Introduction
Parkinson’s disease (PD), dementia with Lewy bodies (DLB), and multiple system atrophy (MSA) are common pathologically overlapping neurodegenerative disorders associated with α-synuclein aggregates at autopsy. Parkinsonism is defined as bradykinesia in combination with rest tremor, rigidity, or both. These features must be clearly demonstrable and not attributable to confounding factors.Reference Postuma, Berg and Stern1 DLB is defined as dementia (cognitive dysfunction affecting activities of daily living), concurrent with or preceding parkinsonism,Reference McKeith, Dickson and Lowe2 associated with the triad of fluctuating attention, hallucinations and parkinsonism with recent inclusion of rapid eye movement (REM) sleep behavior disorder as a supportive criterion.Reference McKeith, Boeve and Dickson3 By contrast, dementia occurring in the setting of well-established parkinsonism, pragmatically defined by at least 1 year of symptoms, is considered to define Parkinson’s disease dementia (PDD), although this “1-year rule” remains contentious.Reference Postuma, Berg and Stern4 The revised diagnostic MSA criteria continue to categorize MSA with predominant parkinsonism (MSA-P) or with predominant cerebellar ataxia (MSA-C). Clinical diagnosis of possible MSA requires rigorously-defined dysautonomia and parkinsonism that is poorly responsive to levodopa, or cerebellar ataxia with neuroimaging abnormalities representing a supporting feature.Reference De Pablo-Fernandez, Lees, Holton and Warner5,Reference Gilman, Wenning and Low6
While motor symptoms define parkinsonism, nonmotor features, including mild cognitive impairment (MCI), neuropsychiatric features, dysautonomia, and REM sleep behavior disorder, can precede synucleinopathy diagnosisReference Tolosa, Gaig, Santamaria and Compta7–Reference Iranzo, Tolosa and Gelpi9 and may enhance diagnostic accuracy.Reference Galvin10 While bradykinesia and rigidity in PD respond to dopaminergic treatment, tremor, gait, and postural impairment do not always improve as consistently; less is known about levodopa response in DLB and MSA, though some response is possible, especially in early MSA-P.Reference Lucetti, Logi and Del Dotto11,Reference Hughes, Colosimo, Kleedorfer, Daniel and Lees12 Nonmotor symptoms (NMS) do not consistently respond to dopaminergic therapy,Reference Schaeffer and Berg13 while the response to motor symptoms becomes less complete as cognitive impairment worsens.Reference Alty, Clissold, McColl, Reardon, Shiff and Kempster14 Some features, such as sleepiness, orthostatic hypotension (OH), and freezing of gait (FOG), may in fact be exacerbated by dopaminergic medications.
Falls are common in synucleinopathies and can lead to significant morbidity and mortality. This underscores the need for fall risk assessment and the implementation of preventative measures in any patient with parkinsonism. Non-synuclein-related conditions, such as corticobasal ganglionic degeneration and progressive supranuclear palsy (PSP), also feature falls and can be misdiagnosed as synucleinopathies.Reference Borm, Krismer and Wenning15 This review focuses on synucleinopathies since it is a distinct group of movement disorders with shared pathogenic features associated with parkinsonism, gait impairment, and dysautonomia that increase fall risk. The aim is to review modifiable risk factors as well as medical, surgical, and nonpharmacological approaches to the prevention and treatment of falls in these disorders.
Falls Epidemiology in Synucleinopathies
Falls are common in synucleinopathies. Thirty-five to 90% of PD patients fell at least once over 12 months (average 60.5%), with two-thirds recurrent fallers.Reference Allen, Schwarzel and Canning16 Likewise, those with DLB sustained sixfold more falls compared to healthy controls.Reference Allan, Ballard, Rowan and Kenny17 In MSA, frequent falls were the commonest milestone of disease advancement and mortality progression, with an estimated probability of at least daily falls of 23% 5 years from disease onset, and years to death of 1–2 years when falls occur.Reference O’Sullivan, Massey and Williams18
Recurrent falls and postural instability (PI) are supportive clinical features of DLB and MSA, and conversely, are considered red flags early in the course of PD. Correspondingly, falls incidence is roughly double in DLB relative to those with PD without dementia.Reference Allan, Ballard, Rowan and Kenny17,Reference O’Sullivan, Massey and Williams18 Studies assessing postmortem-confirmed parkinsonian disorders noted that the latency between symptom onset to recurrent falling was far shorter in DLB and MSA compared with PD (Table 1). The latency between recurrent falling and death was not significantly different between groups, suggesting that recurrent falls herald an advanced stage of disease with poor prognosis.
Table 1: Falls latency (duration from first symptom to falls onset) in the synucleinopathies
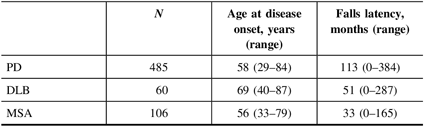
Data adapted from refs. [Reference Wenning, Ebersbach and Verny19,Reference Williams, Watt and Lees20]
PD: Parkinson’s disease; DLB: dementia with Lewy bodies; MSA: multiple system atrophy.
Fall Risk Factors in PD, DLB, and MSA
Fall Risk Factors in PD
Given the impact falls have in synucleinopathies, a systematic way to identify those most likely to fall would be ideal to proactively mitigate risk. Numerous risk factors have been proposed; however, the relative contribution of any factor in particular is difficult to assess given the interplay between them and their tendency to change over time. Many studies have devised prediction models for falling in PD,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21–Reference Parashos, Bloem and Browner31 and although these have highlighted various risk factors, their relative degree of contribution is inconsistent for several reasons. Many studies rely on subjectively collected questionnaires or one-time neurological assessments. Often, falls are self-reported in fall diaries with considerable heterogeneity between subjects. Some studies evaluate not easily accessible or practical physiological measurements.Reference Creaby and Cole32 Recently, a clinical prediction tool to discriminate future fallers from non-fallers was developedReference Paul, Canning, Sherrington, Lord, Close and Fung33 and validated,Reference Duncan, Cavanaugh and Earhart34,Reference Lindholm, Nilsson, Hansson and Hagell35 using a history of at least one fall within the past year, FOG in the past month, and slowed gait speed as fall risk determinants. This allows the simple classification of PD patients into low (17%), moderate (51%), and high (85%) fall risk in the ensuing 6 months, which can be effectively communicated and introduce strategies for fall prevention. This provides a benchmark for the development for other predictive models but doesn’t identify all factors that might be targeted for interventions. Other simple models, such as recent fall history, pull test, tandem gait and dyskinesias, and Frontal Assessment Battery (FAB), have also been examined.Reference Lindholm, Nilsson, Hansson and Hagell35,Reference Lindholm, Eek, Skogar and Hansson36
Preemptively identifying modifiable risk factors to implement preventative strategies is essential. Over the past decade, many small to moderately sized prospective studies have attempted to elucidate this by comparing potential risk factor profiles between fallers and non-fallers (Supplementary Table S1). Prior studies have been reviewed previously.Reference Allen, Schwarzel and Canning16,Reference Canning, Paul and Nieuwboer37 The evidence for the prevention of falls through the remediation of these risk factors is reviewed in the next section.
