Sensory impairments increase with age and are very common among older adults (≥ 65 years of age). The prevalence rates of these impairments are expected to increase over time because the population is aging (Mathers & Loncar, Reference Mathers and Loncar2006). Approximately 65 per cent of Canadians 70 years of age and older have a hearing impairment (HI), with both incidence and prevalence rates rising with each decade of life (Feder, Michaud, Ramage-Morin, McNamee, & Beauregard, Reference Feder, Michaud, Ramage-Morin, McNamee and Beauregard2015). In 2016, 1,500,000 Canadian males 45–85 years of age had at least mild hearing loss, 1,800,000 had at least mild vision loss, and 570,000 had both. Among females, 1,200,000 had at least mild hearing loss, 2,200,000 had at least mild vision loss, and 450,000 had both (Mick et al., Reference Mick, Hämäläinen, Kolisang, Pichora-Fuller, Phillips and Guthrie2020). It has been estimated that HI and vision impairment (VI), respectively, are the second- and third-most common impairments worldwide (Vos, Reference Vos2016).
HI and VI in older adults are particularly important to understand given their influence on multiple health-related outcomes. For example, HI is associated with poor self-rated health (Choi et al., Reference Choi, Betz, Deal, Contrera, Genther and Chen2015) and difficulties with activities of daily living (ADLs) and instrumental ADLs (IADLs) (Chen et al., Reference Chen, Betz, Yaffe, Ayonayon, Kritchevsky and Martin2015; Choi et al., Reference Choi, Betz, Deal, Contrera, Genther and Chen2015; Slaughter, Hopper, Ickert, & Erin, Reference Slaughter, Hopper, Ickert and Erin2014), falls (Campos, Ramkhalawansingh, & Pichora-Fuller, Reference Campos, Ramkhalawansingh and Pichora-Fuller2018; Jiam, Li, & Agrawal, Reference Jiam, Li and Agrawal2016; Lin & Ferrucci, Reference Lin and Ferrucci2012), and is the top potentially modifiable risk factor for dementia (Davies, Cadar, Herbert, Orrell, & Steptoe, Reference Davies, Cadar, Herbert, Orrell and Steptoe2017; Deal et al., Reference Deal, Betz, Yaffe, Harris, Purchase-Helzner and Satterfield2017; Fritze et al., Reference Fritze, Teipel, Ovari, Kilimann, Witt and Doblhammer2016). HI is also associated with lower social support and loneliness (Mick, Parfyonov, Wittich, Phillips, & Pichora-Fuller, Reference Mick, Parfyonov, Wittich, Phillips and Pichora-Fuller2018).
Similarly, VI is associated with an increased risk of mortality (Reuben, Mui, Damesyn, Moore, & Greendale, Reference Reuben, Mui, Damesyn, Moore and Greendale1999; Wang, Mitchell, Simpson, Cumming, & Smith, Reference Wang, Mitchell, Simpson, Cumming and Smith2001), difficulties with ADLs and IADLs (Grue et al., Reference Grue, Finne-Soveri, Stolee, Poss, Sorbye and Noro2009; Reuben et al., Reference Reuben, Mui, Damesyn, Moore and Greendale1999), and difficulties caused by reduced mobility (Wang, Mitchell, Smith, Cumming, & Attebo, Reference Wang, Mitchell, Smith, Cumming and Attebo1999). Individuals with VI also have lower social support, increased loneliness, reduced social participation, and smaller social networks (Grue et al., Reference Grue, Finne-Soveri, Stolee, Poss, Sorbye and Noro2009; Laliberte Rudman et al., Reference Laliberte Rudman, Gold, McGrath, Zuvela, Spafford and Renwick2016). Compared with those who have no VI, they are also more likely to receive community-based supports such as home care and meals-on-wheels (Wang et al., Reference Wang, Mitchell, Smith, Cumming and Attebo1999).
Older people with a combination of VI and HI, known as dual sensory impairment (DSI), are a particularly vulnerable group (Simcock, Reference Simcock2017; Simcock & Wittich, Reference Simcock and Wittich2019; Wittich & Simcock, Reference Wittich, Simcock and Ravenscroft2019). They experience limitations in completing ADLs and IADLs (Smith, Bennett, & Wilson, Reference Smith, Bennett and Wilson2008), are at increased risk for depression (Capella-McDonnall, Reference Capella-McDonnall2009; Fletcher & Guthrie, Reference Fletcher and Guthrie2013; Guthrie, Theriault, & Davidson, Reference Guthrie, Theriault and Davidson2015; Schneider et al., Reference Schneider, Gopinath, McMahon, Leeder, Mitchell and Wang2011) and mortality (Reuben et al., Reference Reuben, Mui, Damesyn, Moore and Greendale1999), have significantly impaired communication function (McDonnall, Crudden, LeJeune, Steverson, & O’Donnell, Reference McDonnall, Crudden, LeJeune, Steverson and O’Donnell2016) and have reduced social participation (Mick et al., Reference Mick, Parfyonov, Wittich, Phillips and Pichora-Fuller2018). Despite these findings, there is very limited research globally on older adults with DSI (Heine & Browning, Reference Heine and Browning2015), and this is also true in Canada (Guthrie et al., Reference Guthrie, Theriault and Davidson2015; Wittich, Watanabe, & Gagné, Reference Wittich, Watanabe and Gagné2011).
Previous research in Canada points to differential outcomes for individuals with VI, HI, or DSI. For example, in cross-sectional analyses, individuals with DSI and cognitive challenges were the most likely to experience communication difficulties, deterioration in communication over time, or a diagnosis of Alzheimer’s dementia, and to have a primary caregiver feeling distressed (Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018). HI has also been shown to interact with impaired cognitive functioning to influence the risk of admission to long-term care. Individuals with an HI, but no cognitive challenges, had a faster time to admission versus clients with both HI and cognitive difficulties (Williams et al., Reference Williams, Phillips, Wittich, Campos, Mick and Orange2020). Home care clients who experienced significant deterioration in their hearing are more likely than those with no changes in their hearing to experience communication difficulties and to have a caregiver who is distressed (Williams, Guthrie, Davidson, Fisher, & Griffith, Reference Williams, Guthrie, Davidson, Fisher and Griffith2018).
The current literature linking sensory impairments and outcomes such as communication difficulties, cognitive decline, and caregiver burden has several gaps. The research designs have been mainly cross-sectional, few studies have included adults with DSI, and little research has been completed in Canada. Previous studies have primarily investigated community-dwelling older adults (Alattar et al., Reference Alattar, Bergstrom, Laughlin, Kritz-Silverstein, Richard and Reas2020; Amieva et al., Reference Amieva, Ouvrard, Giulioli, Meillon, Rullier and Dartigues2015; Davies et al., Reference Davies, Cadar, Herbert, Orrell and Steptoe2017; Deal et al., Reference Deal, Betz, Yaffe, Harris, Purchase-Helzner and Satterfield2017; Lin et al., Reference Lin, Yaffe, Xia, Xue, Harris and Purchase-Helzner2013) or long-term care residents (Yamada et al., Reference Yamada, Vlachova, Richter, Finne-Soveri, Gindin and van der Roest2014), with very few focused on home care recipients (Vengnes Grue et al., Reference Vengnes Grue, Hylen Ranhoff, Noro, Finne-Soveri, Birna Jensdottir and Ljunggren2009).
