Introduction
Epilepsy affects individuals of all ages, but its highest prevalence is in patients >75 years of age.Reference Collins, Shapiro and Ramsay 1 Nevertheless, no aetiology is identified in up to 25-50% of these cases.Reference Collins, Shapiro and Ramsay 1
There is a well-defined association between epilepsy and neurodegenerative diseases, particularly Alzheimer’s disease (AD). Compared to non-demented controls, patients with clinically diagnosed sporadic AD appear to have a six- to tenfold higher seizure or epilepsy risk.Reference Friedman, Honig and Scarmeas 2 While AD is notoriously underdiagnosed, medial temporal lobe atrophy (MTA) has been established as a biomarker on neuroimaging and in elderly subjects can provide indirect evidence of such neurodegenerative processes as AD.Reference Dubois, Feldman, Jacova, Dekosky, Barberger-Gateau and Delacourte 3
MTA is readily assessable on routine neuroimaging.Reference Frisoni, Geroldi, Beltramello, Bianchetti, Binetti and Bordiga 4 The presence of significant MTA in older people most frequently represents degenerative changes, which could constitute an underrecognized aetiology of epilepsy in this group. Consequently, we aimed to evaluate the degree of MTA in older patients with new-onset epilepsy.
Methods
Patients
Magnetic resonance images (MRIs) or computed tomographic (CT) scans were reviewed from consecutive patients ≥65 years of age with new-onset epilepsy of unknown aetiology. The participants were part of a previous retrospective study aiming to review treatment adequacy in older patients with new-onset epilepsy. Patients were all evaluated at the Centre Hospitalier Universitaire de Sherbrooke (CHUS) in Quebec between January of 2001 and October of 2010, and all were started on antiepileptic drug (AED) treatment for new-onset epilepsy. Epilepsy was defined clinically as two or more seizures or one seizure with a high risk for recurrence leading to initiation of AED treatment. All patients underwent MRIs or CT scans within a year of the diagnosis of epilepsy. The exclusion criteria included epilepsy diagnosis at <65 years of age, acute symptomatic seizures, AED treatment before diagnosis, or insufficient information regarding diagnosis and treatment. Classification as new-onset epilepsy of unknown aetiology was made after routine clinical evaluation by the clinician, including brain imaging and EEG (for most patients).
A total of 31 normal healthy older controls without epilepsy or dementia aged between 65 and 85 years as well as 10 neurodegenerative disease controls with clinically diagnosed mild AD were included for comparison. Both control groups were part of another study from the same centre. Older controls were recruited from the general population according to the following exclusion criteria: Mini-Mental State Examination (MMSE) <26 of 30, active smoking, diabetes or glucose intolerance, cardiac, hepatic or renal disease, and untreated hypertension, dyslipidemia or thyroid disease. AD patients were recruited from the CIUSSS–CHUS memory clinic. The study was approved by the local research ethics committee.
Temporal Lobe Atrophy Assessment
Evaluation of MTA was made using two different scales, with evaluators blinded to patient group. First, it was visually graded on a 0-3 scale (0=no atrophy, 1=mild atrophy, 2=moderate atrophy, 3=severe atrophy) looking at sulcal and ventricular dilatation on axial images. This scale was shown to be reproducible with an inter-observer kappa of 0.67-0.76 on MRI imaging.Reference Pasquier, Leys, Weerts, Mounier-Vehier, Barkhof and Scheltens 5 Second, temporal horn radial width (THRW) was measured as a quantitative estimate of TLA. We employed a validated method that has excellent inter-observer agreement and is very sensitive (93-97%) and specific (80-95%) for AD using a cutoff of 5.3 mm for THRW.Reference Frisoni, Geroldi, Beltramello, Bianchetti, Binetti and Bordiga 4 However, we adapted this parameter to the available images, which were acquired with about 20 and 12° differences from the original method, respectively, for CT and MRI. Slices were also thicker (3-4 mm) than the 2 mm by Frisoni et al.Reference Frisoni, Geroldi, Beltramello, Bianchetti, Binetti and Bordiga 4
Comparison of MTA was made to healthy older controls, non-epileptic AD individuals, and patients from the same cohort with epilepsy attributed, as judged by clinicians, to neurodegenerative disease causing cognitive impairment.