Non-modifiable risk factors inconsistently associated with increased fall risk in PD include advanced ageReference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Lamont, Morris, Menz, McGinley and Brauer38–Reference Allcock, Rowan, Steen, Wesnes, Kenny and Burn45 and gender,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Lamont, Morris, Menz, McGinley and Brauer38–Reference Mak and Pang44,Reference Voss, Elm and Wielinski46,Reference Mak, Wong and Pang47 although they are known risk factors in the general population.Reference Deandrea, Lucenteforte, Bravi, Foschi, La Vecchia and Negri48 Conversely, the rate at which nonmotor and motor symptoms develop can depend on sex and age at diagnosis, with dramatic rates in the development of axial symptoms after age 70.Reference Prange, Danaila and Laurencin49 Thus, the patient population studied may influence findings in observational studies. Other risk factors consistently associated with falls in PD include disease severity as measured by H&Y scale,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna and Yarnall25–Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Parashos, Bloem and Browner31,Reference Lamont, Morris, Menz, McGinley and Brauer38,Reference Cole, Rippey, Naughton and Silburn42–Reference Mak and Pang44,Reference Voss, Elm and Wielinski46,Reference Mak, Wong and Pang47,Reference Rudzinska, Bukowczan and Stozek50–Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52 UPDRS total score,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Latt, Lord, Morris and Fung23,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Mak and Pang44–Reference Voss, Elm and Wielinski46,Reference Rudzinska, Bukowczan and Stozek50,Reference Sakushima, Yamazaki and Fukuma51,Reference Kataoka and Ueno53 and UPDRS motor subscore.Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21–Reference Latt, Lord, Morris and Fung23,Reference Lord, Galna and Yarnall25,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Lamont, Morris, Menz, McGinley and Brauer38,Reference Smulders, Esselink, Weiss, Kessels, Geurts and Bloem41,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak, Wong and Pang47,Reference Sakushima, Yamazaki and Fukuma51,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52,Reference Lindholm, Hagell, Hansson and Nilsson54,Reference Almeida, Sherrington and Allen55 Studies where no association was foundReference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Mak and Auyeung43,Reference Mak and Pang44,Reference Kataoka and Ueno53 may be explained by the fact that as motor features progress, the fall risk decreases as the patient becomes more sedentary. Moreover, tremor might decrease leading to lower scores in the setting of worsening gait and PI subscores with paradoxically better overall scores in fallers.Reference Mactier, Lord, Godfrey, Burn and Rochester26 Similarly, proxies for disease severity, including higher levodopa equivalent dosesReference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22–Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak and Pang44,Reference Mak, Wong and Pang47,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52,Reference Kataoka and Ueno53,Reference Almeida, Sherrington and Allen55 and disease duration, have been variably identified as risk factors,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21–Reference Latt, Lord, Morris and Fung23,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Parashos, Bloem and Browner31,Reference Lamont, Morris, Menz, McGinley and Brauer38,Reference Heinzel, Maechtel and Hasmann40,Reference Cole, Rippey, Naughton and Silburn42–Reference Mak and Pang44,Reference Voss, Elm and Wielinski46,Reference Mak, Wong and Pang47,Reference Rudzinska, Bukowczan and Stozek50–Reference Almeida, Sherrington and Allen55 although the degree of levodopa response is not usually directly assessed. Falls can be observed preceding diagnosisReference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Lord, Galna and Yarnall25 and prior to initiation of levodopa,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21 particularly in those with the postural instability and gait difficulties (PIGD) subtype.Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Lord, Galna and Yarnall25,Reference Chou, Elm and Wielinski30,Reference Cole, Rippey, Naughton and Silburn42,Reference Voss, Elm and Wielinski46 Injurious falls and hip fractures may in fact precede PD diagnosis by a decade or more.Reference Nyström, Nordström and Nordström56
Certain motor symptoms and signs of parkinsonism are potentially modifiable risk factors. Numerous studies have identified impaired balance as a key risk factor by assessing composite measures of PI,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Lord, Galna and Yarnall25,Reference Chou, Elm and Wielinski30,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak and Auyeung43,Reference Voss, Elm and Wielinski46,Reference Mak, Wong and Pang47,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52–Reference Almeida, Sherrington and Allen55,Reference Brandmeir, Brandmeir, Kuzma and McInerney57 anticipatory balance maneuvers,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22–Reference Lord, Galna and Yarnall25,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Parashos, Bloem and Browner31,Reference Smulders, Esselink, Weiss, Kessels, Geurts and Bloem41,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak and Pang44,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52,Reference Almeida, Sherrington and Allen55,Reference Brandmeir, Brandmeir, Kuzma and McInerney57–Reference Paul, Allen and Sherrington59 reactive balance maneuvers,Reference Lindholm, Nilsson, Hansson and Hagell35,Reference Lindholm, Hagell, Hansson and Nilsson54,Reference Brandmeir, Brandmeir, Kuzma and McInerney57–Reference Paul, Allen and Sherrington59 and slowed mobility.Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24–Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Smulders, Esselink, Weiss, Kessels, Geurts and Bloem41,Reference Mak and Pang44,Reference Lindholm, Hagell, Hansson and Nilsson54,Reference Paul, Sherrington, Canning, Fung, Close and Lord58 FOG is also reliably associated,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21–Reference Latt, Lord, Morris and Fung23,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Lindholm, Nilsson, Hansson and Hagell35,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak and Auyeung43,Reference Mak, Wong and Pang47,Reference Rudzinska, Bukowczan and Stozek50,Reference Lindholm, Hagell, Hansson and Nilsson54,Reference Almeida, Sherrington and Allen55,Reference Paul, Sherrington, Canning, Fung, Close and Lord58–Reference Camicioli and Majumdar60 while conversely, tremor, axial rigidity, and dyskinesias are not convincingly associated.Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22–Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Parashos, Bloem and Browner31,Reference Rudzinska, Bukowczan and Stozek50,Reference Lindholm, Hagell, Hansson and Nilsson54,Reference Almeida, Sherrington and Allen55,Reference Paul, Sherrington, Canning, Fung, Close and Lord58