Home care is an important sector to study for several reasons. Roughly 2,000,000 Canadians receive home care annually, of whom approximately 40 per cent are 65 years of age and older (Sinha & Bleakney, Reference Sinha and Bleakney2014). The federal, provincial, and territorial governments recently endorsed A Common Statement of Principles on Shared Health Priorities, accompanied by an $11 billion federal investment to improve Canadians’ access to home and community care (Government of Canada, 2019). Evidence to better understand this sector is important, because the majority of older adults prefer to “age in place” and remain in their own homes for as long as possible (Wiles, Leibing, Guberman, Reeve, & Allen, Reference Wiles, Leibing, Guberman, Reeve and Allen2012). Finally, older persons receiving home care are typically more impaired in their cognitive and physical functioning than community-dwelling older adults. The prevalence of cognitive difficulties is approximately 20–30 per cent (de Almeida Mello et al., Reference de Almeida Mello, Ces, Vanneste, Van Durme, Van Audenhove and Macq2020; Garms-Homolova et al., Reference Garms-Homolova, Notthoff, Declercq, van der Roest, Onder and Jonsson2017; Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018) in home care and roughly 3 per cent among physically healthy, community-dwelling older persons (Mery, Wodchis, & Laporte, Reference Mery, Wodchis and Laporte2016). The same is true for functional abilities, with prevalence of ADL impairment as high as 32 per cent in home care (Brown, McAvay, Raue, Moses, & Bruce, Reference Brown, McAvay, Raue, Moses and Bruce2003; Foebel et al., Reference Foebel, van Hout, van der Roest, Topinkova, Garms-Homolova and Frijters2015) versus roughly 10 per cent among those living in the community (Mery et al., Reference Mery, Wodchis and Laporte2016; Raina, Wolfson, Kirkland, & Griffith, 2010–Reference Raina, Wolfson, Kirkland and Griffith2015). This group is important to study because they are under-represented in health services research in Canada.
The present study aimed to address several of the gaps in the current literature. A longitudinal design was utilized to enable the identification of the onset of new sensory impairments. Assessing home care clients over time allowed for an evaluation of risk versus simply understanding cross-sectional relationships. We created multiple unique cohorts to explore how the identification of the onset of new sensory impairments influences the risk of several important outcomes including communication difficulties, a deterioration in cognitive performance, and the risk of caregiver burden. We chose to focus on individuals with a newly identified sensory impairment, because this group may be in an adjustment phase and may not have had time to develop compensatory strategies (e.g., seeking advice and looking into and purchasing assistive devices), whereas people with longer-term impairments may have had time to do so. Developing these compensatory strategies may also be more difficult among home care recipients who are also dealing with multiple co-morbidities.
Based on our previous work and other literature, we anticipated that older adults with a newly identified DSI would show a greater risk for multiple negative outcomes than individuals with VI or HI only. For example, we expected that this group would be more likely to experience communication difficulties, worsening cognitive performance, and caregiver burden, even after adjusting for multiple control variables.
Design and Methods
Data Source
We conducted secondary analyses of data collected using the Resident Assessment Instrument for Home Care (RAI-HC) in Ontario. All data included in this article came from the RAI-HC, and no other supplementary assessments or data were used. The RAI-HC is a standardized assessment that is routinely used to assess clients who are expected to receive at least 60 days of service (Ontario Ministry of Health and Long-Term Care, 2007). The assessment has established validity and reliability and includes roughly 300 items to capture domains including, but not limited to communication, sensory impairments, cognitive status, and functional ability (Hirdes et al., Reference Hirdes, Ljunggren, Morris, Frijters, Finne-Soveri and Gray2008). Assessments are completed by trained care coordinators (typically registered nurses) through an interview with the client and their informal caregivers, and in consultation with other health professionals, as needed. Re-assessments are completed every 6–12 months or following a clinically important change in health status. Because the assessment is mandated in Ontario, the software used to capture the completed assessments does not allow the assessor to leave fields blank. Therefore, there are virtually no missing data in this data set. In Ontario, all assessments are shared with the Canadian Institute for Health Information (CIHI). Researchers can apply to the CIHI for a copy of the anonymized data, as was done for the present study. The Research Ethics Board at Wilfrid Laurier University reviewed and approved the design of this study (REB #4184).
Sample
The sample included all home care clients who were 65 years of age and older and who had completed at least two RAI-HC assessments between 2009 and 2014 (n = 106,920). Each pair of assessments that was completed within 60 months was examined to find the first point in time when a sensory impairment was newly identified for a client. The time frame between assessments was chosen in order to maximize the length of follow-up within the data set. For example, if an individual had three assessments, and at their first assessment had no sensory impairment, but were identified as having a new HI at their third assessment (i.e., no new impairment at their second), then their third assessment would be considered T2 and their second assessment would be considered T1. We followed each person forward to the first instance when a new sensory impairment occurred, which was deemed their T2 assessment, whereas the assessment just previous to this one was T1. For those who did not develop a new impairment at any of their assessments, we used their two most recent assessments in the database as T1 and T2.
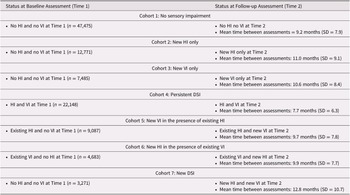
We defined seven mutually exclusive cohorts of individuals based on their sensory status at baseline (T1) and how this status may have changed by follow-up (T2) (Table 1). The seven cohorts included those with (1) no sensory impairments at either T1 or T2 (n = 47,475), (2) a newly identified HI at T2 (n = 12,771), (3) a newly identified VI at T2 (n = 7,485), (4) “persistent” DSI that was present at both T1 and T2 (n = 22,148), (5) an existing HI and a newly identified VI at T2 (n = 9,087), (6) an existing VI and a newly identified HI at T2 (n = 4,683), and (7) no baseline impairments and a newly identified DSI at T2 (n = 3,271).
