Introduction
Dementia affects more than 50 million people around the world (Alzheimer Disease International, 2020), and it is characterized by a progressive decline in cognition (American Psychiatric Association, 2013; World Health Organization, 2012), particularly affecting people 65 years and older. The symptomatology associated with dementia and its progression can vary, depending on the severity of the disease and from person to person. Dementia can affect almost all activities of daily living and the ability to manage personal finances (Hsu & Willis, Reference Hsu and Willis2013), transportation (Carr & Ott, Reference Carr and Ott2010), and socializing over time (Honda, Meguro, Meguro, & Akanuma, Reference Honda, Meguro, Meguro and Akanuma2013). An aging population with an increasing prevalence of dementia has created the need for primary care clinicians to take on a more essential and active role in dementia care in collaboration with specialists and community agencies (Hogan et al., Reference Hogan, Borrie, Basran, Chung, Jarrett and Morais2012).
In 2019, the Canadian Consensus Conference on the Diagnosis and Treatment of Dementia (CCCDTD) met for the fifth time (Ismail et al., Reference Ismail, Black, Camicioli, Chertkow, Herrmann and Laforce2020). Since 1989, this conference has provided updated recommendations to guide Canadian health care professionals, including primary care practitioners, in the management of persons living with dementia (PLWD) and their caregivers. The Canadian health care system infrastructure places primary care as the main entry for health care services. Thus, primary care providers oversee the detection, diagnosis, and treatment of dementia. Furthermore, the previous CCCDTD statements and the 2019 Canadian Academy of Health Sciences recommended that the diagnosis and management of patients with dementia should mainly be the responsibility of primary health care (Canadian Academy of Health Science, 2019; CCCDTD3, 2007).
In Canada, according to the 2015 Commonwealth Fund Survey of Family Physicians, more than 86 per cent of family physicians “often” or “sometimes” provided medical care for PLWD (Canadian Institute for Health Information, 2022; The Common Wealth Fund, 2015). We know that primary care providers are motivated to provide care for PLWD (Arsenault-Lapierre et al., Reference Arsenault-Lapierre, Henein, Rojas-Rozo, Bergman, Couturier and Vedel2021). However, it is well known that some family physicians and primary care providers have difficulties getting involved with the dementia population due to multiple factors, such as time constraints (Arsenault-Lapierre et al., Reference Arsenault-Lapierre, Henein, Rojas-Rozo, Bergman, Couturier and Vedel2021) and stigma (Bacsu, Mateen, Johnson, Viger, & Hackett, Reference Bacsu, Mateen, Johnson, Viger and Hackett2020), and requiring more support and training (Arsenault-Lapierre et al., Reference Arsenault-Lapierre, Henein, Rojas-Rozo, Bergman, Couturier and Vedel2021). As a result, family physicians, despite the availability of guidelines for the treatment of dementia, continue to feel unequipped for providing adequate care for PLWD (Bacsu et al., Reference Bacsu, Mateen, Johnson, Viger and Hackett2020). Physicians often indicate that guidelines are typically lengthy and complex to read (Gupta et al., Reference Gupta, Rai, Bhattacharrya, Cheng, Connelly and Boulet2016). The issues mentioned above not only affect family physicians and primary care providers in their practices, but also PLWD and their care partners still perceive that their needs are unmet when receiving care in primary care settings. It is thus essential to present the latest recommendations from the fifth CCCDTD (CCCTDT5) in a format that is relevant and usable to Canadian primary care practitioners (Black et al., Reference Black, Johnston, Rabins, Morrison, Lyketsos and Samus2013; Khanassov, Rojas-Rozo, Sourial, Yang, & Vedel, Reference Khanassov, Rojas-Rozo, Sourial, Yang and Vedel2021).
To continue to provide relevant and up-to-date information for all professionals involved in the care of PLWD, the CCCDTD5 made recommendations regarding diagnosis and management, including dementia case finding and detection, use of neuroimaging in dementia diagnoses, risk reduction and prevention of dementia, and medication use. Furthermore, for the first time during these conferences, a working group was formed to specifically address psychosocial and non-pharmacological interventions for PLWD and their caregivers (Ismail et al., Reference Ismail, Black, Camicioli, Chertkow, Herrmann and Laforce2020). However, these guidelines are extensive, with more than 30 pages of recommendations for the treatment and management of dementia. As a result, in the present article, we selected and tailored recommendations that are relevant for the practice of family practitioners and interdisciplinary primary care teams (that could include several health care professionals such as social workers, nurse practitioners, and psychologists).
