We present a case of brain abscess caused by a rare organism, group A streptococci (GAS), in an adult male manifesting with acute-onset lateralized neurologic deficits mimicking stroke and a misleading initial brain CT scan suggestive of tumor. To our knowledge, there are only five adult patients with Streptococcus pyogenes brain abscess reported in the literature. In addition to the case presentation, we have also summarized and compared characteristics of all other five cases of brain abscess due to GAS in adults published since 1980, which provides important information on its manifestations and managements.
A 49-year-old male presented with sudden-onset slurred speech, left leg weakness, and severe headache. He presented to hospital within 7 h of onset and was triaged as a possible acute stroke. On initial examination, he was afebrile (36.7°C), alert though not fully oriented, had mild left-sided facial droop, dysarthria, and expressive aphasia. Initial examination of his left leg was limited due to pain, attributed to known left L5 radiculopathy on recent spine MRI for which he was using high potency analgesics. No signs of meningeal irritation were appreciated. He had intermittent headaches for 2 weeks and unintentional 20-lbs weight loss over 4 months. Other than prediabetes and shingles 1 year ago, he did not have other past medical history.
Head CT scan with and without contrast and CT perfusion at the time of admission (Figure 1(A–D)) showed a nonenhancing expansile subcortical hypodense lesion and low cerebral blood volume in the right frontal lobe suggestive of either a low-grade glioma or demyelination. Abscess was felt unlikely by the radiologist due to lack of ring enhancement. Sinuses and parameningeal structures were normal. He had mild leukocytosis (WBC 12.1), otherwise initial laboratories were unremarkable. Blood cultures were drawn. MRI brain, neurosurgical consultation, and lumbar puncture were planned. Six hours postadmission his level of consciousness (LOC) progressively declined, and he developed high fever (39.0°C), hypertension, tachycardia, and tachypnea. He was empirically started on intravenous vancomycin, ceftriaxone, ampicillin, and acyclovir. His LOC further