), global areflexia (with no post-exercise accentuation of reflexes), right up-going plantar response, diffuse proximal greater than distal, lower extremity greater than upper extremity motor weakness, and glove and stocking gradient distribution loss of sensation to pinprick. Collateral history from the daughter confirmed an insidious onset, 3-month progressive functional decline with symptoms of autonomic dysfunction (early satiety, constipation, and sicca symptoms). There was a recent canned goods exposure, for which the husband also was exposed, the latter revealed no similar findings. Given the subacute presentation over 3 months, with a mixture of central nervous system (gaze reversing nystagmus, proximal greater then distal, and up-going plantar) and peripheral nervous system signs (autonomic dysfunction, third nerve enhancement on MRI brain, and global areflexia and stocking distribution sensory changes) we surmised a paraneoplastic process.
Initial Edx performed at post-admission day (PAD) 7 revealed a mild, symmetric, motor greater than sensory, axonal polyneuropathy. In retrospect, the motor amplitudes may have been smaller compared to the relatively preserved sensory amplitudes. The minimal Edx abnormality did not correlate with the profound degree of clinical weakness. Further testing was prompted. Cerebrospinal fluid analysis revealed a lymphocytic pleocytosis with 30 × 106 WBC (high), with normal glucose, protein, and electrophoresis with no banding. Cytology was negative. Brain and cervical spine MRI revealed gadolinium enhancement of the right third cranial nerve. CT chest, abdomen, and pelvis did not reveal any malignancy. Extended serologic paraneoplastic panel revealed SOX-1 autoantibodies to be medium positive and VGCC antibody (P/Q type) to be positive at >30 pmol/L (normal range <30 pmol/L). Positron emission tomography CT body revealed a low-grade metabolically active pulmonary nodule. Wedge resection revealed a small cell lung cancer (SCLC).
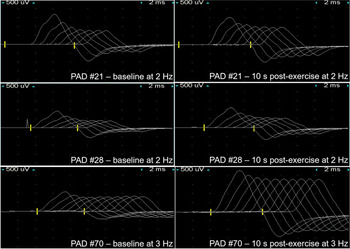
Repeat Edx at PAD 21 and 28 revealed progressive reduction in CMAP’s diffusely with preserved SNAP’s, and significant, reproducible, straight decrement >50%-70% seen on slow-rate (2 Hz) RNS of the ulnar nerve recording over the abductor digiti minimi, and 9.5%-32% decrement after brief exercise. High-rate (50 Hz) RNS was not done at these two visits (see Table 1).
Table 1. Summary of motor nerve studies of right ulnar nerve to abductor digiti minimi at supramaximal stimulation at the wrist during multiple visits

Edx on PAD 70 confirmed persistently low CMAP’s diffusely, resolution of significant decrement on slow-rate (2 Hz) RNS, and documented incremental response after brief exercise (116%) and high-rate (50 Hz) RNS (96%).
Despite wedge resection, short-term corticosteroids, and plasma exchange, she continued to decline. 3,4-diaminopyridine and long-term immune suppression were offered, but not accepted by family given severity of clinical state. The patient expired 3 months PAD.
We have serial Edx from this patient at PAD 7, 21, 28, and 70 which demonstrates how these studies progress through a LEMS patient’s clinical course. An incremental response after brief exercise or high-rate stimulation on RNS is a critical Edx criterion that must be met in order to fulfill a diagnosis of LEMS. All patients with LEMS in a study by Hatanaka and Oh Reference Hatanaka and Oh4 demonstrated at least 60% increment following exercise of 10 s. Oh et al Reference Oh, Kurokawa, Claussen and Ryan5 described lowering the typical 100% increment threshold to a minimum normal limit of increment to 60% post brief 10 s of exercise or high-rate RNS. This more sensitive increment threshold was able to identify 97% of patients with LEMS. The AAEM Quality Assurance Committee Reference Chiou-Tan, Tim and Gilchrist6 describes a minimum increment of 25% required for the diagnosis of LEMS.
There are two uncommon features we would like to emphasize from our serial Edx on this patient. First, in our PAD 21 and PAD 28 study, brief exercise yielded a decrement of 9.5%–32% in the CMAP amplitude from baseline. Oh Reference Shin7 has recently described four patients with such an atypical pattern (type 2) with low CMAP at rest, decrement at slow-rate RNS, initial decrement with high-rate RNS, and only later increment with high-rate RNS in his 50-year experience (see Table 1).
Secondly, as shown by Baslo, Reference Baslo, Deymeer and Serdaroglu8 on slow-rate RNS our serial Edx revealed CMAP amplitude decreased progressively from the first to the seventh response (straight decrement) often seen in LEMS patients, unlike myasthenia gravis patients where the CMAP amplitude demonstrates an improvement in initial decrement (U-shape decrement) between the first and fourth or fifth response (see Figure 1).

Figure 1: Low-frequency repetitive nerve stimulation of right ulnar nerve to adductor digiti minimi pre- and post-10 s exercise facilitation post-admission day (PAD) 21, 28 and 70.
Limitations from our series of Edx include lack of both brief exercise and high-rate RNS done early in the PAD, and for RNS sampling both a proximal and distal muscle would have been optimal. Having said that Lambert first diagnosed 97% of his cases based on ADM studies alone. Reference Baslo, Deymeer and Serdaroglu8 Although not routinely performed by all electromyographers, single fibre electromyography could be used earlier if NMJ is suspected, given its positivity on the first study. Reference Oh and Ohira9
In addition, SOX1 is an immunogenic antigen identified in SCLC with a 67% sensitivity and 95% specificity to discriminate between paraneoplastic LEMS and non-tumor LEMS. Reference Titulaer, Klooster and Potman10
Conflict of Interest
The authors have no conflicts of interest to declare.
Statement of Authorship
SS - primary author (wrote the majority of content and contributed to literature review); SH - secondary author (made table and figures as well as contributing to literature review); RJ - primarily acted as role of content reviewer and provided additional electrodiagnostic expertise.