Methodology
As explained in detail elsewhere (Ismail et al., Reference Ismail, Black, Camicioli, Chertkow, Herrmann and Laforce2020), the methodology used during the CCCDTD5 was based on the Appraisal of Guidelines for Research and Evaluation (AGREE) II collaboration (Brouwers et al., Reference Brouwers, Kho, Browman, Burgers, Cluzeau and Feder2010). Recommendations were rated using the Grade of Recommendations, Assessment, Development, and Evaluations (GRADE) framework. A recommendation was accepted if it was approved by 80 per cent or more of the participants from the panel of experts (Ismail et al., Reference Ismail, Black, Camicioli, Chertkow, Herrmann and Laforce2020).
The CCCDTD5’s lead members recommended knowledge translation efforts for tailoring the recommendations for primary care. With the aid of family physicians who were part of the conference and/or of the Canadian Consortium on Neurodegeneration in Aging (CCNA), the most relevant recommendations for primary care and family physicians were selected. With the aim of making these recommendations more practical and easier to consult, we tailored and summarized the recommendations most relevant for family practitioners and interdisciplinary primary care teams (see Figure 1 for a summary and Table 1 for the detailed recommendations with their respective GRADE classifications as well as the approval percentage from the panel of experts). As part of our knowledge translation strategy, we also created tables accompanying the different recommendations, as well as visual representations to help better understand where in the dementia journey they may be applied.

Figure 1. CCCDTD5 recommendations most relevant for primary care clinicians.
Table 1. The most relevant CCCDTD 5 recommendations for primary care clinicians with GRADE classification and approval percentage by the panel of experts
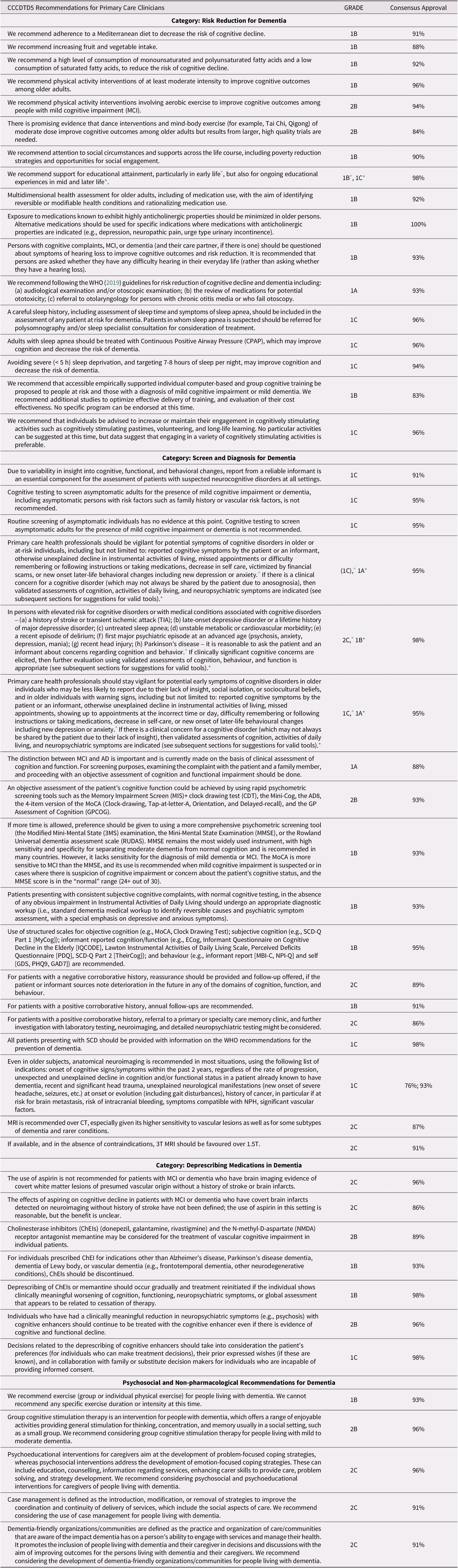
Recommendations of the 5th Canadian Consensus Conferences on the Diagnosis and Treatment of Dementia (adapted from Ismail Z, et al.); Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimer’s & Dementia. 2020;16(8):1182–1189.