Dementia is a significant fall risk factor in the general population and in PD,Reference Shaw61 although data are less comprehensive in MCI. Some studies correlated increased fall risk with lower scores on global cognitive assessmentsReference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21,Reference Latt, Lord, Morris and Fung23,Reference Chou, Elm and Wielinski30,Reference Smulders, Esselink, Weiss, Kessels, Geurts and Bloem41,Reference Rudzinska, Bukowczan and Stozek50,Reference Paul, Sherrington, Canning, Fung, Close and Lord58,Reference Camicioli and Majumdar60 while others have not.Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24–Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Lamont, Morris, Menz, McGinley and Brauer38,Reference Heinzel, Maechtel and Hasmann40,Reference Mak and Auyeung43,Reference Mak and Pang44,Reference Rudzinska, Bukowczan and Stozek50,Reference Sakushima, Yamazaki and Fukuma51,Reference Kataoka and Ueno53,Reference Almeida, Sherrington and Allen55 However, many studies excluded patients with cognitive impairment a priori based on mini-Mental State Exam (MMSE) screening (i.e., less than 24).Reference Amar, Stack, Fitton, Ashburn and Roberts62 This is problematic since MCI and dementia are common in PD,Reference Domingos, Godinho and Dean63 and the MMSE is insensitive to attention and executive function, both of which are more prominently affected in PD-related cognitive impairment.Reference Yogev, Giladi, Peretz, Springer, Simon and Hausdorff64 The few studies that examined specific cognitive domains found mixed data to suggest possible correlations with frontal,Reference Latt, Lord, Morris and Fung23,Reference Lord, Galna and Yarnall25,Reference Allcock, Rowan, Steen, Wesnes, Kenny and Burn45,Reference Mak, Wong and Pang47,Reference Kataoka and Ueno53,Reference Paul, Sherrington, Canning, Fung, Close and Lord58 visuospatial,Reference Lord, Galna and Yarnall25 working memory,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Parashos, Bloem and Browner31,Reference Paul, Sherrington, Canning, Fung, Close and Lord58 verbal function,Reference Parashos, Bloem and Browner31,Reference Paul, Sherrington, Canning, Fung, Close and Lord58 and dual-tasking impairment.Reference Heinzel, Maechtel and Hasmann40,Reference Smulders, Esselink, Weiss, Kessels, Geurts and Bloem41,Reference Lindholm, Hagell, Hansson and Nilsson54
OH is prevalent in PD and may have a reversible effect upon cognition secondary to CNS hypoperfusion.Reference Centi, Freeman, Gibbons, Neargarder, Canova and Cronin-Golomb65 A few studies showed involvement of OH in falls.Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Allcock, Rowan, Steen, Wesnes, Kenny and Burn45,Reference Sakushima, Yamazaki and Fukuma51,Reference Kataoka and Ueno53 One recent study focusing on cardiovascular dysautonomia in PD fallers found an adjusted tenfold increased probability of falling in those with OH.Reference Romagnolo, Zibetti and Merola66 Dopaminergic medications can exacerbate OH and further increase fall risks. Likewise, one study investigating urinary dysfunction in PD fallers found mild (but not severe) urinary urgency was associated with a fivefold increased fall risk,Reference Sakushima, Yamazaki and Fukuma51 presumably given the balance between the need to ambulate quickly versus the presence of activity and environmental factors.
With respect to neuropsychiatric symptoms, depression has been proposed as a potential factor, as it may influence motivation to ambulate in addition to psychomotor slowing,Reference Hiorth, Alves, Larsen, Schulz, Tysnes and Pedersen21,Reference Voss, Elm and Wielinski46,Reference Sakushima, Yamazaki and Fukuma51 although numerous other studies have not found a correlation.Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Heinzel, Maechtel and Hasmann40,Reference Mak and Auyeung43,Reference Mak and Pang44,Reference Mak, Wong and Pang47,Reference Kataoka and Ueno53 Depression is a risk factor for falls in older people without PD.Reference Deandrea, Lucenteforte, Bravi, Foschi, La Vecchia and Negri48
Other potentially modifiable risk factors include a fear of falling, which can be related to anxiety,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24–Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Chou, Elm and Wielinski30,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Cole, Rippey, Naughton and Silburn42,Reference Mak and Pang44,Reference Voss, Elm and Wielinski46,Reference Mak, Wong and Pang47,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52–Reference Almeida, Sherrington and Allen55 impaired activities of daily living and reduced quality of life,Reference Kerr, Worringham, Cole, Lacherez, Wood and Silburn22,Reference Lord, Galna, Yarnall, Coleman, Burn and Rochester24,Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Matinolli, Korpelainen, Sotaniemi, Myllylä and Korpelainen27,Reference Chou, Elm and Wielinski30,Reference Parashos, Bloem and Browner31,Reference Lamont, Morris, Menz, McGinley and Brauer38,Reference Gazibara, Kisic Tepavcevic and Svetel39,Reference Cole, Rippey, Naughton and Silburn42,Reference Voss, Elm and Wielinski46,Reference Rudzinska, Bukowczan and Stozek50,Reference Almeida, Valenca, Negreiros, Pinto and Oliveira-Filho52–Reference Almeida, Sherrington and Allen55 reduced lower extremity strength,Reference Latt, Lord, Morris and Fung23,Reference Paul, Sherrington, Canning, Fung, Close and Lord58 and sensory dysfunction.Reference Latt, Lord, Morris and Fung23,Reference Voss, Elm and Wielinski46,Reference Sakushima, Yamazaki and Fukuma51,Reference Paul, Sherrington, Canning, Fung, Close and Lord58
Fall Risk Factors in DLB
The onset of recurrent falling is much shorter in DLB relative to PD, with repeated falls a supportive criterion for DLB. Although both are neuropathologically characterized by Lewy bodies and Lewy neurites, their symptomatology and clinical course differ substantially. Similar to PD, the presenting features may be broadly subdivided into four categories: cognitive impairment, physical symptoms, dysautonomia, and neuropsychiatric phenomena.