Table 1. Summary of the seven different cohorts and their status at baseline and at follow-up
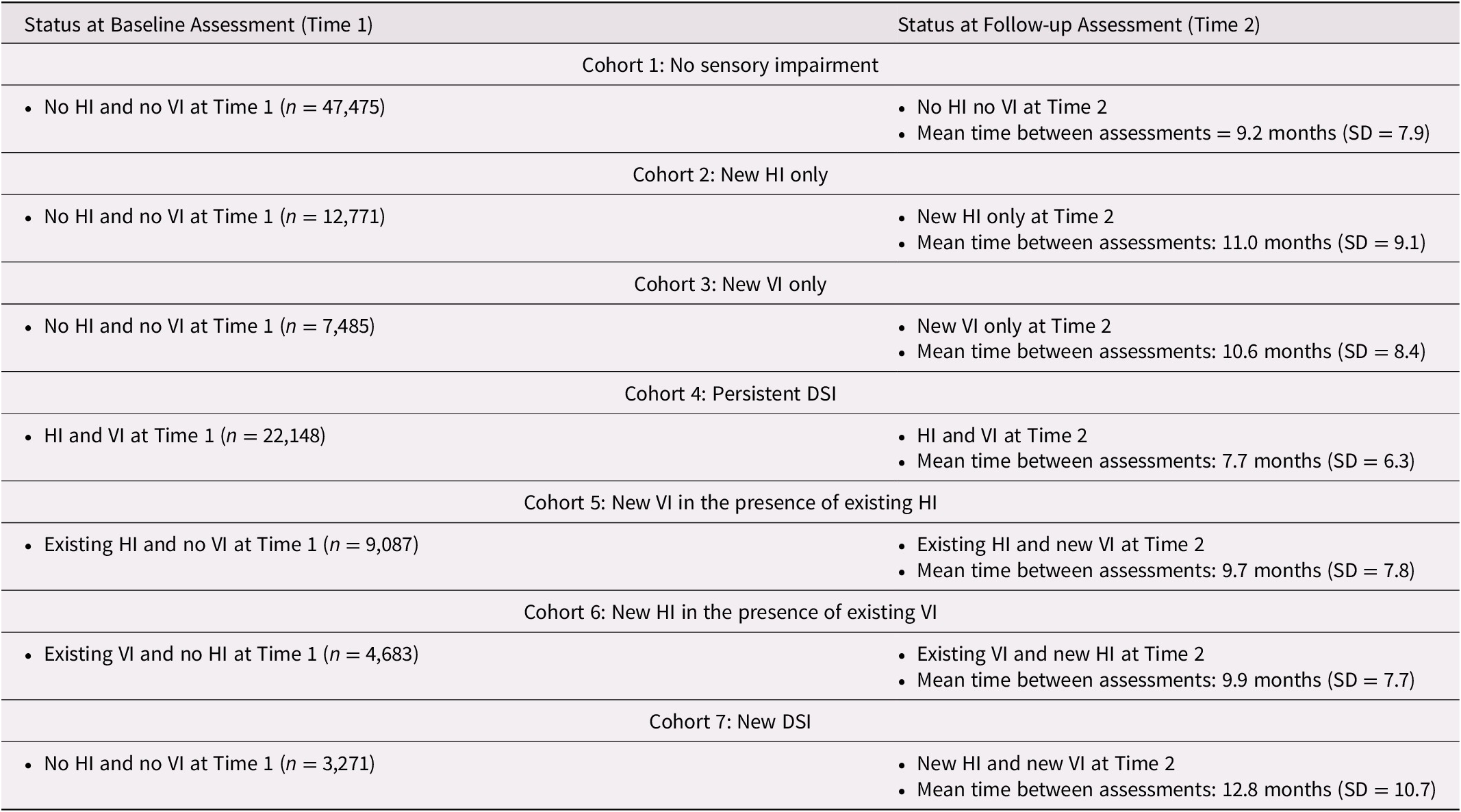
Note. HI = hearing impairment; VI = vision impairment; DSI = dual sensory impairment; SD = standard deviation
Sensory Measures
Care coordinators complete the RAI-HC based on a standardized manual developed by interRAI, the group who holds the copyright for the instrument (Morris et al., Reference Morris, Bernabei, Ikegami, Gilgen, Frijters and Hirdes1999). They determine a client’s hearing and vision status based on a combination of self-assessment, discussions with caregivers, consultations with other care providers, and review of any available medical records, as appropriate. For the measures related to vision and hearing (one item each on the RAI-HC), assessors are instructed to evaluate sensory impairments when the client is using their existing aids or devices (i.e., while using hearing aids or glasses).
The presence of HI was defined as a score of one or higher on a single item on the RAI-HC. This item scores hearing ability from zero (no impairment) to three (highly impaired). Similarly, VI was defined as a score of one or higher on a single item that scores vision from zero (no impairment) to four (severely impaired). Finally, DSI was defined as a score of three or higher on the Deafblind Severity Index (DbSI). The DbSI uses the two items that measure vision and hearing to identify clients with at least minimal losses in both senses (i.e., a score of one or higher on both items) (Dalby et al., Reference Dalby, Hirdes, Stolee, Strong, Poss and Tjam2009). The hearing and vision items have good test–retest reliability (hearing: κ = 0.83; vision: κ = 0.85) (Dalby et al., Reference Dalby, Hirdes, Stolee, Strong, Poss and Tjam2009) and correlate well with gold standard objective measures (Urqueta Alfaro et al., Reference Urqueta Alfaro, Guthrie, Phillips, Pichora-Fuller, Mick and McGraw2019).
Outcome Measures
The RAI-HC includes two items on communication, one to capture expressive and the other to capture receptive communication. The first item, on expressive communication, scores the person’s ability to express themselves orally, using sign language, in writing, or some combination of these techniques. The other item targets the person’s capacity to understand information communicated with the person orally, in writing, through sign language, or in braille. In both cases, the items are scored from zero to four, where a score of one or higher was used to define difficulties with communication. A deterioration in communication was defined as at least a one-point increase on the item over time.
The Caregiver Risk Evaluation (CaRE) algorithm is a decision-support tool that differentiates the risk of caregiver burden among informal caregivers (Guthrie et al., Reference Guthrie, Williams, Beach, Maxwell, Mills and Mitchell2021). The algorithm is created using items from within the RAI-HC and assigns caregivers to one of four unique groups, ranging from low risk (score of zero) to very high risk (score of four) of experiencing burden. Individuals were grouped into two categories, including those with low or moderate risk (scores of 1 and 2; roughly 60% of individuals across the seven cohorts) and compared with those with a high or very high risk of experiencing caregiver burden (scores of 3 and 4; roughly 40%).
The Cognitive Performance Scale (CPS) is a hierarchical scale which includes two items found on traditional cognitive assessments (e.g., short-term memory, daily decision making) and two items reflecting functional status (e.g., expressive communication, independence in eating). The scale ranges from zero to six (0 = no cognitive impairment, 1 = borderline intact, 2 = mild impairment, 3 = moderate impairment, 4 = moderately severe impairment, 5 = severe impairment, and 6 = very severe impairment), has excellent inter-rater reliability (average κ = 0.85), and is correlated with performance on two cognitive screening measures; namely, the Montreal Cognitive Assessment (Jones, Perlman, Hirdes, & Scott, Reference Jones, Perlman, Hirdes and Scott2010) and the Mini Mental State Exam (Gruber-Baldini, Zimmerman, Mortimore, & Magaziner, Reference Gruber-Baldini, Zimmerman, Mortimore and Magaziner2000). It was designed to be a functional measure and to act as a brief screen for impaired cognitive performance.
We focused on four main dependent measures; namely, a deterioration in: (1) cognitive performance (measured with the CPS scale), (2) receptive communication, (3) expressive communication, and (4) the CaRE algorithm. In all cases, a minimum of a one-point change on the item or scale was considered to be a meaningful decline. These four outcomes were chosen because they are highly relevant to the functioning of individuals who are experiencing sensory changes over time. Furthermore, they enabled us to build upon our earlier cross-sectional analyses which showed associations among these outcomes(Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018).