* MMSE remains the most widely used instrument, with high sensitivity and specificity for separating moderate dementia from normal cognition and is recommended in many countries.
+ The MoCA is more sensitive to MCI than the MMSE, and its use is recommended when mild cognitive impairment is suspected or in cases where there is suspicion of cognitive impairment or concern about the patient’s cognitive status, and the MMSE score is in the “normal” range (24+ out of 30).
Recommendations more relevant to other specialists and research endeavours were not selected for this article as they did not prove as useful for primary care, for instance, specific imaging guidelines and definitions more suited to specialists. The complete list of recommendations is published elsewhere, with specific information on the evidence for each recommendation as well as the consensus percentage approval (Gauthier et al., Reference Gauthier, Chertkow, Theriault, Chayer, Ménard and Lacombe2020; Ismail et al., Reference Ismail, Black, Camicioli, Chertkow, Herrmann and Laforce2020; Vedel et al., Reference Vedel, Sheets, McAiney, Clare, Brodaty and Mann2020).
In the text below, we summarize recommendation key points and offer practical guidance on how to implement them. After the recommendations, we provide a list of public resources to support primary care providers in their practices and to support their patients, as well as two infographics depicting the dementia journey for both health and social care providers (Figure 2) and PLWD (Figure 3), developed in collaboration with the Alzheimer Society of Canada.

Figure 2. Dementia journey transition points: family doctor (adapted from figure developed in collaboration with the Alzheimer Society of Canada).
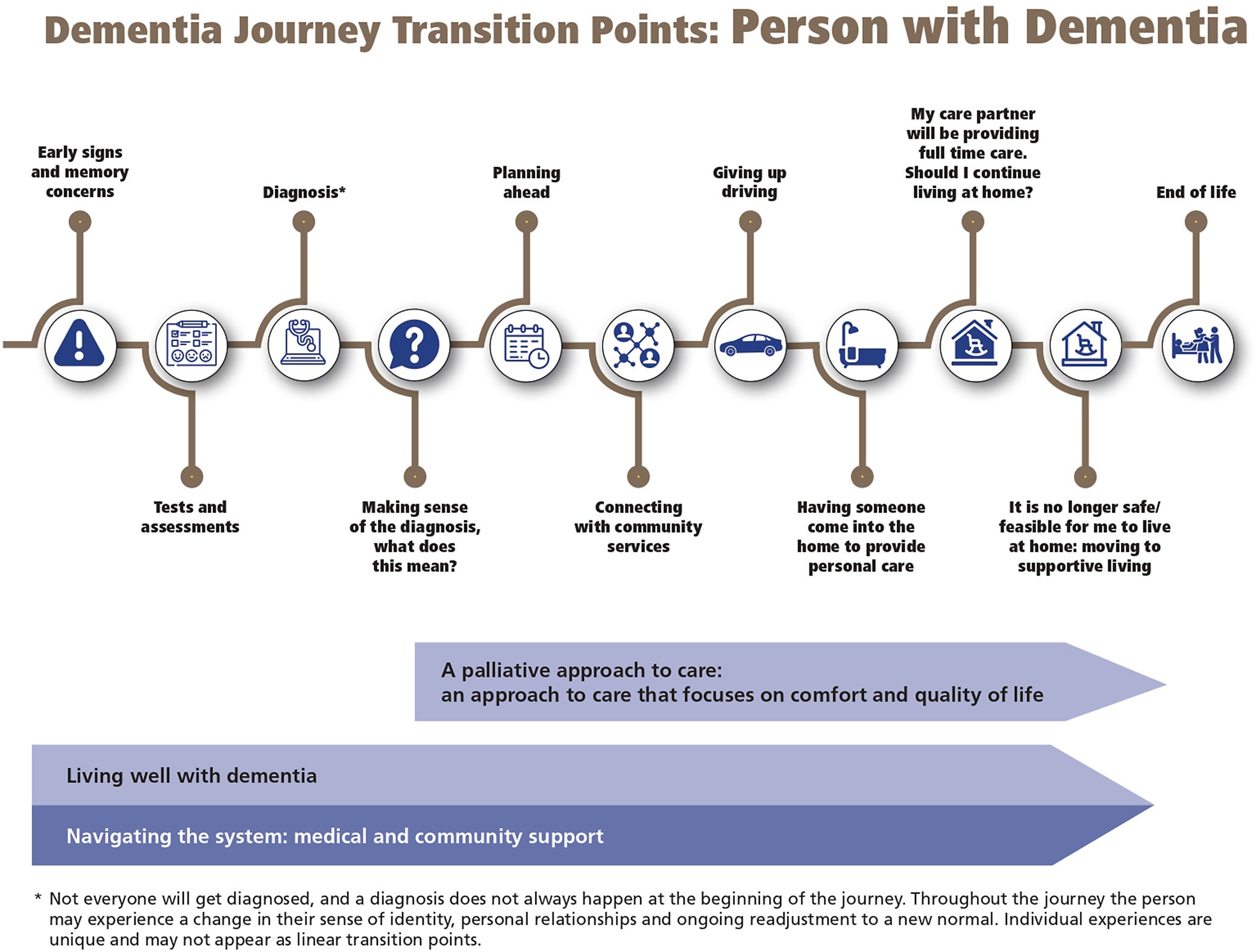
Figure 3. Dementia journey transition points: person living with dementia (developed in collaboration with the Alzheimer Society of Canada).
CCCDTD5 Recommendations Relevant for Primary Care Clinicians
Risk reduction for dementia: Recommendations for all patients
Nutrition