DLB is the second-most common form of neurodegenerative dementia, after Alzheimer’s disease (AD), and consequently, studies of fall risk tend to directly compare the two. Distinct from AD, in which early anterograde amnesia is sine qua non, those with DLB display variable memory impairment; fluctuating cognitive impairment; and early dysfunction of attention, executive, and visuospatial domains.Reference McKeith, Boeve and Dickson3 While a decline in global cognitive function is associated with increasing fall risk in heterogeneous general populations,Reference Anstey, Von Sanden and Luszcz67–Reference Martin, Blizzard and Wood70 a more careful examination of dementia subtypes has demonstrated a far greater fall risk and a shorter latency period from diagnosis to onset of recurrent falls in DLB relative to AD.Reference Allan, Ballard, Rowan and Kenny17,Reference Allan, Ballard, Burn and Kenny71–Reference Hanyu, Sato, Hirao, Kanetaka, Sakurai and Iwamoto73 The reason does not seem to be from a more aggressive rate of overall cognitive decline in DLB.Reference Hanyu, Sato, Hirao, Kanetaka, Sakurai and Iwamoto73,Reference Breitve, Chwiszczuk and Hynninen74 More likely, the decline of specific cognitive domains characteristic of DLB, as well as autonomic and motor features, might explain the discrepancy. Patients with DLB have disproportionately more severe visuospatial impairment.Reference Mosimann, Mather, Wesnes, O’Brien, Burn and McKeith75 This was substantiated by one study that demonstrated dysfunction in constructional praxis increased fall risk in DLB, and in particular, those without parkinsonism.Reference Kudo, Imamura, Sato and Endo76 Other disproportionately affected cognitive domains in DLB, such as judgment and executive function, likely contribute to fall risk as they do in the general population.Reference Martin, Blizzard and Wood70,Reference Springer, Giladi, Peretz, Yogev, Simon and Hausdorff77 The positive effects of cholinesterase inhibitors upon cognition and behaviorReference Wang, Yu and Tang78 may extend to reducing fall risk, although this is not clearly demonstrated.Reference Kim, Brown, Ding, Kiel and Berry79 Finally, fluctuating cognition in DLB is likely to impact upon fall risk, as fall-related injuries may occur during cognitive “down” periods in patients without evidence of parkinsonism or PI.Reference Imamura, Hirono and Hashimoto80
More severe parkinsonism is associated with an increased falls in DLB.Reference Ballard, Shaw, Lowery, McKeith and Kenny81 However, while the essential motor features of PD can be present in DLB, they are not strictly required to diagnose “probable DLB,”Reference McKeith, Boeve and Dickson3 and as only one cardinal feature is required to meet criteria for parkinsonism in DLB, many patients would not meet criteria for clinical PD.Reference Boeve, Dickson and Duda82 In fact, cross-sectional studies have shown that approximately 20%–30% of patients have minimal or no signs of parkinsonism during their disease course.Reference Aarsland, Ballard, McKeith, Perry and Larsen83,Reference Del Ser, McKeith, Anand, Cicin-Sain, Ferrara and Spiegel84 When parkinsonism is present, extrapyramidal motor features in DLB more often take the form of prominent axial symptoms rather than resting tremor as compared with PD.Reference Goldman, Goetz, Brandabur, Sanfilippo and Stebbins85,Reference Burn, Rowan and Minett86 Correspondingly, a cross-sectional analysis found that those with Lewy body dementias (either DLB or PDD) had a greater fall risk and poorer scores on gait and balance relative to those with AD.Reference Allan, Ballard, Burn and Kenny71 Other studies have similarly shown poorer scores on gait and balance when comparing those with DLB versus AD and PDD, and likewise correlated these measures to fall risk.Reference Scharre, Chang and Nagaraja87,Reference Fritz, Kegelmeyer and Kloos88 A prospective assessment of baseline risk factors in DLB recapitulated these findings and noted that abnormalities in gait and balance correlated with increasing fall risk.Reference Allan, Ballard, Rowan and Kenny17 FOG is usually a sign of advanced PD and is frequently seen in atypical parkinsonism, including DLB.Reference Factor89,Reference Palermo, Frosini and Corsi90
The NMS profile of DLB includes dysautonomia, neuropsychiatric symptoms, and REM sleep behavior disorders. A prospective assessment of baseline risk factors in DLB demonstrated that fall risk was related to the duration of dementia, use of cardioactive medications, and dysautonomia.Reference Fritz, Kegelmeyer and Kloos88 Dysautonomia, particularly OH and exaggerated carotid sinus reflex, is more common in DLB compared with PD and other forms of dementia,Reference Kenny, Kalaria and Ballard91,Reference Thaisetthawatkul, Boeve and Benarroch92 and together caused the majority of syncopal episodes in those with DLB in a prospective observational study.Reference Ungar, Mussi and Ceccofiglio93 Part of this may relate to the exaggerated period of hypotension following orthostatic challenge seen in DLB relative to other forms of dementia.Reference Andersson, Hansson, Minthon, Ballard and Londos94 Furthermore, dysautonomia was associated with significantly reduced survival within 3 years of a prospective longitudinal study.Reference Stubendorff, Aarsland, Minthon and Londos95 Persistently untreated OH may in fact precipitate or worsen dementia secondary to prolonged regional cerebral hypoperfusion.Reference Tanaka, Shimo and Yamashiro96,Reference Robertson, Messner and Shirzadi97 OH and consequent falls can be further exacerbated by medications used to treat the neuropsychiatric manifestations of DLB, including psychotropic medication, and the use of psychotropic medications is an independent risk factor in falls in DLB.Reference Allan, Ballard, Rowan and Kenny17 Cholinesterase inhibitors may induce bradycardia and syncope leading to fall-related injuries including hip fractures in patients with dementia,Reference Gill, Anderson and Fischer98 which is of particular significance in DLB considering the association with neurocardiovascular instability.Reference Kenny, Kalaria and Ballard91 Benzodiazepines are often used to treat REM sleep behavior disorder but also increase falls risk and hip fractures in the elderly population.Reference Donnelly, Bracchi, Hewitt, Routledge and Carter99 Finally, dopaminergic drugs are also well recognized to cause hypotension or exacerbations of OHReference Noack, Schroeder, Heusser and Lipp100 that lead to increased falls risk.
Fall Risk Factors in MSA
Fall risks have not been as extensively studied in MSA when compared to PD, which may reflect the early onset and high frequency of falls in this diseaseReference Wenning, Ebersbach and Verny19 in addition to its relative rarity. Moreover, its rapid progression, particularly early in the course of the disease, necessitates the use of gait aids and confinement to wheelchair by 3 and 5 years, respectively.Reference Watanabe, Saito and Terao101 One retrospective study examined fall risk factors in atypical parkinsonism, including MSA.Reference Williams, Watt and Lees20 In this study, univariate analysis distinguished fallers from non-fallers using clinical features of MSA including limb rigidity, speech disturbance, dysphagia, and pyramidal tract signs. Further, the latency to first fall was independently influenced by age of disease onset and PI. The presence of cerebellar signs, as seen prominently in MSA-C, is likely also to be a risk factor, particularly given the broad-based ataxic gait commonly associated with this condition,Reference Gilman, Wenning and Low6 but was underrepresented in this study sample. Finally, dysautonomia was not found to be a fall risk factor; however, this is likely a reflection of its early and severe prominence in both fallers and non-fallers, particularly in the urogenital and cardiovascular domains.Reference Kollensperger, Geser and Ndayisaba102
Other prominent features of MSA that may be early risk factors for falls may be extrapolated from the preceding discussions on PD and DLB. Motor risks include PI (89% at presentation), FOG (38%), postural tremor (54%), and dystonia (10%).Reference Kollensperger, Geser and Ndayisaba102 Likewise, nonmotor potential risks include depression (41% at presentation), executive dysfunction (49%), and dementia (4.5%).Reference Kollensperger, Geser and Ndayisaba102,Reference Stankovic, Krismer and Jesic103
Approaches to Fall Prevention
Although the majority of the evidence toward disease management comes from studies in PD, there is substantial overlap in the challenges faced, and therefore, the strategies are broadly applicable to other synucleinopathies (Table 2). Pharmacological, nonpharmacological, and surgical treatment options are described later and are summarized in Table 3.