Control Variables
There are five health index scales embedded within the RAI-HC. For all scales, a higher score indicates worse functioning (i.e., greater impairment). The scales have been described in detail previously (Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018) and are summarized.here.
-
1. The Activities of Daily Living (ADL) Self-Performance Hierarchy Scale (ADL-H) includes items on bathing and dressing with scores ranging from a score of zero (independent) to six (total dependence) (Morris, Fries, & Morris, Reference Morris, Fries and Morris1999). A score of two or higher, representing the point at which an individual can no longer complete all of their ADLs independently, was used to define moderate/severe ADL impairment, in line with previous research (Davidson & Guthrie, Reference Davidson and Guthrie2017; Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018; Williams, Jamal, & Guthrie, Reference Williams, Jamal and Guthrie2018).
-
2. The Instrumental Activities of Daily Living (IADL) Involvement Scale has scores ranging from 0 to 21 and includes items on telephone use, managing medications, and meal preparation. A cut-point of 14 or higher was used to indicate at least moderate impairment in performing these tasks, in line with previous studies (Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018; Williams, Jamal, & Guthrie, Reference Williams, Jamal and Guthrie2018).
-
3. The Depression Rating Scale (DRS) is a summative scale with scores ranging from 0 to 14. A score of three or higher, the cut-point we have chosen, is predictive of a clinical diagnosis of depression (Martin et al., Reference Martin, Poss, Hirdes, Jones, Stones and Fries2008).
-
4. The Pain Scale includes two items which capture the frequency and intensity of pain. The scale ranges from zero to four and has been validated against the vertical version of the Visual Analog Scale (VAS). A score of two or higher was used to indicate severe/daily pain, because research has shown an important increase in the VAS score among those with a score of two or higher (Fries, Simon, Morris, Flodstrom, & Bookstein, Reference Fries, Simon, Morris, Flodstrom and Bookstein2001).
-
5. The Changes in Health, End-Stage Disease and Signs and Symptoms (CHESS) Scale is scored from zero to five and is a measure of health instability. For every one-point increase on the scale, there is a nearly two-fold increased risk of mortality. A cut-point of two or higher was used to determine health instability based on previous research showing a marked increase in the hazard ratio for mortality among those scoring two or higher compared with those scoring less than two (Hirdes, Poss, Mitchell, Korngut, & Heckman, Reference Hirdes, Poss, Mitchell, Korngut and Heckman2014).
Several other dichotomous variables (measured as yes/no) were examined, including self-reported loneliness, and items related to diagnoses (Alzheimer’s dementia/another type of dementia, stroke, diabetes, hypertension, coronary artery disease, congestive heart failure, Parkinson’s disease, irregularly irregular pulse, peripheral vascular disease, cataracts, and glaucoma). A co-morbidity count was developed (having at least one diagnosis vs. none) from the preceding list.
Univariate Analysis
The preliminary descriptive analysis examined the absolute percent change between T1 and T2 across multiple outcomes and across the seven cohorts. We focused on change over time, as we have previously explored similar outcomes, but using cross-sectional data (Guthrie et al., Reference Guthrie, Davidson, Williams, Campos, Hunter and Mick2018). Given the large sample size and potential for type I error, we used an absolute standardized difference (stdiff, similar to a z-score) of 0.2 or higher to identify statistically meaningful differences between individuals with and those without impairments (Yang & Dalton, Reference Yang and Dalton2012). Standardized differences are one metric by which to understand the effect size when comparing two proportions (Azuero, Reference Azuero2016). Our chosen cut-point identifies a difference representing at least a small effect size. The standardized difference is defined as the difference in two proportions divided by an estimate of the prevalence of the covariate in each of the two groups (Austin, Reference Austin2009). Given that we considered the raw baseline data (T1) to be supplementary to our main question, we did not calculate any standardized differences to compare the seven cohorts at T1.
For each of the four outcomes (described previously), the analysis was then stratified by both the type of sensory change (i.e., the seven unique cohorts), as well as by age (5-year increments). We did a similar analysis, stratified by sensory cohort and by sex, which failed to show any significant differences (data not shown); therefore, all results presented here are collapsed across sex.
Multivariate Analysis
Logistic regression was used to examine the association between the identification of a new impairment (i.e., new HI-only, new VI-only, or a new DSI) across the four different outcomes of interest. A total of four multivariate models were constructed, in which each model represented at least a one-point decline (i.e., worsening) in the outcome of interest between T1 and T2: (1) a decline in receptive communication, (2) a decline in expressive communication, (3) a decline on the CaRE algorithm, and (4) a decline on the CPS score. The reference group for each outcome was the absence of the event (e.g., for a decline on the CPS score, the reference group represented individuals who did not experience a decline in their score between T1 and T2). Each model was adjusted for the following control variables: age (continuous variable), sex (male/female), marital status (married/never married/widowed/separated or divorced), level of education (post-secondary/less than high school/high school), a score of three or higher on the DRS, a co-morbidity count of one or higher, and a score of one or higher on the CPS scale (only applicable for a decline in receptive communication outcome). Because we were interested in examining the association between a new impairment across the four outcomes, all of the control variables were based on data from T1, whereas the outcomes of interest were the change in scores (decline vs. no decline) between T1 and T2. All analyses were completed using SAS Enterprise Guide, version 7.1, and the study followed the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines (von Elm et al., Reference von Elm, Egger, Altman, Pocock, Gotzsche and Vandenbroucke2007).
Results
In this group of home care clients, 33.6% (n = 35,918) had a sensory impairment at T1 and an additional 34.9% (n = 37,297) were identified as having the onset of a new sensory impairment between T1 and T2. A small proportion (3.1%) were identified as having developed a new impairment in both their vision and hearing. The average time between T1 and T2 was very similar across the cohorts, ranging from a mean 9.7 months (cohort 5: existing HI/new VI) to 12.8 months (cohort 7: new DSI; Table 1).
At T1, the cohorts identified as having sensory challenges tended to be at least 85 years of age. The proportion in this age group varied by cohort, ranging from 26.7 per cent (new VI) to 64.1 per cent (persistent DSI), compared with 27 per cent in those without any sensory impairments. At least 60 per cent in each of the seven cohorts were female, with little variation across these groups (ranging from 61.7% to 71.1%). Between 33.5 per cent (persistent DSI) and 43.2 per cent (no impairments) were married. Based on the proportions alone (i.e., without any statistical testing), clients with existing sensory impairments, and in particular the persistent DSI cohort, were relatively more likely to experience impaired ADLs and IADLs, to experience health instability, to have difficulties in both receptive and expressive communication, and to experience some level of difficulty with cognitive performance, measured with the CPS scale (Table 2). We opted not to conduct any formal testing of statistical significance between these proportions, because these analyses were not meant to address our primary research question, which focused on change between T1 and T2.