To decrease the risk of cognitive decline, it is recommended that persons adhere to a Mediterranean diet, increase their fruit and vegetable intake, and consume a high level of monounsaturated and polyunsaturated fatty acids (e.g., walnuts, flaxseeds), as well as consume a low level of saturated fatty acids (e.g., beef, cream, butter).
Physical exercise
Physical exercise is recommended for reducing the risk of cognitive decline. Experts currently recommend no specific exercises, and the recommendation should be tailored for each case. Exercise of at least moderate intensity should be recommended for older adults, such as brisk walking. In this type of exercise, the patient should still be able to talk. Family practitioners can recommend aerobic exercise (e.g., swimming, walking), resistance training (e.g., deadlifts, working with all major groups – legs, hips, back abdomen), dance, and mind-body exercise (e.g., Tai Chi, Qigong).
Social engagement and education
It is recommended that attention be given to social circumstances and supports across the life course, including poverty reduction strategies and opportunities for social engagement (e.g., meeting friends and family in person, talking on the phone), as well as support for educational attainment (e.g., access to education) and ongoing educational experiences.
Medications
Older people should have a multidimensional health assessment, including medication use. Family practitioners should aim to minimize patients’ exposure to medications known to have highly anticholinergic properties and replace these with other medications when necessary and feasible. See Table 2 for a list of medications with anticholinergic properties to be avoided based on the American Geriatrics Society Beers Criteria for potentially inappropriate medication use in older adults (The American Geriatrics Society Beers Criteria Update Expert Panel, 2019).
Table 2. List of medication with anticholinergic properties to be avoided based on the American Geriatrics Society Beer’s Criteria for potentially inappropriate medication use in the older adults

Modified from the American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. Journal of the American Geriatrics Society. 2019;67(4):674–694.