Table 2: Therapy interventions and monitoring of falls in PD and DLB
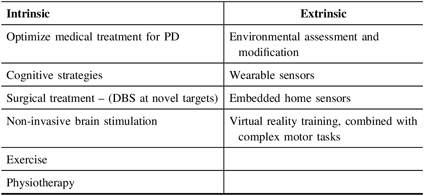
Table 3: Pharmacological, nonpharmacological, and surgical interventions
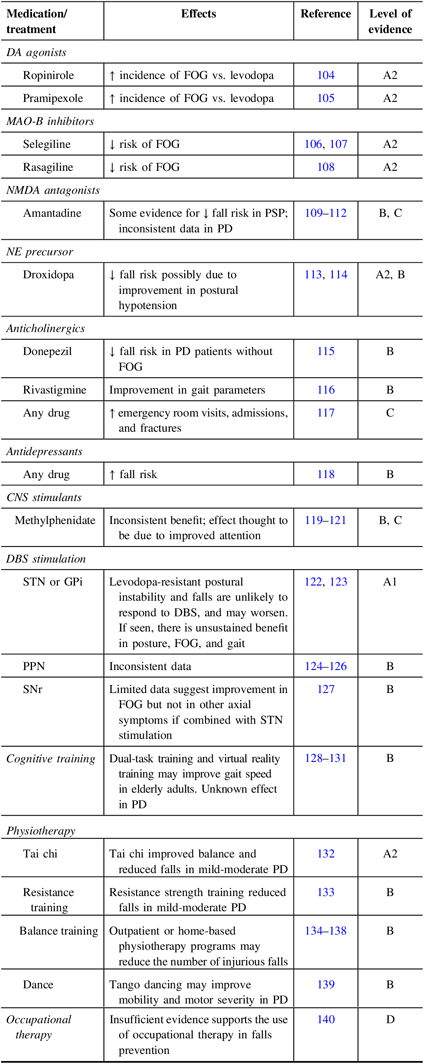
A1=meta-analysis containing at least some trials with evidence level A2, with consistency in trial results; A2=good quality randomized comparative clinical trials (randomized double-blind controlled trials) of sufficient size and consistency; B=moderate (weak) quality randomized clinical trials of insufficient size or other comparative trials (non-randomized trials, cohort studies, patient-control studies); C=non-comparative trials; D=expert opinion.
Clinical Approaches to Fall Classification
Key to establishing a treatment plan in such a multifactorial phenomenon is to classify falls to understand and manage risk. In practice, establishing fall circumstances can be challenging, as this is often self-reported and retrospective, and therefore subject to the vagaries of memory,Reference Hale, Delaney and Cable141 particularly in a population with cognitive challenges. Moreover, it is important to document the events leading toward a fall, rather than simply assessing fall frequency, as the latter does not have a clear relationship with fall etiology.Reference Mactier, Lord, Godfrey, Burn and Rochester26 Instead, the use of fall diaries to record both the frequency and circumstances of falling can be successfully used, at least in those without major cognitive impairment.Reference Ashburn, Stack, Ballinger, Fazakarley and Fitton142,Reference Hunter, Rochester, Morris and Lord143
Establishing the environment and type of movements leading to a fall is important, as for example, those falling during transitional movements or in the home tend to have a higher burden of disease than those who fall during higher-risk activities or in the community.Reference Mactier, Lord, Godfrey, Burn and Rochester26,Reference Lamont, Morris, Menz, McGinley and Brauer38 Falls occurring during multitasking, at a particular time suggestive of cognitive fluctuations, or in the presence of neuropsychiatric disturbances might suggest the need for neurocognitive treatment. A review of medications and “on” or “off” status can be helpful for medication selection and titration. Preceding symptoms, such as presyncope or dizziness, may suggest the need for management of OH, bearing in mind that OH can be asymptomatic. Finally, other mitigating circumstances, such as urinary urgency, proprioceptive loss, or visual impairment, can trigger management of comorbid disease.
Recently, a simplified approach toward falls classification was developed, which divides falls into those involving transitional movements (e.g., rising from a chair), complex motor activities (e.g., skiing), and combined movements (e.g., garden work).Reference Ross, Yarnall, Rochester and Lord144 Describing falls in this manner may simplify the approach toward management by categorizing physical differences between fallers and thereby allowing the detection and targeting of fall risk factors. For example, those with consistent transitional falls may benefit from strength or simple balance training, while those with consistent falls during combined movements may benefit from targeted neurocognitive strategies.