deteriorated in the next hours such that he required intubation and transfer to ICU. MRI brain (Figure 1(E–F)) following clinical deterioration demonstrated diffuse leptomeningeal enhancement and right frontal opercular intra-axial mass suggestive of an atypical abscess. His antimicrobial regimen was then switched to vancomycin, ceftriaxone, and metronidazole. Lumbar puncture showed purulent cerebrospinal fluid (CSF) with 19,700 × 106/L WBC (90% neutrophils), high protein, and low glucose (12% of serum glucose) (Table 1). Subsequent blood and CSF cultures yielded S. pyogenes (group A streptococcus (GAS)). Further physical exam and investigations for potential source of GAS infection (e.g., transesophageal echocardiography for endocarditis) were unremarkable.
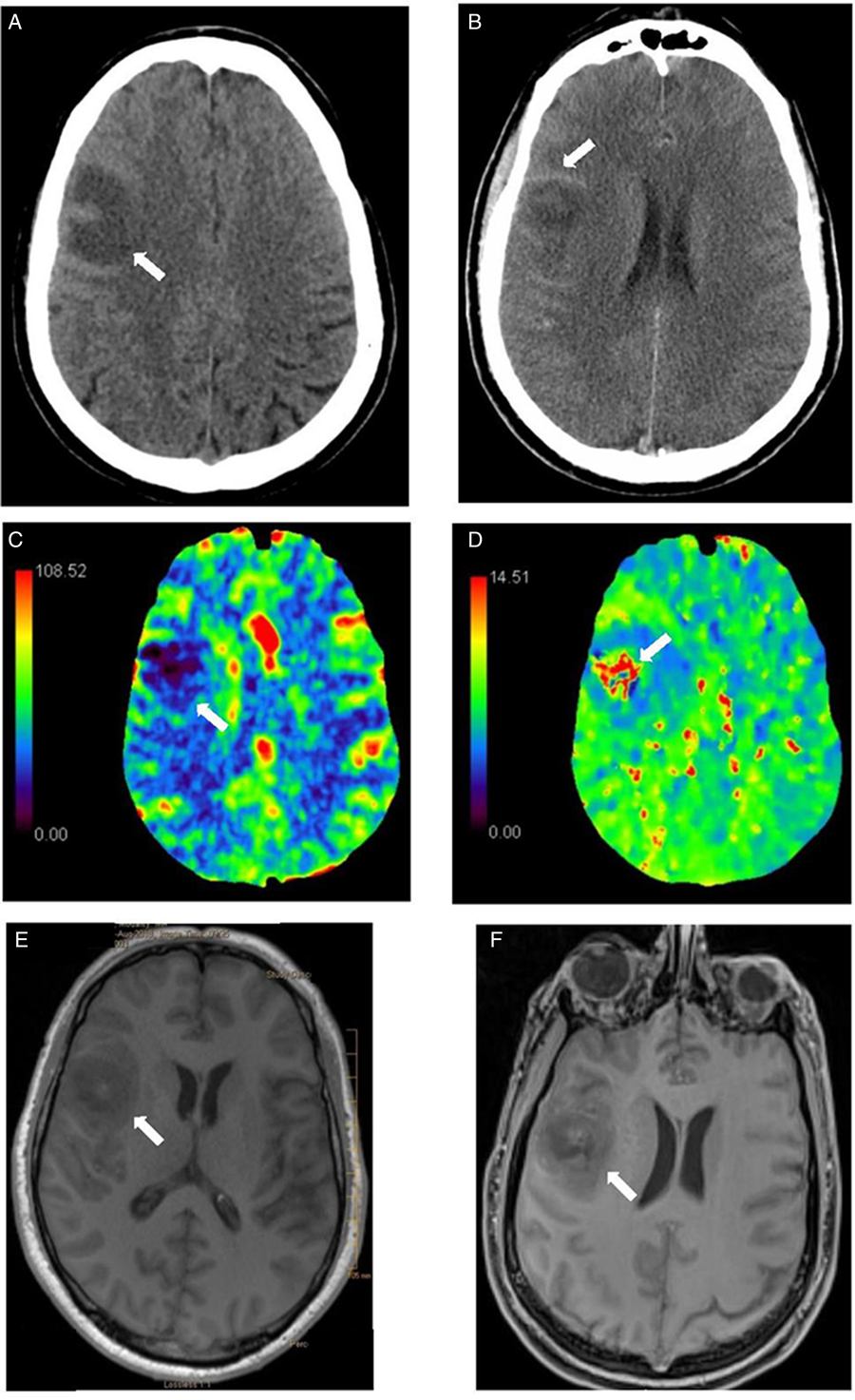
Figure 1: Head CT scan without (A) and with contrast (B): right frontal subcortical expansile hypodensity with subtle thin ring enhancement. (C-D) Head CT perfusion: decreased cerebral blood flow (CBF) (C) and cerebral blood volume (CBV) in the right frontal lesion with corresponding prolonged time-to-peak (TTP) (D). Differential considerations included low-grade glioma and demyelinating lesion given low cerebral blood volume. Note that abscess can have low CBV and therefore, can be difficult to be distinguished from glioma. Brain MRI without (E) and with Gadolinium enhancement-T1 cyberKnife (F) (performed 15 hours later): right frontal opercular intra-axial mass with surrounding vasogenic edema, peripheral rim of petechial hemorrhage and diffuse leptomeningeal enhancement. There was no restricted diffusion in the center of the lesion. The combination of findings was felt to be due to diffuse meningitis with cerebritis and evolving phlegmon/early abscess changes in the context of CNS bacterial infection. Given diffuse leptomeningeal enhancement post-Gd, neoplasm such as primary glioma was less likely. The appearance was not compatible with demyelinating disease.
Table 1: Case reports of GAS brain abscesses in adults during 1966–2018 (age > 18 years)
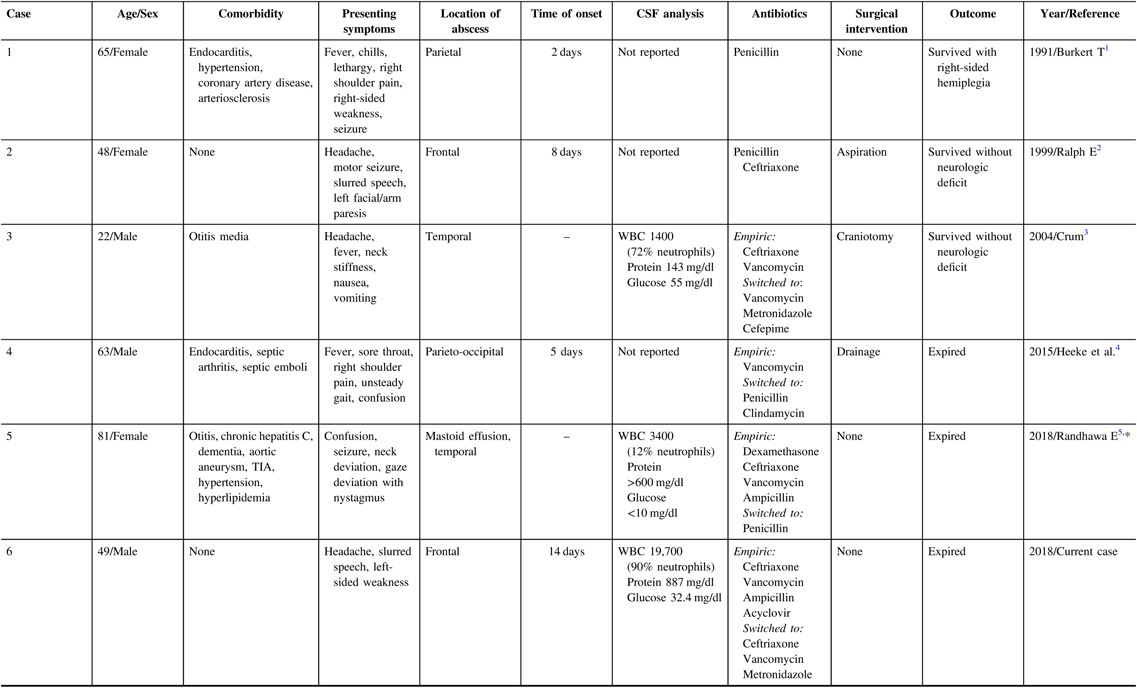
CSF=cerebrospinal fluid; GAS=group A streptococci; TIA=transient ischemic attack; WBC=white blood cells.