MRIs and CT scans were analyzed using the two visual rating scales. Both temporal lobes were rated, and the side with the most severe MTA on CT or MRI was utilized for analysis. There was no significant difference between CT and MRI measurements in patients in whom both were performed (κ=0.73 and 0.75 for visual assessment of right and left temporal lobes, respectively; intra-class coefficients equal to 0.88 and 0.89 for THRW of the right and left temporal lobes).
Statistical Analyses
Visual scale results were compared between groups using Fisher’s exact test. The Mann–Whitney U test was employed for THRW mean comparison. Both analyses were adjusted for age and gender with ordinal and multiple linear regression, respectively. The chi-squared test (or Fisher’s exact test for comparison with the AD group) was calculated for dichotomized visual scale atrophy and dichotomized THRW. Again, adjustment for age and gender was made using logistic regression. Regarding dichotomization, scores of 0-1 and a THRW ≤5.3 mm were considered non-significant atrophy. Significant atrophy was defined as 2-3 on the visual scale and a THRW >5.3 mm. Cohen’s κ coefficient between the two scales was also calculated.
Results
Study Participants’ Characteristics
From the original cohort, 112 patients with epilepsy of unknown aetiology and 26 patients with epilepsy plus neurodegenerative disease satisfied our inclusion criteria (Table 1). Among those with cognitive dysfunction, 25 of 26 had clinical dementia, 15 of 25 had AD, and 1 had mild cognitive impairment. MRIs were available for 57 of 138 (41%) of participants from the original cohort and for all healthy older and AD controls.
Table 1 Baseline Characteristics of the Study Participants

AD=Alzheimer’s disease; MMSE=Mini-Mental State Examination; N/A=not available; incl. sleep rec.=including sleep recording.
Temporal Lobe Atrophy
Measurements were made by both a neurologist specialized in cognitive neurology and a neurology resident. The second rater’s evaluations were used for analyses, after excellent inter-rater agreement (intra-class coefficient ≥0.882 on 20 random ratings). A Cohen’s κ coefficient of 0.779 (obtained using dichotomized results) indicated substantial concordance between both scales (p<0.001).
On the basis of non-dichotomized data, patients with epilepsy of unknown cause had significantly higher THRW and visual scale scores than healthy older controls. They also had significantly lower THRW and visual scale scores than patients with both epilepsy and cognitive dysfunction before and after adjustment for age and gender (Tables 2–3).
Table 2 Visual Scale for Temporal Lobe Atrophy in Older People with Epilepsy and Controls
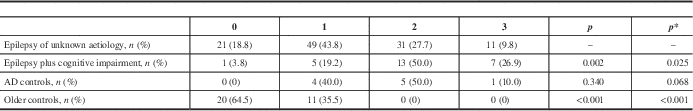
The p values reflect comparisons made between patients with epilepsy of unknown aetiology and each other individual group.
* p adjusted for age and gender; AD=Alzheimer’s disease.
Table 3 THRW in Older People with Epilepsy and Controls

The p values reflect comparisons made between patients with epilepsy of unknown aetiology and each individual other group.
THRW=temporal horn radial width; AD=Alzheimer’s disease.
**p adjusted for age and gender.
Analysis of dichotomized data showed that 37.5% (visual scale) and 38.1% (THRW) of patients with epilepsy of unknown aetiology presented significant temporal lobe atrophy on brain imaging (Figures 1–2). This was statistically more than in healthy older controls but less than in patients with epilepsy associated with cognitive disorders, both before and after adjustment by logistic regression.

Figure 1 Visual scale, dichotomized results, for temporal lobe atrophy in older people with epilepsy and older controls.