Optimizing Medical Management of Parkinsonism
Levodopa remains the agent of choice in the treatment of parkinsonism, particularly in PD, although short-lived, if less robust, responses are seen in as many as half of patients with DLB and MSA.Reference Lucetti, Logi and Del Dotto11,Reference Hughes, Colosimo, Kleedorfer, Daniel and Lees12,Reference Kollensperger, Geser and Ndayisaba102 However, PI may respond less reliably,Reference Deandrea, Lucenteforte, Bravi, Foschi, La Vecchia and Negri48,Reference Prange, Danaila and Laurencin49,Reference Klawans145 and fall risk and PI may persist and progressively worsen despite levodopa.Reference Agid, Graybiel and Ruberg146 This may, in part, arise from levodopa-induced dyskinesias.Reference Curtze, Nutt, Carlson-Kuhta, Mancini and Horak147 In patients responding well to antiparkinsonian drugs (i.e., >30% change in UPDRS-III with treatment), axial features were found to respond partially to levodopa.Reference Bejjani, Gervais and Arnulf148 Decreased levodopa efficacy with disease progression has been attributed to worsening axial symptoms (i.e., gait disorders and PI)Reference Klawans145,Reference Bonnet, Loria, Saint-Hilaire, Lhermitte and Agid149 and is considered to result from the increasing severity of non-dopaminergic deficits affecting brain regions and systems localized outside of the striatal output pathways.Reference Agid150 Conversely, levodopa improves rigidity and bradykinesia, and thus enhances patient mobility. As PD fallers have higher impulsivity scores compared to non-fallers,Reference Smulders, Esselink, Cools and Bloem151 this imbalance between the persistence of PI and improved ambulation with levodopa can potentially increase the likelihood of falling.Reference Mactier, Lord, Godfrey, Burn and Rochester26
FOG is common in synucleinopathies and remains difficult to treat.Reference Factor89,Reference Palermo, Frosini and Corsi90 In PD, FOG can occur in the “on” or “off” states, with off-freezing potentially improving with dopaminergic medications. Levodopa has been shown to be a better agent of choice compared to dopamine agonists: an increased risk of freezing was observed in a pramipexole group compared with the levodopa group (hazard ratio 1:7) in a phase-III, prospective, double-blind, placebo-controlled trial.Reference Holloway, Shoulson and Fahn152 A similar observation was reported with ropinirole in patients with early PD in a prospective, double-blind, placebo-controlled trial with a 5-year follow-up.Reference Rascol, Brooks, Korczyn, De Deyn, Clarke and Lang104 Selegiline was effective in reducing the risk of developing FOG,Reference Giladi, McDermott and Fahn106 and in decreasing the number of patients who develop FOG as a late complication of disease progression in a 2-year prospective follow-up.Reference Shoulson107 Rasagiline showed a 1.17 point improvement on the FOG-Questionnaire (FOG-Q) total score, in a prospective, double blind, placebo-controlled study.Reference Rascol, Brooks and Melamed108 In both studies, however, falls were not directly addressed, and therefore the clinical significance is unclear. In the case of DLB, dopamine agonists are not preferable because of their propensity to cause hallucinations and somnolence.Reference Fernandez, Wu and Ott153 Moreover, monoamine oxidase type-B inhibitors may cause OH and thus might precipitate falls. There are conflicting results regarding Amantadine in FOG and subsequent falls risk, but there is some evidence for fall risk reduction in PSP.Reference Kondo109 The norepinephrine precursor, droxidopa, was shown to reduce fall risk in PD,Reference Hauser, Heritier, Rowse, Hewitt and Isaacson113 with the mechanism attributed to improvement in postural hypotension, though other effects have not been extensively examined.
Optimizing Surgical Management
Multiple randomized controlled studies have demonstrated that deep brain stimulation (DBS) of the subthalamic nucleus (STN) and globus pallidus interna (GPi) is superior to medical treatment alone in the treatment of a number of cardinal PD symptoms and motor complications from therapy.Reference Benabid, Pollak, Louveau, Henry and de Rougemont154–Reference Rizzone, Fasano and Daniele156 The benefit of DBS on axial symptoms, however, is less clear.Reference Ferraye, Deba and Pollak157 Several reports have indicated improvement of posture, gait, and balance control after STN- or GPi-DBS, when these symptoms were responsive to levodopa treatment before DBS surgery;Reference Castrioto, Lozano, Poon, Lang, Fallis and Moro158–Reference Rodriguez-Oroz, Obeso and Lang163 however, the benefit on PI and gait is not sustained.Reference Castrioto, Lozano, Poon, Lang, Fallis and Moro158 Moreover, a significant number of patients report post-operative worsening of gait despite concurrent improvement in motor scores and global outcomes after bilateral STN-DBS.Reference Karachi, Cormier-Dequaire and Grabli164 Further, falls risk has been demonstrated to increase and levodopa-resistant FOG to persist or worsen.Reference Follett, Weaver and Stern165–Reference Xu, Ma, Huang, Qiu and Sun171 To complicate matters further, stimulation parameters (i.e., high-frequency stimulation) can also lead to adverse axial effects in patients.
The pedunculopontine nucleus (PPN) is considered a key component of the mesencephalic locomotor region (MLR).Reference Tattersall, Stratton and Coyne172 Widespread projections involving the PPN include direct glutamatergic inputs from the motor cortex, and GABAergic inputs from substantia nigra, GPi, STN, and deep nuclei of the cerebellum.Reference Lee, Rinne and Marsden173 Previous work suggests that the PPN is underactive in PD due to degeneration and inhibition, and that this underactivity relates to axial motor impairment.Reference Thevathasan and Moro174 Since the first report of PPN-DBS,Reference Mazzone, Lozano and Stanzione175 multiple studies have shown clinical improvement in patients with PD who have PI and FOG, but results have been variable.Reference Stefani, Lozano and Peppe176–Reference Khan, Mooney and Plaha179 Despite more than 10 years of experience, PPN-DBS remains experimental and the number of implanted patients remains limited since the outcome varies considerably. A heterogeneous dataset has been published so far including case reports, open label series, double-blinded single time point single-center study, and longer-term double-blinded studies. The heterogeneous study designs, differences in outcome measures to assess FOG, falls, and PI, the different targeting strategies and stimulation sites (caudal vs. rostral PPN), the variability in DBS settings (including unilateral PPN vs. bilateral, lone bilateral PPN-DBS, and combined bilateral PPN-DBS with other targets, i.e., STN or caudal zona Incerta) may explain the inconsistencies. In addition, different stimulation parameters contribute to variable results. For instance, low-frequency PPN-DBS can improve axial motor symptoms presumably by partly reversing PPN underactivity.Reference Thevathasan, Silburn and Brooker178,Reference Nosko, Ferraye and Fraix180 More precise targeting strategies with improved technology (i.e., improved imaging and programming) are required. Structural and functional neuroimaging may have a role in patient selection to determine if PPN degeneration is too severe for PPN-DBS to work.Reference Boisgontier, Cheval and Chalavi181 In addition, to improve axial symptoms, that is, FOG, multitarget DBS strategies have been trialed allowing the modulation of cortico-basal ganglia loops, and subsequently aiming to improve falls.Reference Castrioto and Moro182 Since falls and gait impairment are likely related to multiple failing neural circuits, combined PPN and STN stimulation may have greater beneficial effects on gait and PI than either target alone.Reference Stefani, Lozano and Peppe176 Targeting multiple sites can potentially synchronize different circuits and promote neuroplasticity; however, the risk/benefit trade-offs have yet to be determined.Reference Udupa and Chen183 Although there is scarce evidence of PPN-DBS reducing falls and FOG in PD, whether and how to modulate the PPN remains to be determined.
The substantia nigra pars reticulata (SNr) is another key player in the MLR, via its significant efferent GABAergic input to the PPN.Reference Takakusaki, Chiba, Nozu and Okumura184 Axial motor symptomatology, including gait impairment and PI, responds favorably to SNr stimulation in the literature.Reference Weiss, Walach and Meisner127,Reference Brosius, Gonzalez, Shuresh and Walker185–Reference Weiss, Klotz and Govindan187 One of the more recent double-blind, cross-over, randomized controlled trials with combined STN and SNr stimulation showed significant improvement in FOG, but not in other axial symptoms when compared to STN-DBS alone.Reference Weiss, Walach and Meisner127 With SNr-DBS, one should be cautious about the possibility of worsening akinesia, as increased immobility and recurrent falls were reported with combined STN and SNr stimulation.Reference Weiss, Walach and Meisner127
Spinal cord stimulation has recently been investigated in PD.Reference Gilat, Ligia Silva de Lima, Bloem, Shine, Nonnekes and Lewis188 Although presently studied only in small open-label case studies, there has been early evidence to suggest improvement in FOG and PI,Reference Samotus, Parrent and Jog189,Reference Pinto de Souza, Hamani and Oliveira Souza190 although falls were not assessed as an outcome.