Risk reduction for dementia: Recommendations for patients at risk of dementia
Hearing
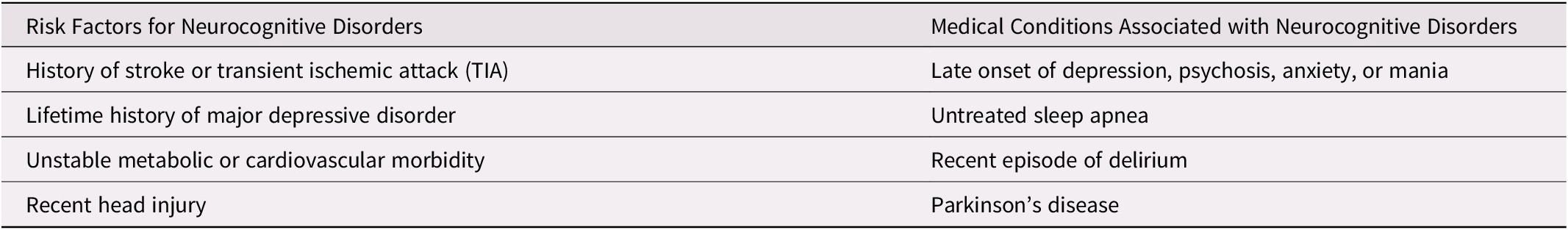
It is recommended that persons who are at risk of dementia (see Table 1), as well as their caregivers be asked whether they have any difficulty hearing in their everyday life (rather than asking whether they are experiencing hearing loss). If symptoms of hearing loss are reported, then hearing loss should be confirmed by audiometry conducted by an audiologist meeting provincial regulations for the practice of audiology. If confirmed, audiological rehabilitation and/or hearing aids or other devices should be considered.
All persons at risk of dementia (see Table 3) should have an audiological and/or otoscopic examination and review of medications for potential ototoxicity. If the patient has chronic otitis or fails the otoscopy, that patient should have a referral to otolaryngology.
Table 3. Risk factors for, and medical conditions associated with, neurocognitive disorders
Sleep
A careful sleep history, including assessment of sleep time and symptoms of sleep apnea, should be included in the assessment of any patient at risk for dementia (see Table 3). Patients for whom sleep apnea is suspected should be referred for polysomnography and/or a sleep specialist consultation for consideration of treatment with a CPAP machine. It is recommended that persons avoid severe sleep deprivation (< 5 hours) and aim to have 7 to 8 hours of sleep per night.
Cognitive training and stimulation
It is recommended that persons at risk increase or maintain engagement in cognitively stimulating activities such as volunteering, long-life learning, and other cognitively stimulating pastimes. When accessible, empirically supported individual computer-based and group cognitive training may be proposed for people at risk and those with mild cognitive impairment (MCI) or dementia. However, further studies are needed; no specific program can be endorsed at this time.
Screening and diagnosis
Screening for people at risk of dementia or with symptoms suggestive of cognitive decline
Cognitive testing is not recommended for screening asymptomatic adults for the presence of MCI or dementia, even in the presence of risk factors. However, if persons are at elevated risk for cognitive disorders (see Table 3), it is reasonable to ask the person and an informant (e.g., caregiver, spouse, child, relative, close friend) about concerns regarding memory and, if present, to evaluate using validated cognitive screening tools (see Table 4). As well, given the underrecognition of dementia in the community, clinicians should be aware of signs and symptoms suggestive of cognitive decline (see Table 5), particularly if reported by family or friends. If signs or symptoms are present, clinicians should consider evaluating them with cognitive screening tools. In the context of the current coronavirus disease (COVID-19) pandemic, recommendations have also been made elsewhere regarding the use of virtual cognitive testing (Geddes et al., Reference Geddes, O’Connell, Fisk, Gauthier, Camicioli and Ismail2020).
Table 4. Psychometric screening tools for assessing cognitive function

* MMSE remains the most widely used instrument, with high sensitivity and specificity for separating moderate dementia from normal cognition and is recommended in many countries.
+ The MoCA is more sensitive to MCI than the MMSE, and its use is recommended when mild cognitive impairment is suspected or in cases where there is suspicion of cognitive impairment or concern about the patient’s cognitive status, and the MMSE score is in the “normal” range (24+ out of 30).
Table 5. Potential symptoms of neurocognitive decline in older individuals

Diagnosis and use of cognitive tests
Screening tools are used to increase the pre-test probability of diagnosis; they alone should not be used to make the diagnosis. The diagnosis requires clinical interviews with the individual and corroborated history with the caregiver, if available, a focused physical examination, and consideration of cognitive test results, which usually require more comprehensive cognitive test tools (Molnar, Benjamin, Hawkins, Briscoe, & Ehsan, Reference Molnar, Benjamin, Hawkins, Briscoe and Ehsan2020). Brief assessment screening tools can be easily administered in family practice. However, for diagnostic purposes, when time permits, more comprehensive tools should be considered. These are listed in Table 4. The diagnosis of MCI or dementia should not be solely based on the results of cognitive screening tests; assessment of functional abilities is important.