Table 2. Comparison of demographic and clinical characteristics across the seven cohorts at baseline (T1)
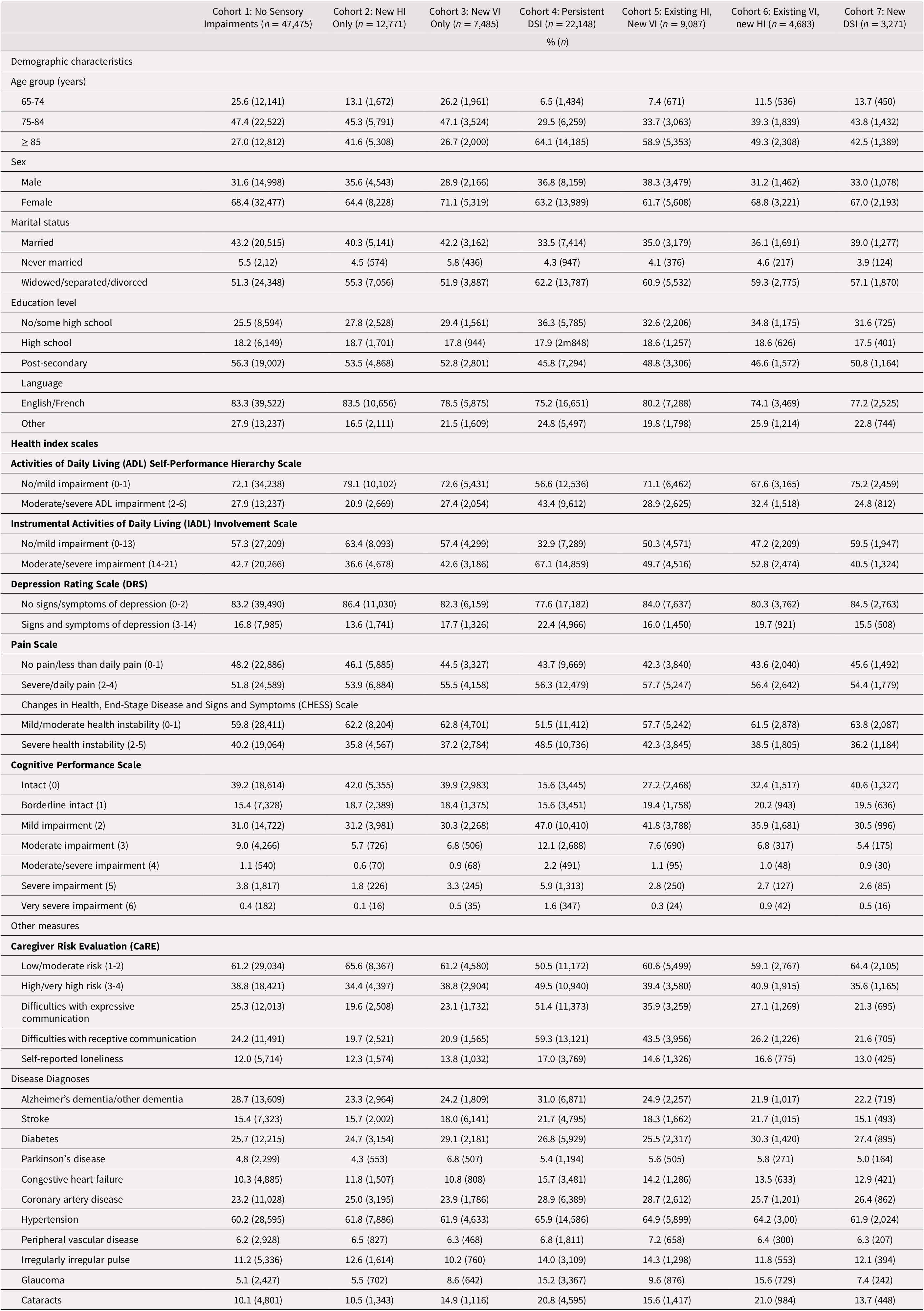
Note. HI = hearing impairment; VI = vision impairment; DSI = dual sensory impairment.
In examining change over time, a clear pattern emerged whereby the new DSI cohort had the highest proportion of individuals who showed a deterioration over time across multiple outcomes. For this reason, we calculated the standardized difference comparing the new DSI cohort and those with no sensory impairments, to highlight which of these differences were statistically meaningful. Those in the new DSI cohort were significantly more likely to experience a deterioration over time in terms of receptive communication (stdiff = 0.68), expressive communication (stdiff = 0.61), cognitive performance (stdiff = 0.49), ADLs (stdiff = 0.36), IADLs (stdiff = 0.39), and symptoms of depression (stdiff = 0.36). Those with a newly identified DSI were also more likely to have caregivers who, over time, were in the highest risk groups for caregiver burden (stdiff = 0.38). Across the nine disease categories, only two met our threshold for statistical significance; namely, the presence of Alzheimer’s dementia/another type of dementia (stdiff = 0.25) and cataracts (stdiff = 0.26; Table 3).
Table 3. Percent of participants who experienced a change between baseline (T1) and onset of a new impairment (T2) across the seven cohortsa
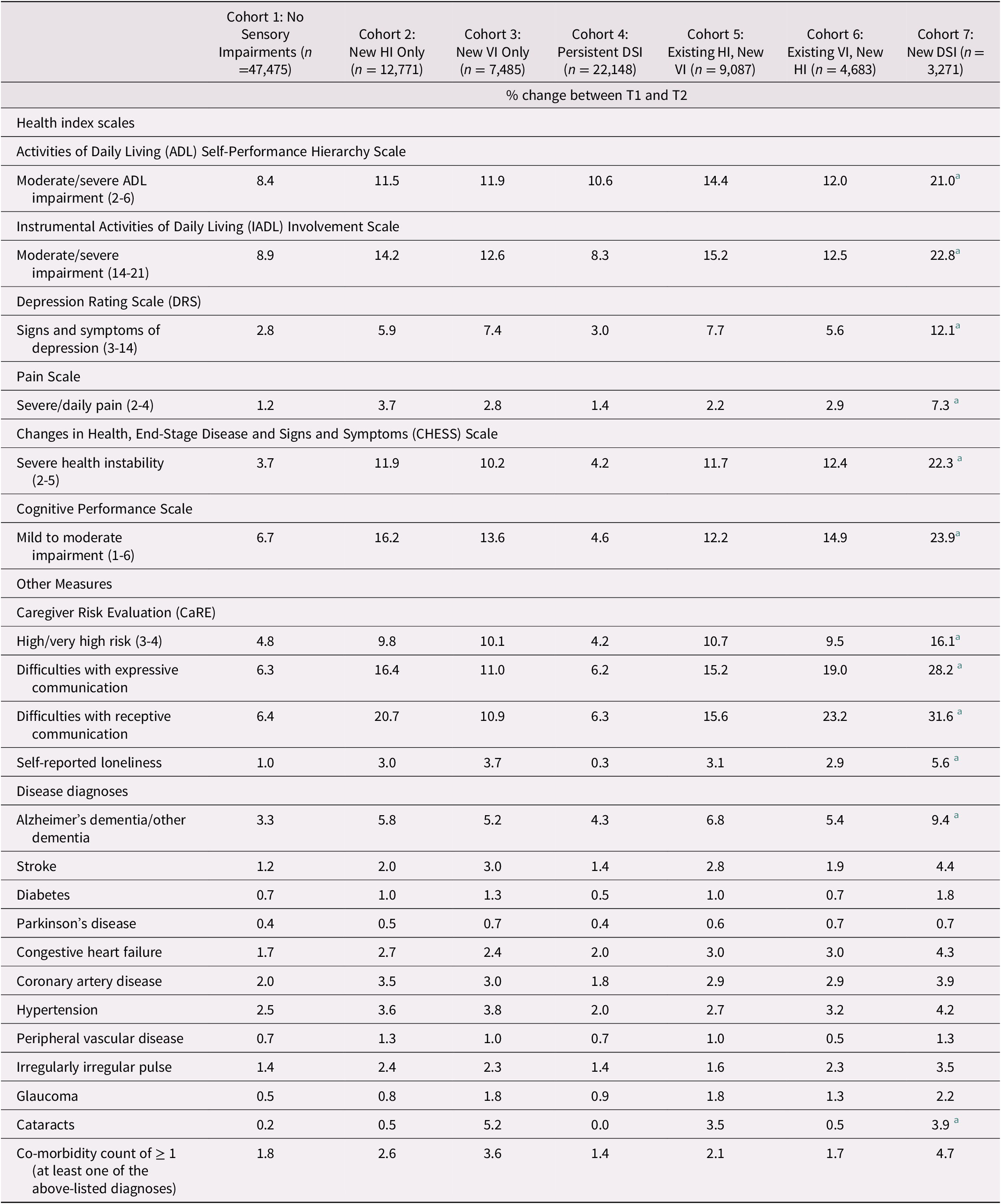
Note. Negative values in the table indicate a decrease in the proportion in that group over time.
