Cerebral microbleeds (CMBs) are small regions of intraparenchymal bleeding that presumably result from microvascular fragility and/or increase permeability. CMBs are most often encountered in age-related cerebral small vessel diseases (CSVDs); however, they are increasingly reported in varied cerebral vasculopathies. Reference Charidimou, Krishnan, Werring and Rolf Jager1 CMBs can occur rapidly in acute stroke, Reference Shoamanesh, Yan and Charidimou2 and imaging-pathology correlation studies have demonstrated intact erythrocytes, confirming recent formation in a subset. Reference Shoamanesh, Kwok and Benavente3 We report a case of rapid CMB formation in reversible cerebral vasoconstriction syndrome (RCVS).
A 45-year-old woman presented with 4 days of progressive headache with associated mild confusion, nausea, and photophobia. She suffered a brief generalized seizure upon presentation to the emergency department. Her symptoms were preceded by a thunderclap headache 2 weeks prior to presentation. Review of systems was normal. She was afebrile, blood pressure was 115/58 mmHg, and neurological examination was non-focal, but demonstrated mild drowsiness and disorientation to time.
Her past medical history included remote history of alcohol withdrawal seizures, migraine headaches, and depression. Medications at presentation included dilantin, lamotrigine, and lorazepam as required. She was a habitual marijuana smoker and on a selective serotonin reuptake inhibitor (SSRI; citalopram 20 mg daily). She denied any recent head trauma, and antithrombotic or nasal decongestant use. Her toxicology was negative apart for cannabis.
Head CT showed a right parafalcine posterior parietal intracerebral hemorrhage with overlying convexal subarachnoid hemorrhage (SAH) (Figure 1). CT angiogram (CTA) demonstrated diffuse cerebral vasospasm (Figure 1). Dural venous sinuses opacified normally. An initial MRI 4 days into her admission did not show any CMBs on susceptibility-weighted imaging (Figure 2), and there was no evidence of vasogenic edema beyond the perihematomal region or white matter hyperintensities on fluid attenuated inversion recovery (FLAIR) (Figure 1). Her cerebral spinal fluid, echocardiogram, and markers of systemic vasculitis/inflammation were unremarkable.
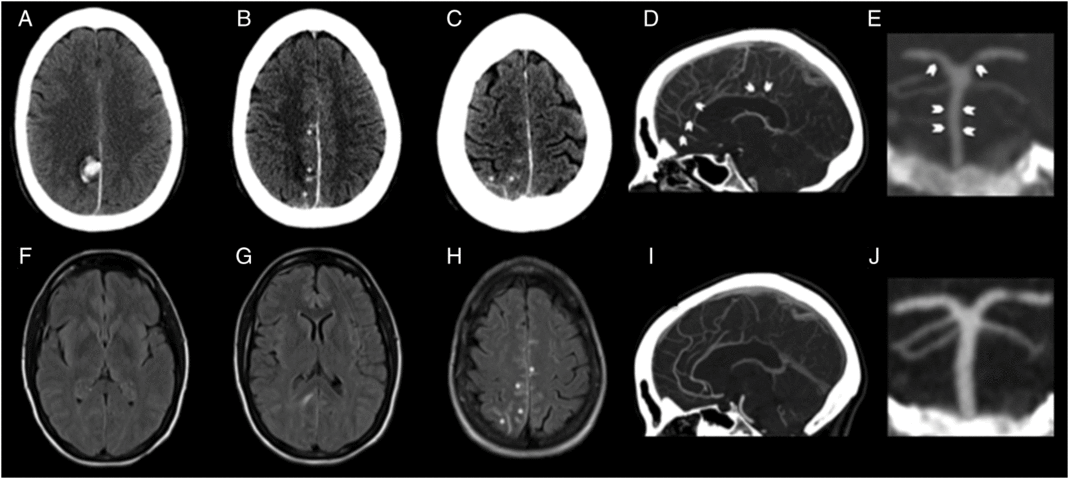
Figure 1: CT and fluid attenuation inversion recovery images. Baseline CT images (A–C) showing right parafalcine parietal intraparenchymal hemorrhage (A) with overlying convexal SAH (asterisk; B, C). Baseline MRI FLAIR images (F–H) showing absence of microangiopathy in form of white matter disease, absence of vasogenic edema beyond the perihematomal region (D, E) and presence of convexal SAH as sulcal T2-hyperintensity (asterisk; F). CTA at baseline showing baseline smooth/segmental narrowing of the distal anterior cerebral artery (arrows; D) and smooth narrowing of the basilar artery and segmental narrowing of the posterior cerebral artery (arrows; E). Repeat CTA at 9 months shows complete resolution of these changes confirming reversible vasoconstriction (I, J).