Subjective cognitive decline
Subjective cognitive decline (SCD) is a condition in which persons complain about worsening of cognition, but both their function and performance on objective cognitive testing are within normal limits (Studart & Nitrini, Reference Studart and Nitrini2016).
Persons presenting with consistent subjective cognitive complaints, with normal cognitive testing, and absence of any obvious impairment in Instrumental Activities of Daily Living should undergo an appropriate diagnostic workup (i.e., standard dementia medical workup to identify reversible causes, as well as psychiatric assessment – with a particular emphasis on depressive and anxious symptoms).
It is recommended to use structure scales for the evaluation of objective cognition, subjective cognition (such as the SCD-Q Part 1), the informant reported cognition (IQCODE, Lawton Instrumental Activities of Daily Living Scale, PDQ), and informant and self-reported behaviour (see Table 1 for more details on this recommendation).
Corroborated history from a reliable informant is essential (e.g., caregivers, spouses, children, relatives, close friends). For patients with negative corroborative history, reassurance should be provided, and a follow-up should be offered if the patient or informant sources note deterioration in the future in any of the domains of cognition, function, or behaviour. For patients with a positive corroborative history, a referral to a primary or specialty care memory clinic and further investigation with laboratory testing, neuroimaging, and detailed neuropsychiatric testing might be considered. All patients with SCD should be provided with information on World Health Organization recommendations for the prevention of dementia (World Health Organization, 2019).
Use of neuroimaging for the diagnosis and follow-up of dementia
The CCCDTD5 recommends the use of anatomical neuroimaging for most situations (see Table 1 for specific situations needing imaging tests). An MRI is recommended in most situations over CT because of its higher sensitivity for vascular lesions as well as for some subtypes of dementia and rarer conditions (favour 3t MRI over 1.5t MRI when available and non-contraindicated).
Deprescribing in dementia
Aspirin
Without a history of stroke or brain infarcts and in the absence of well-established indications (e.g., history of coronary artery disease), acetylsalicylic acid (Aspirin) is not recommended for patients with MCI or dementia, even if there is imaging evidence of covert white matter lesions of presumed vascular origin.
Cognitive enhancers
The CCCDTD5 builds on the recommendations of the CCCDTD4, which recommended that cholinesterase inhibitors (ChEIs) be considered in most persons with Alzheimer’s disease (mild, moderate, and severe) as well as those with Alzheimer’s disease with a component of cerebrovascular disease (mixed Alzheimer’s disease – vascular dementia being most common) and Parkinson’s disease dementia (Moore, Patterson, Lee, Vedel, & Bergman, Reference Moore, Patterson, Lee, Vedel and Bergman2014).
The CCCDTD5 recommends that, in some specific cases, a trial of ChEIs can be considered for the treatment of vascular dementia. Furthermore, the CCCDTD5 offers complementary recommendations on deprescribing ChEIs where appropriate for individuals taking a ChEI for Alzheimer’s disease, Parkinson’s disease dementia, Lewy body dementia, or vascular dementia for > 12 months. See Table 1 for specific situations in which deprescribing should be considered.
If the patient has been prescribed a ChEI or memantine for MCI or for a type of dementia other than Alzheimer’s disease, Parkinson’s disease dementia, Lewy body dementia, and vascular dementia, it should be discontinued.
Deprescribing of ChEIs or memantine should occur gradually and treatment reinitiated if the individual shows clinically meaningful worsening of cognition, functioning, neuropsychiatric symptoms, or global assessment that appears to be related to cessation of therapy. Individuals who have had a clinically meaningful reduction in neuropsychiatric symptoms (e.g., psychosis) with cognitive enhancers should continue to be treated with the cognitive enhancer, even if there is evidence of cognitive and functional decline.
All deprescribing decisions of these medications should take into consideration the patient’s preference and include consultation with a family member or substitute decision maker for persons who cannot consent.