a Standardized difference between Cohort 1 and Cohort 7 of at least 0.2 (absolute value).
HI = hearing impairment; VI = vision impairment; DSI = dual sensory impairment.
Across all seven cohorts, the prevalence of experiencing a deterioration on the CPS score was nearly 20 per cent, regardless of age, and the rate tended to increase with age within each cohort. When assessing each individual cohort, very little difference was seen with age. The exception to this was the cohort with newly identified DSI. Within this cohort, the percent experiencing a deterioration on the CPS score ranged from 41.7 per cent in the youngest group (65–69 years of age) to 64.0% in the oldest age group (≥ 95 years of age; Figure 1).
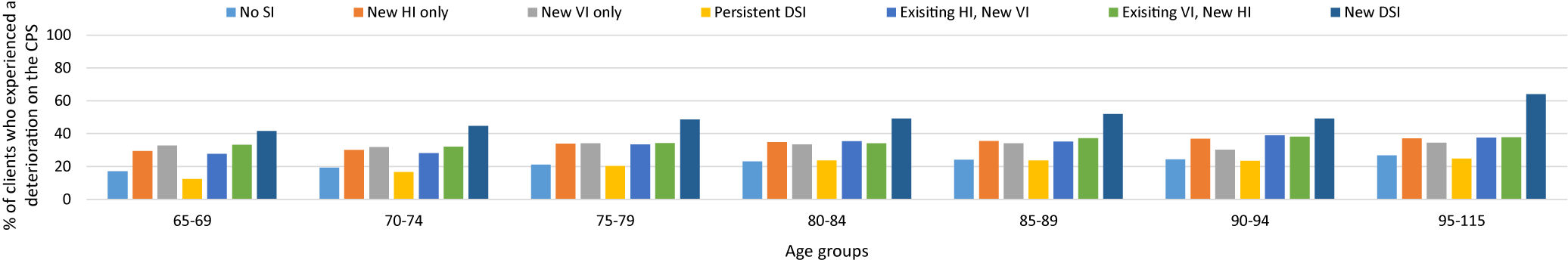
Figure 1. Individuals who experienced any deterioration on the Cognitive Performance Scale (CPS) (any one-point increase) between time 1 and time 2
A very similar pattern was observed for both expressive and receptive communication. In both types, the newly identified DSI cohort had the highest prevalence, of declines in communication, in each of the age groups. There was an absolute difference, between proportions, of roughly 10 per cent when comparing the youngest with the oldest age groups. Across most age groups, the rates were next highest in the existing HI/new VI cohort. This cohort also showed the largest difference across the age groups at 13.8 per cent for deterioration in expressive communication, and a difference of 15.1 per cent for deterioration in receptive communication. In this cohort, there was an increase in the proportion across each age cohort (Figures 2 and 3). A newly identified VI in those with an existing HI exhibited roughly the same effect on communication as a newly identified DSI.
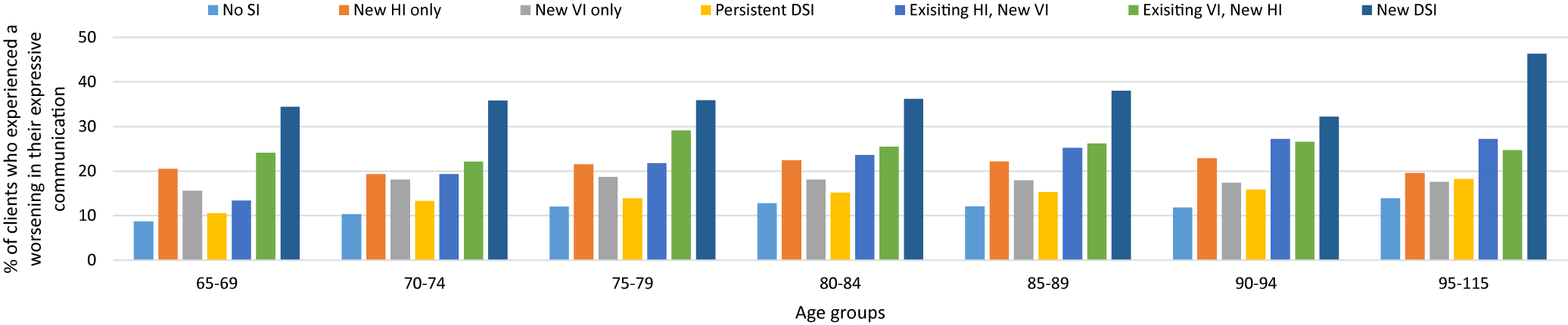
Figure 2. Comparison of the clients with and without sensory impairments who experienced a deterioration in expressive communication
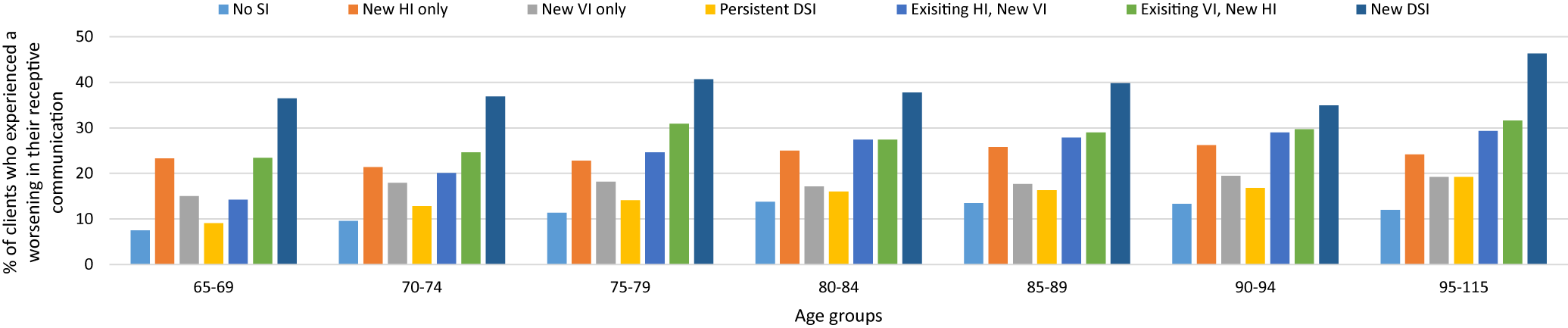
Figure 3. Comparison of clients with and without sensory impairments who experienced a deterioration in their receptive communication
The final outcome, stratified by age, was deterioration on the CaRE algorithm. The results were unremarkable except for the newly identified DSI cohort. This cohort had the highest risk of caregiver burden in all but one of the age groups. Like the other outcomes we studied, those with newly identified DSI showed the most difference when comparing the two extreme age groups, with a difference of 8.5 per cent (65–69 years of age: 16.7% vs. ≥ 95 years of age: 25.2%; Figure 4).