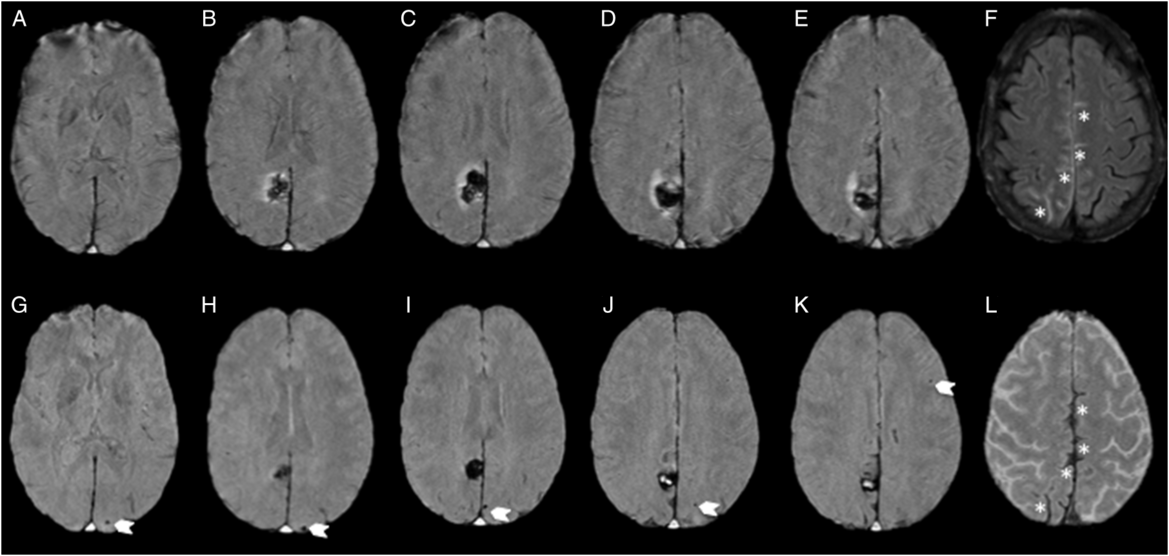
Figure 2: MRI at baseline and 1 month follow-up. Susceptibility-weighted images showing absence of CMBs at baseline (A–E) and incident (six total) posterior-predominant strictly lobar CMBs (arrows; G–K; one occipital CMB not shown) and evolving cortical superficial siderosis (asterisk; L), in regions of convexal SAH at initial presentation (asterisk; F).
Her SSRI was discontinued and she was initiated on a calcium channel blocker (verapamil). She made a complete recovery and had not experienced further seizures at discharge 10 days following presentation. She was normotensive throughout her hospitalization and outpatient follow-up.
A repeat MRI at 29 days showed evolving cortical superficial siderosis in previous regions of convexal SAH and multiple incident lobar (occipital predominant) CMBs in the contralateral hemisphere, without any evidence of adjacent mass effect (Figure 2). A repeat CTA at 9 months showed complete resolution of the angiographic findings confirming the diagnosis of RCVS (Figure 1). She has abstained from SSRI treatment and marijuana since her admission. No recurrence has occurred during 4 years of follow-up to date.
RCVS is a vasculopathy characterized by recurrent thunderclap headaches with reversible vasoconstriction of the intracranial arteries. In addition, patients can often develop ischemic strokes and intracerebral hemorrhage in the first weeks following the initial headache. Serotonergic medications and cannabis use are among the exogenous substances known to provoke RCVS. Reference Ducros4
Our patient presented with lobar hemorrhage, overlying convexal SAH and rapid formation of incident posterior-predominant strictly lobar CMBs from RCVS. This particular profile of concomitant intracranial hemorrhagic lesions is often attributed to cerebral amyloid angiopathy. Our case highlights the need to consider a broad differential diagnosis, including RCVS, in stroke patients presenting with CMBs, particularly in younger age.
Isolated convexal SAH has been well described in RCVS. Reference Kumar, Goddeau and Selim5 To our knowledge, this is the first report of rapid CMB formation in this condition. CMBs have been reported to develop rapidly in stroke patients. Reference Shoamanesh, Yan and Charidimou2 The strongest predictor for new CMBs is baseline CMBs and/or other MRI markers of underlying CSVD. As our patient did not have baseline pre-existing CSVD features on MRI, we believe the CMBs resulted from blood–brain barrier breakdown and increased vascular permeability from RCVS. Reference Lee, Cha and Choi6 Posterior reversible encephalopathy syndrome has been reported to result in rapid CMB formation Reference Charidimou, Krishnan, Werring and Rolf Jager1 and can coincide with RCVS. However, our patient was non-hypertensive and did not have any vasogenic edema beyond the perihematomal region. Acute CMBs have also been reported from increased vascular permeability during refractory status epilepticus, Reference Jeon, Parikh and Choi7 but in our case the seizure is unlikely to have contributed to the CMBs seen on repeat imaging, as it was brief, did not recur, and baseline MRI performed 4 days following the event was devoid of CMBs.
To conclude, CMBs may be a third intracranial hemorrhage manifestation of RCVS and clinicians should maintain a broad differential when encountering CMBs in stroke patients. Our report further highlights the propensity to form CMBs rapidly in the absence of apparent CSVD, with broader implications pertaining to our understanding of the degree of heterogeneity in CMB pathogenesis. Further studies are required to confirm our preliminary observation.
Funding
Dr. AS is supported by the Marta and Owen Boris Chair in Stroke Research and Care.
Disclosures
The authors report no relevant disclosures.