Psychosocial and non-pharmacological interventions
Exercise
Group or individual physical exercise for PLWD is recommended, but there is no specific recommendation on the duration or intensity. This should be discussed and planned based on individual circumstances and preferences.
Cognitive stimulation therapy
Cognitive stimulation therapy, such as engaging in hobbies or volunteering, is the most widely used non-pharmacological intervention for dementia (Piras et al., Reference Piras, Carbone, Faggian, Salvalaio, Gardini and Borella2017) and may stimulate information processing in PLWD (Clarkson et al., Reference Clarkson, Hughes, Xie, Larbey, Roe and Giebel2017). Based on implicit learning, it stimulates language and executive functioning, focusing on orientation, reminiscence, new ideas, thoughts, and associations (Piras et al., Reference Piras, Carbone, Faggian, Salvalaio, Gardini and Borella2017). Group cognitive stimulation therapy is recommended for persons living with mild to moderate dementia. Primary care clinicians should have information for patients on these services where available.
Psychoeducational interventions
Psychoeducational interventions provide information to the caregivers on the disease and adequate management of dementia, helping them develop coping skills and strategies (Ponce et al., Reference Ponce, Ordonez, Lima-Silva, Dos Santos, de Fátima Viola and Nunes2011). Psychosocial and psychoeducational interventions for caregivers of PLWD include education, counselling, information regarding services, enhancing carer skills to provide care, problem-solving, and strategy development. Primary care clinicians should have information for patients on these services when available.
Case management
Case management is an intervention that may improve the quality of care that patients and caregivers receive by managing and coordinating services (Khanassov, Vedel, & Pluye, Reference Khanassov, Vedel and Pluye2014). Case management uses a collaborative process for managing dementia cases (including the assessment, planning, care coordination, and advocacy for options and services to meet the patients’ and caregivers’ needs) (Khanassov et al., Reference Khanassov, Vedel and Pluye2014). Where available, implementation of a case management approach should be considered in primary care practice for PLWD.
Dementia-friendly communities and organizations
Dementia-friendly organizations aim to preserve the safety and well-being of PLWD (Hebert & Scales, Reference Hebert and Scales2019). In health care, a dementia-friendly organization is aware of the impact dementia has on a person’s ability to engage with services and manage their health. Dementia-friendly organizations implement activities and changes to promote the inclusion of PLWD and caregivers in decisions and discussions to improve outcomes (Vedel et al., Reference Vedel, Sheets, McAiney, Clare, Brodaty and Mann2020). Primary care practices should also consider advocating for and becoming a dementia-friendly organization.
Additional Resources
Below, we compiled a small list of selected available resources that could potentially aid primary care clinicians in their practice with dementia patients. These resources are not present on the CCCDTD5 list of recommendations and are in the present article to provide tailored information for primary care providers. Figures 2 and 3 were developed in collaboration with the Alzheimer Society of Canada. These figures include two dementia journeys, one for the patient and one for the health and social care provider.
As seen above, the care of PLWD requires a comprehensive network of professionals. We recommend consulting the Interdisciplinary Primary Care Pathways (Interdisciplinary Clinical Process – Family Medicine Group (FMG), 2020a; Interdisciplinary Clinical Process – Family Medicine Group (FMG), 2020b), divided into two components: diagnosis and follow-up. Although it was developed for the context of primary care groups in the province of Quebec, coupled with the Dementia Journeys for health and social care providers and PLWD (see Figures 2 and 3), it can be a good resource for understanding and getting an overall view of the importance of collaborating closely with a multidisciplinary team and providing examples of when other professionals can be involved in the care of PLWD.
The Forward with Dementia website (available in French and English: www.forward-avancer.ca and www.forwardwithdementia.ca) is a resource created by an international team effort and with the input of PLWD, care partners, and health and social care providers. To provide a message of hope and guidance for PLWD and their care partners after the diagnosis of dementia, it offers tailored information regarding several topics, including nutrition, exercise, and social engagement activities. There are also resources for health and social care providers.