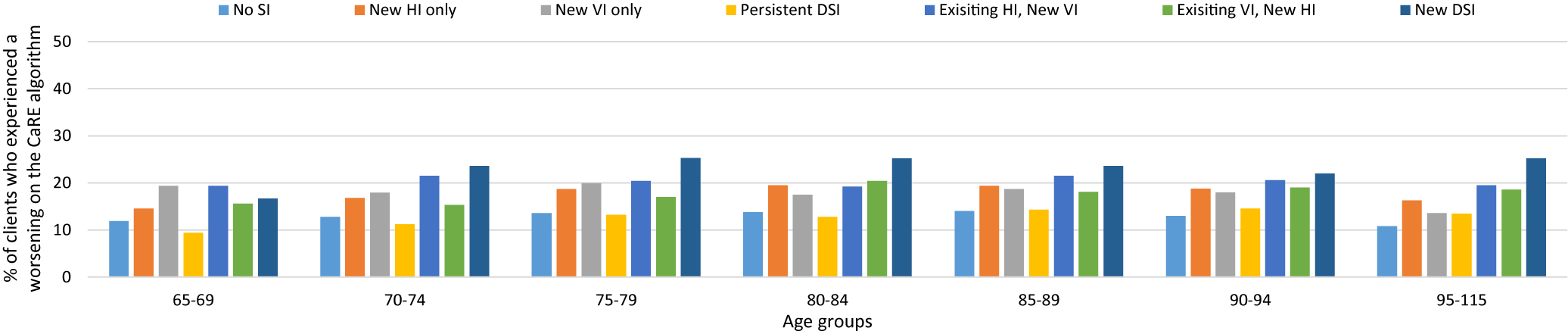
Figure 4. Comparison of clients with and without sensory impairments with a caregiver who experienced a deterioration on the Caregiver Risk Evaluation (CaRE) algorithm
Given that there was an influence of age across several of these outcomes and given the known association among age, cognitive status, and sensory functioning, we explored these outcomes using multivariate regression. After adjusting for multiple control variables, individuals with a newly identified DSI (compared with those without DSI) were significantly more likely to experience each outcome. This was particularly true for both types of communication in which the newly identified DSI cohort had at least a four-fold increase in the odds of experiencing a decline in expressive communication (odds ratio [OR] = 4.13; 95% confidence interval [CI]: 3.69, 4.62) as well as a decline in receptive communication (OR = 5.22; CI: 4.63, 5.87). The newly identified HI only cohort also had a significant increase in the odds of having communication difficulties, with a two-fold increase for a decline in receptive communication (OR = 2.61; CI: 2.43, 2.80), compared with those without a new HI. The odds were lower, but still significant, for difficulties with expressive communication (OR = 2.00; CI: 1.86, 2.15). Similar, but lower odds were also observed for the newly identified VI cohort for both a decline in receptive (OR = 1.53; CI: 1.39, 1.68) and expressive communication (OR = 1.52; CI: 1.39, 1.67). The newly identified DSI cohort also had a roughly 80 per cent increase in the risk of caregiver burden (OR = 1.81; CI: 1.60, 2.06), compared with roughly 40 per cent in the newly identified HI cohort (OR = 1.43; CI: 1.32, 1.53) and newly identified VI cohorts (OR = 1.38; CI: 1.26, 1.51). Finally, the newly identified DSI cohort was approximately three times as likely to experience a worsening on the CPS score (OR = 3.11; CI: 2.80, 3.46), and again, the odds ratios were lower in the two other single-impairment cohorts (Table 4).
Table 4. Logistic regression models for the association between newly acquired sensory impairments and the odds of the event across four unique outcomes
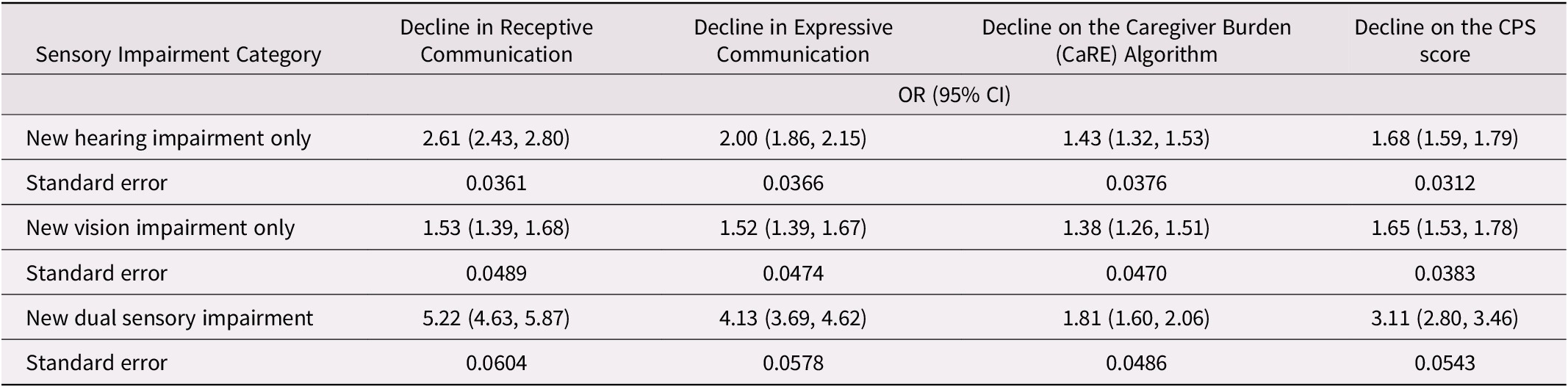
Note. All models were adjusted for age, sex, level of education, marital status, Depression Rating Scale (DRS) score of ≥ 3, co-morbidity (sum of disease diagnoses listed in Table 1) and Cognitive Performance Scale (CPS) score (only for decline in receptive communication outcome).