Managing Orthostatic Hypotension
The approach to treating OH includes correcting aggravating factors (discontinuing hypertensive medications and correcting anemia) and implementing nonpharmacological measures and pharmacological treatment. Levodopa and dopamine agonists have been variably reported to contribute toward OH (reviewed inReference Sanchez-Ferro, Benito-Leon and Gomez-Esteban191), which should be reviewed with patients when making dose adjustments. Nonpharmacological measures with volume expansion, lifestyle management, activity level, and diet adjustment are first-line recommendations. Physical counter-maneuvers are helpful.Reference Fanciulli and Wenning192
In patients with severe OH, pharmacologic therapies are advisable to minimize the fall risks leading to injuries.Reference Palma and Kaufmann193 Efficacious agents include the synthetic mineralocorticoid fludrocortisone and the pressor agents midodrine or droxidopa. All drugs that raise blood pressure when standing also raise blood pressure when supine, increasing the risk of supine hypertension. Although there are no specific reports on cardio- and cerebro-vascular adverse events induced by supine hypertension in this cohort, one should be cautious about this potential side effect.
Cognitive Strategies
Emerging evidence indicates that deficits in multiple cognitive domains, such as attention, executive function, and working memory, are associated with low gait velocity, stability, and falls.Reference Camicioli and Majumdar60 Medical treatment addressing the motor deficits in PD dementia and DLB can have cognitive side effects (i.e., decreasing verbal short-term memory, attention, reaction time and set-shifting, increasing impulsivity/worsened decision-making, impaired distractor resistance, hallucinations) that increase the propensity to fall.Reference Poletti and Bonuccelli194 Dopamine agonists and anti-cholinergics should be avoided in this population, given adverse effects on alertness and general cognition, and less improvement in overall motor function compared to levodopa. DLB patients are at heightened risk for complications of drug therapy, since they are prone to psychosis triggered by dopaminergic therapies and more susceptible to side effects of antipsychotics used to treat hallucinations. Atypical agents, such as olanzapine or quetiapine, are not risk-free but are preferred when an antipsychotic drug is indicated.Reference Fernandez, Wu and Ott153,Reference Fischer, Bozanovic, Atkins and Rourke195 While quetiapine has less propensity to worsen parkinsonism, it has sedating and anti-cholinergic effects that might increase fall risk.
Nonpharmacologic treatment strategies for DLB are the same as in other dementia syndromes,Reference Carlson, Fleming, Smith and Evans196,Reference Teri, Logsdon and McCurry197 with a focus on ameliorating environmental, medical, psychological, and social factors that may exacerbate problem behaviors leading to falls. Many interventions for fall prevention do not translate successfully from cognitively normal older adults to those with dementia,Reference Montero-Odasso, Verghese, Beauchet and Hausdorff198 likely due to different underlying mechanisms for falls and treatments unable to adequately address cognitive deficits. Studies using nonpharmacological methods, including single motor task, dual-task, and complex motor task training, have shown some benefit reducing fall risk;Reference Verghese, Mahoney, Ambrose, Wang and Holtzer128–Reference You, Shetty, Jones, Shields, Belay and Brown131 however, results vary from study to study. Large-scale studies with more standardized protocols are needed.
Cholinesterase inhibitors can offer clinically meaningful benefits to patients with cognitive issues, particularly in the domains of apathy, confusion, hallucinations, and somnolence,Reference Samuel, Caligiuri and Galasko199 and thus may improve fall risk. A small trial of donepezil suggested a reduction of falls in PD patients without FOG.Reference Chung, Lobb, Nutt and Horak115 Another study showed improvements in gait parameters, but may have been inadequately powered to examine the impact on falls.Reference Henderson, Lord and Brodie116 Memantine was investigated in patients with mild to moderate PD and DLB, with cognitive measures improving in the memantine group, but increased falls was a documented adverse effect.Reference Emre, Tsolaki and Bonuccelli200,Reference Aarsland, Ballard and Walker201
Conversely, anti-cholinergic burden is associated with adverse events, including emergency room visits, fractures, and falls.Reference Crispo, Willis and Thibault117 Consistent with studies in older patients, antidepressants are associated with increased fall risk in PD,Reference Martinez-Ramirez, Giugni and Almeida118 but it is not clear if this is related to comorbid pathology or a medication effect. Methylphenidate was shown to improve FOG in PD presumably through improving attention,Reference Auriel, Hausdorff, Herman, Simon and Giladi119,Reference Pollak, Dobronevsky, Prohorov, Bahunker and Rabey120 although a randomized clinical trial showed negative results.Reference Espay, Dwivedi and Payne121
Exercise/Physiotherapy
There is abundant evidence showing the benefits of physical activity for PD, and exercise can regulate neurotrophic factors and therefore potentially promote neuroprotection in PD.Reference Campos, Rocha, Lattari, Paes, Nardi and Machado202,Reference da Silva, Domingues, de Carvalho, Allodi and Correa203 Strength and balance training can reduce falls among the community-dwelling elderly.Reference Goodwin, Abbott and Whear204–Reference Rimland, Abraha and Dell’Aquila208 Rhythmic visual or auditory clues209–Reference Arias and Cudeiro211 and mental singing during walking may also improve gait in PD.Reference Satoh and Kuzuhara212 Randomized control trials have shown falls reduction with Tai Chi,Reference Li, Harmer and Fitzgerald132 and exercise programs for muscle strengthening and movement strategies.Reference Morris, Menz and McGinley133
Physical therapy can improve fall prevention,Reference Sparrow, DeAngelis, Hendron, Thomas, Saint-Hilaire and Ellis134–Reference Shen and Mak136 but benefit may be limited and short-lasting.Reference Morris, Martin and McGinley137,Reference Frazzitta, Maestri, Uccellini, Bertotti and Abelli138 Dosage, training intensity, and duration may also affect results. A recent randomized trial of home program with strength and movement strategy training and falls education did not prevent falls.Reference Morris, Taylor and Watts213 Dance therapy can be beneficial and may have longer-lasting effects.Reference McNeely, Mai, Duncan and Earhart139,Reference McKay, Ting and Hackney214,Reference Duncan and Earhart215 In particular, tango training improved mobility and other motor domains.Reference McNeely, Mai, Duncan and Earhart139
One should be cautious in applying the results of therapeutic intervention studies to practice. Regular exercise, physiotherapy, and dance are helpful in mobility, but the right dosage and type of exercise should be individualized. Future studies should also focus on therapy content, repetitions, and effective duration, to optimize outcomes and cost-effectiveness for PD patients to further guide treatment. Falls should be included as on outcome in such studies.