* Not indexed in MEDLINE.
His clinical status further deteriorated, and repeat CT scan showed diffuse cerebral edema and transtentorial herniation with midbrain/pontine descent. On the fourth day of admission, his brainstem reflexes were absent and brain death was pronounced.
Alpha-hemolytic streptococci are commonly found in brain abscesses; however, isolation of GAS is rareReference Ralph and Shoemaker2 with only five adult cases published in the literature. Recent evidence suggests that the incidence of invasive GAS, including brain abscess, is increasing particularly among children and adolescents.Reference Capua, Klivitsky and Bilavsky6 In brain abscesses, the routes of infection are unknown in 15% of cases.Reference Brouwer, Coutinho and van de Beek7 While GAS is often associated with ear–nose–throat infections, only rarely presents with secondary intracranial infections. In such cases, meningitis or subdural empyema are usually the most common complications.Reference Lucas, Brouwer, Bovenkerk, Man, van der Ende and van de Beek8 Among the previously reported GAS brain abscesses in adulthood (Table 1), possible sources include septic emboli from GAS endocarditis in two casesReference Burkert and Watanakunakorn1,Reference Heeke, Blumberg and Perry4 and otogenic spread in another two.Reference Crum3,Reference Randhawa, Sibliss and Woytanowski5 In other patientsReference Ralph and Shoemaker2 (including our present case), no obvious infectious source was identified.
Clinical features, location of the lesion, and comorbid conditions varied among the five adult cases of GAS brain abscess. In addition to meningeal symptoms, slurred speech, hemiparesis, and hemiplegia were reported in three cases. Most patients were immunologically intact with two patients healthy at baseline.
Empiric treatment of undifferentiated pyogenic brain abscesses usually includes a third-generation cephalosporin and metronidazole. Vancomycin can be added to cover abscesses induced by hematogenous spread, cranial trauma, postneurosurgical procedure, suspected methicillin-resistant staphylococcus aureus, and unknown source. Intravenous penicillin or ceftriaxone in combination with clindamycin has been suggested for tailored treatment of invasive GAS infection. Duration of antimicrobials should be at least 4–8 weeks to ensure eradication of any residual infection.Reference Bodilsen, Brouwer, Nielsen and Van De Beek9 Surgical procedures including aspiration, drainage or abscess resection and open craniotomy are typically performed in abscesses >2 cm. The best outcome, survival without neurological deficit, occurred in cases with shorter onset and early diagnosis in whom appropriate medical and neurosurgical interventions were performed. Yet, one patientReference Heeke, Blumberg and Perry4 who underwent surgical drainage also died.
Despite being a common pathogen, a brain abscess from S. pyogenes is unusual and may show a fulminant course with rapid progression creating a life-threatening clinical condition. Patients may present with sudden onset of aphasia, hemiplegia, and hemiparesis, which may mimic acute stroke. In our case, initial history, physical exam, laboratory investigations, and imaging were attributed to brain tumor, which mislead his initial care. With several weeks’ history of subacute headache, fatigue, and weight loss followed by sudden neurological decline, a growing cryptogenic GAS brain abscess that secondarily ruptured was the most likely diagnosis. Given invasiveness of GAS infection, a timely diagnosis to provide early treatment (i.e., medical and neurosurgical interventions) is critical for survival. Based on the limited number of cases in the literature (Table 1), clinical presentation of GAS brain abscess does not differ remarkably from other causes of brain abscess. Clinicians should consider a late-presenting infectious etiology (e.g., brain abscess), in the differential diagnosis of acute stroke or brain tumor in the acute phase when further imaging or laboratory assessment is time-consuming, not yet available or showing equivocal findings.
Acknowledgements
The authors would like to thank the patient’s family for their permission, as well as all physicians, residents, and nurses who were involved in his care.
Conflict of Interest
The authors have no conflict of interest to disclose.
Statement of Authorship
All authors (S-MF, MM, JIB, and VP) were involved in the clinical assessment of the case. Data collection was done by S-MF and MM. S-MF and JIB performed literature review and S-MF summarized similar cases in a summary table. S-MF, MM, and VP drafted the manuscript for intellectual content, and all authors (S-MF, MM, JIB, and VP) revised the final version of the manuscript.