Figure 2 Temporal horn radial width, dichotomized results, in older people with epilepsy versus older controls.
In comparison to individuals with established mild AD, analysis only revealed a statistically significant difference for THRW measurements before adjustment of non-dichotomized data (p=0.032) and after adjustment of dichotomized results (p=0.040) (Table 3, Figure 2).
Discussion
This single-centre series of consecutive older patients with new-onset epilepsy of “undetermined aetiology” suggests that almost 40% of such patients show significant MTA. These patients had more atrophy than healthy older controls, but less than patients with AD. We also found significantly greater atrophy for THRW dichotomized data when comparing our group of interest to patients with cognitive impairment and epilepsy.
Focal epileptiform discharges, when found on EEG, were mostly temporal for patients with epilepsy and cognitive impairment as well as patients with epilepsy of unknown aetiology (Table 1). This reinforces the idea of a link between MTA and epilepsy.
Whether this association between MTA and epilepsy of unknown aetiology reflects a condition underlying epilepsy or is a consequence of this epilepsy cannot be established by our study. However, MTA is a recognized biomarker for AD, which is an important risk factor for epilepsy in older patients. The possibility of under-diagnosed early AD being the common factor between MTA and epilepsy thus deserves further investigation. Other epilepsy causes associated with MTA also have to be considered, mainly idiopathic or secondary hippocampal sclerosis (HS). However, these aetiologies were not observed in our cohort of elderly with new-onset epilepsy. Furthermore, idiopathic HS usually manifests in childhood or early adulthood, and only a few case reports of secondary HS have been reported in older patients.Reference Morillo 6 Nonetheless, HS cases might have been missed because MRI was obtained only in 41% of our epilepsy patients.
In our study, patients with epilepsy of unknown cause had in fact an intermediate degree of MTA between that of normal older persons and AD patients. These patients could be in a subclinical, early phase of such a neurodegenerative disease. Longitudinal follow-up and measurement of other biomarkers should be obtained to confirm this hypothesis, but epidemiological and neuropathological arguments support the probability that MTA in this age group is most often caused by AD. Epilepsy is mostly associated with late-stage AD, but it has recently been reported to occur in amnestic mild cognitive impairment and to predate AD diagnosis.Reference Vossel, Beagle, Rabinovici, Shu, Lee and Naasan 7 However, to our knowledge, this is the first study focusing specifically on imaging temporal lobe characteristics of older patients with otherwise unexplained epilepsy.
Along with its retrospective design, the limitations of this study include the heterogeneity of imaging modalities and the disparity in comorbidities between groups. Vascular risk factors are known to contribute to temporal and global cerebral atrophy but were not as prevalent in the elderly controls in our study.Reference Meyer, Rauch, Crawford, Rauch, Konno and Akiyama 8 , Reference Beauchet, Celle, Roche, Bartha, Montero-Odasso and Allali 9 The comparison of MTA with AD patients is limited by the small sample size of the AD group. Complete cognitive assessment was also lacking in this dataset. Basic cognitive testing with the MMSE was only available for a small subset of our cohort, and the timing of this evaluation was unspecified in most patients.
Disclosures
Emmanuelle Lapointe, Christian Bocti, Charles Deacon, Louis Royer-Perron and Christian-Alexandre Castellano do not have anything to disclose.
Stephen Cunnane has the following disclosures: Centre de Recherche sur le Vieillissement, researcher, research support; Canada Foundation of Innovation: researcher, research support; Canadian Institutes of Health Research: researcher, research support; Fond de Rechereche Santé Québec: researcher, research support; Université de Sherbrooke: researcher, research support; Keto Products: ad hoc advisor, consulting fee.
Acknowledgments
Dr. Cunnane would like to thank the Centre de Recherche sur le Vieillissement, the Canada Foundation for Innovation, the Canadian Institutes of Health Research, the Fond de Recherche Santé Québec, and the Université de Sherbrooke for financial support. He also received a consulting fee from Keto Products.