Although the CCCDTD5 recommends exercise both as prevention and as part of the treatment of dementia, it does not provide enough evidence to support one type of exercise above others. However, resources are available for the general population of ages 65 years and older for recommending exercise. For instance, the guidelines developed by the Canadian 24-hour movement, tailored for adults ages 65 years and older (The Canadian Society for Exercise Physiology, 2021), as well as the resources of Western University in Canada to improve balance (Canadian Centre for Activity and Aging – Western University, 2022) can be a good complement: https://csepguidelines.ca/guidelines/adults-65/ and https://www.uwo.ca/ccaa/programs/videos/balance_flexibility.html.
Discussion
The present article tailors the results of the CCCDTD5 recommendations for primary care providers, which are crucial for the management and treatment of dementia in Canada. Primary care practitioners need evidence-based relevant recommendations to guide the diagnosis and management of dementia in family practice. In addition, with the help of knowledge translation experts, we created visual representations that can help primary care providers visualize when, in the journey of dementia, they can apply these recommendations. The new recommendations in the CCCDTD5 can help guide screening, diagnosis, and pharmacological and non-pharmacological interventions for PLWD and their caregivers in primary care settings.
Future guidelines should focus on emerging diagnostic, therapeutic interventions, and better long-term management supports. For example, while the CCCDTD5 could not recommend specific exercises or cognitive training, there is much ongoing research in this field, and we expect many advances in the coming years to guide better dementia care practices (Livingston et al., Reference Livingston, Sommerlad, Orgeta, Costafreda, Huntley and Ames2017). It is critical that primary care practitioners remain up-to-date and skilled in dementia care to better meet the needs of the aging Canadian population we serve. Future iterations of this conference (e.g., CCCDTD6) could cover additional topics essential for dementia care in primary and specialized care, such as medical assistance in dying (including the ethical and human rights implications and considerations), virtual dementia care and its appropriateness for this population, driving assessment for persons living with dementia, and awareness and respect of human rights for dementia patients with particular emphasis on patients with severe cognitive impairment at the time of shortage of medical care (i.e., during the SARS-CoV-2 [COVID-19] pandemic). In addition, sex and gender were not considered during the CCCDTD5. Future iterations should consider these to better tailor the recommendations to specific populations. Furthermore, additional efforts in knowledge translation should continue to be made for future dementia guidelines to answer to the needs of health care providers in primary care in Canada.
Conclusion
The present article tailored the recommendations from the CCCDTD5 for primary care in a more practical manner to consult. The discussed knowledge translation strategy helps these important recommendations reach knowledge users. The development of recommendations for the ongoing management of dementia is an iterative process as new evidence on interventions for dementia is published. The present article’s recommendations are not only focused on treating dementia after the diagnosis. Primary care providers can start action plans to reduce the risk of dementia in the general population and those at risk. It is not recommended to screen asymptomatic patients. However, special attention should be provided to those with symptoms, including patients complaining of subjective cognitive decline. In addition, medications for dementia should be approached based on the patient’s characteristics, as not all persons with dementia should be prescribed medications such as aspirin or cholinesterase inhibitors. These recommendations on risk reduction, screening and diagnosis, deprescription of dementia medications, and non-pharmacological interventions for dementia are important in the primary care setting as it is the entry point for PLWD into the health system.
Acknowledgements
We want to thank all members of the CCCDTD5’s panel of experts. We would also like to thank Juliette Champoux-Pellegrin, Administrative Coordinator for ROSA Team at the Lady Davis Institute for Medical Research, Jewish General Hospital in Montreal, who helped with the conception of Figure 1 and the grammar revision and formatting of the article. We would like to thank the Alzheimer Society of Canada for collaborating with us in the development of Figures 2 and 3 on the dementia journey transition points.
Funding
The work of Laura Rojas-Rozo and Juliette Champoux-Pellegrin was supported by the Canadian Consortium on Neurodegeneration in Aging (CCNA), which is supported by a grant from the Canadian Institutes of Health Research with funding from several partners (grant number CCNA137794).
The CCCCDTD5 meeting was supported financially by the CCNA, the Réseau des cliniques mémoire du Québec, the Réseau Québecois de Recherche sur le Vieillissement.
Competing interest
The authors report no conflicts of interest.