Risk Reduction in Osteoporosis
PD patients are older and therefore have a higher risk for metabolic bone disease. This predisposes PD patients to fall-related injuries, including fractures.Reference Gregson, Dennison and Compston216,Reference Johnell and Sernbo217 There are no specific guidelines related to management of osteoporosis and PD. A combination of bisphosphonates and vitamin D is recommended along with regular intake of calcium-containing food or supplements.Reference Metta, Sanchez and Padmakumar218 Medical treatment is mainly based on computed X-ray densitometry (DEXA; T score < −2.5) and FRAX scores (10-year risk for fracture > 3% hip, > 20% of any major osteoporotic fracture).Reference Metta, Sanchez and Padmakumar218 Two main types of pharmacological treatments are available to treat patients with osteoporosis: antiresorptive agents and anabolic agents. Dietary and nutritional manipulation is important, and vitamin D, B12, and folate status needs to be addressed and corrected if required. Lifestyle-related management, including smoking cessation and decreasing alcohol consumption and exercise, should be encouraged.
Extrinsic Risk Reduction
For PD, PDD, DLB, and MSA patients, fall prevention can benefit from occupation therapy input for improvement of home environment. This includes footwear, floor slipperiness, creating safe spaces and familiarization with furniture layouts, lighting, handrails conditions, bedroom/bathroom/kitchen adaptations.Reference Binks and Dobson219 Patient education for behavioral adaptations is also essential to avoid multitasking and to take time with postural change to prevent postural dizziness.
Other Risk Factors
Several studies have identified the need for multimodal interdisciplinary methods for fall prevention and the need for more accurate risk assessment instruments.Reference Chang, Morton and Rubenstein206,Reference Cumming220–Reference Gillespie, Gillespie, Robertson, Lamb, Cumming and Rowe223 Multimodal assessments with targeted intervention reduced fall risk by 37%, and exercise interventions reduced fall risk by 14%.Reference Chang, Morton and Rubenstein206 A meta-analysis identified a benefit on fall prevention in the community when individualized management tailored to address individual risk factors was added to exercise interventions.Reference Hill-Westmoreland and Gruber-Baldini224
Other individual prevention strategies include cataract surgery and cardiac pacing. Cataracts are common in PD and PD-related disorders,Reference Nowacka, Lubinski, Honczarenko, Potemkowski and Safranow225 and cataract disease is associated with a higher risk of developing PD.Reference Lai, Lin, Liao and Chang-Ou226 Cataract surgery for the first eye was found to reduce the fall rate by 34% in general population, but not the number of fallers.Reference Harwood, Foss, Osborn, Gregson, Zaman and Masud227 Moreover, surgery for the second eye did not reduce the fall rate further. Cardiac pacemakers in patients with cardio-inhibitory carotid sinus hypersensitivity reduced fall rate by 58%, but not the number of fallers.Reference Kenny, Richardson, Steen, Bexton, Shaw and Bond228,Reference Parry, Watt-Watson, Hodnett, Tranmer, Dennis and Brooks229 For MSA with significant dysautonomia, cardiopulmonary arrest can occur.Reference Zhang, Cao and Zou230,Reference Papapetropoulos, Tuchman, Laufer, Papatsoris, Papapetropoulos and Mash231 Physicians should carefully review the history and risk factors and assess patients for indications for cardiac pacing.
Novel Experimental Approaches to Falls Prevention
Wearable Sensors
Wearable sensors (accelerometers and gyroscopes) have been used to test mobility. Gait and balance analysis can be extracted through daily activities for a real-time fall risk assessment.Reference Mirelman, Giladi and Hausdorff232–Reference Weiss, Herman, Giladi and Hausdorff234 Such evaluations are potentially more sensitive than conventional tests for recording activity.Reference Fasano, Canning, Hausdorff, Lord and Rochester235,Reference Buchman, Leurgans and Weiss236 Embedded home sensors are another approach to continuously monitor fall risk.Reference Austin, Hayes, Kaye, Mattek and Pavel237,Reference Kaye, Mattek and Dodge238 Experimental motion sensors to feedback and help with rehabilitation have been used. Virtual reality training, combined with complex motor tasks, has also been shown to provide some benefit to falls prevention.Reference Mirelman, Rochester and Maidan239
Non-Invasive Stimulation
Non-invasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS), have been trialed in various neurologic and psychiatric disorders.Reference Wassermann and Lisanby240 One recent meta-analysis demonstrated that rTMS can improve motor symptoms in PD with a moderate effect size.Reference Chou, Hickey, Sundman, Song and Chen241 However, few rTMS studies have focused on FOG and falls in PD.Reference Dagan, Herman, Mirelman, Giladi and Hausdorff242–Reference Kim, Chang and Cho246 One study with high-frequency rTMS over the lower leg primary motor cortex showed significant reduction in subjective FOG and improved gait performance.Reference Kim, Chang and Cho246 However, results are inconsistent across studies with different stimulation targets, frequencies, and parameters having been applied. Further well-designed, large-scale studies are needed.
Transcranial direct current stimulation (tDCS) is another non-invasive stimulation technique. To date, a few studies have shown some benefit of tDCS in FOG. A double-blind, crossover, randomized sham-controlled study showed that applying 20-minute-long anodal 2 mA tDCS sessions on M1 during rest over 5 consecutive days significantly reduced dopamine-resistant FOG in 10 PD patients, and the benefit persisted at 1-month follow-up.Reference Valentino, Cosentino and Brighina247 Simultaneous stimulation over M1 and left dorsolateral prefrontal cortex with tDCS was able to modulate consecutive motor and cognitive function and further improved FOG in 20 PD individuals with FOG when compared to sham or tDCS of either target alone.Reference Dagan, Herman and Harrison248 In practice, given the simplicity of the technology and affordability of tDCS compared to TMS, the former may be a safer, more cost-efficient tool for treatment once stronger evidence is established.
Future Directions
The successful future treatment and research of falls in synucleinopathies will need to consider and integrate cognitive, behavioral, pharmacological, device-based and surgical treatments along with the support of a multidisciplinary team. Further research examining functional neuroanatomy and neurochemistry as well as novel imaging modalities will help with our understanding of the pathophysiology underlying gait disorders and falls, and guide better treatment strategies in the future. Clinical trials of interventions should include falls as an outcome and safety measure.
Disclosures
The authors have no relevant disclosures.
Statement of Authorship
All authors are responsible for writing the manuscript, for discussions and for reviewing the manuscript.
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/cjn.2019.287.