OR = odds ratio comparing those with the impairment to those without it; CI = confidence interval
Overall, the newly identified DSI cohort stands out as being more likely to experience multiple negative outcomes related to declines in communication, worsening on the CPS score, and an increased risk of caregiver burden, even after adjusting for many other covariates that may be potential confounders in these associations.
Discussion
The goal of the present study was to describe how a new onset of sensory impairments may influence the risk of negative outcomes such as communication difficulties, changes in cognitive performance, and caregiver burden in a group of older home care clients. In this sample of roughly 100,000 older clients who were receiving home care, 35 per cent were newly identified as having a sensory impairment between the baseline assessment and a re-assessment. The average time frame between the two assessments was approximately 12 months. This is not a trivial proportion of older adults for whom sensory challenges emerge during the delivery of home care. To our knowledge, this is one of the first Canadian studies to explore the influence of single and dual sensory impairments on multiple health-related outcomes over time. The fact that these impairments took roughly a year to be identified implies that there is a window of opportunity within which to assess the person for changes in sensory functioning and to implement strategies and provide rehabilitative services to maximize their ability to function using their residual vision and hearing. This window of opportunity also applies to family caregivers. These caregivers also require access to supportive services and strategies to maintain their own well-being while helping their loved one with the emerging sensory impairments that could exacerbate many aspects of that person’s health and wellness.
At baseline, clients with sensory impairments were more likely to experience functional impairment, health instability, to have difficulties in communication, and to experience cognitive challenges. This is in line with multiple longitudinal studies supporting HI as an important risk factor for the onset of cognitive impairment (Davies et al., Reference Davies, Cadar, Herbert, Orrell and Steptoe2017; Deal et al., Reference Deal, Betz, Yaffe, Harris, Purchase-Helzner and Satterfield2017; Fischer et al., Reference Fischer, Cruickshanks, Schubert, Pinto, Carlsson and Klein2016; Fritze et al., Reference Fritze, Teipel, Ovari, Kilimann, Witt and Doblhammer2016; Gurgel et al., Reference Gurgel, Ward, Schwartz, Norton, Foster and Tschanz2014; Lin et al., Reference Lin, Metter, O’Brien, Resnick, Zonderman and Ferrucci2011, Reference Lin, Yaffe, Xia, Xue, Harris and Purchase-Helzner2013) or a deterioration in cognitive performance (Alattar et al., Reference Alattar, Bergstrom, Laughlin, Kritz-Silverstein, Richard and Reas2020; Amieva et al., Reference Amieva, Ouvrard, Giulioli, Meillon, Rullier and Dartigues2015). A smaller number of studies likewise support VI as a risk factor for dementia or cognitive decline (Barnes et al., Reference Barnes, Cauley, Lui, Fink, McCulloch and Stone2007; Reyes‐Ortiz et al., Reference Reyes‐Ortiz, Kuo, DiNuzzo, Ray, Raji and Markides2005; Rogers & Langa, Reference Rogers and Langa2010; Zheng et al., Reference Zheng, Swenor, Christ, West, Lam and Lee2018). A limited number of studies have been published that examine DSI as a factor for cognitive decline, and all reported a positive association (Lin et al., Reference Lin, Gutierrez, Stone, Yaffe, Ensrud and Fink2004; Liu, Cohen, Fillenbaum, Burchett, & Whitson, Reference Liu, Cohen, Fillenbaum, Burchett and Whitson2016; Maharani et al., Reference Maharani, Dawes, Nazroo, Tampubolon and Pendleton2018; Yamada et al., Reference Yamada, Denkinger, Onder, Henrard, van der Roest and Finne-Soveri2015).
Regardless of the sensory cohort, and irrespective of age, roughly 20 per cent of the home care clients in the present study had a worsening in their CPS score over time. The actual change in the proportion showing this deterioration (i.e., comparing the proportion at T1 with the proportion at T2) was relatively flat, except in the newly identified DSI cohort. Although this cohort represents a relatively small proportion of the sample (3%), it represents a sample of just over 3,200 unique individuals. To our knowledge, this is larger than in most recent publications, which tend to focus on more clinical and rehabilitation populations (Roets-Merken, Zuidema, Vernooij-Dassen, & Kempen, Reference Roets-Merken, Zuidema, Vernooij-Dassen and Kempen2014; Roets-Merken et al., Reference Roets-Merken, Zuidema, Vernooij-Dassen, Teerenstra, Hermsen and Kempen2017; Schneider et al., Reference Schneider, McMahon, Gopinath, Kifley, Barton and Mitchell2014; Shakarchi et al., Reference Shakarchi, Assi, Ehrlich, Deal, Reed and Swenor2020; Urqueta Alfaro, Guthrie, McGraw, & Wittich, Reference Urqueta Alfaro, Guthrie, McGraw and Wittich2020).
Those in the new DSI cohort represent a uniquely impaired group of older adults. More so than any other cohort, they were significantly more likely to experience a deterioration in multiple areas, including communication, cognitive performance, functional abilities, and symptoms of depression. Even after adjusting for multiple control variables, this cohort had an increased risk across all four of the key outcomes measured, including the risk for caregiver burden. These results are in line with previous research showing individuals with DSI to be at increased risk of functional impairment (Smith et al., Reference Smith, Bennett and Wilson2008), depression (Capella-McDonnall, Reference Capella-McDonnall2009; Fletcher & Guthrie, Reference Fletcher and Guthrie2013; Guthrie et al., Reference Guthrie, Theriault and Davidson2015; Schneider et al., Reference Schneider, Gopinath, McMahon, Leeder, Mitchell and Wang2011) and communication difficulties (McDonnall et al., Reference McDonnall, Crudden, LeJeune, Steverson and O’Donnell2016). However, what we cannot tell from our data is exactly when the DSI was first evident, because we only know that it was identified within a specific time frame between two assessments. This is a limitation to our work, because we cannot differentiate individuals who had DSI caused by a single event (e.g., a stroke) from those for whom the onset was more gradual.
The consequences of newly identified sensory changes on caregiver burden is particularly noteworthy given the evidence that the degree of vision loss has been shown to be positively correlated with the caregivers’ perceived level of burden (Braich, Lal, Hollands, & Almeida, Reference Braich, Lal, Hollands and Almeida2012; Khan et al., Reference Khan, Braich, Rahim, Rayat, Xing and Iqbal2016). Three other studies also found a relationship between more severe vision loss and the risk of depression in the caregiver (Bambara et al., Reference Bambara, Owsley, Wadley, Martin, Porter and Dreer2009; Braich et al., Reference Braich, Lal, Hollands and Almeida2012, Reference Braich, Jackson, Knohl, Bhoiwala, Gandham and Almeida2016). A single Canadian study, the only one we found looking at hearing loss, reported that higher caregiver burden was experienced by caregivers who cared for someone without HI than by caregivers of someone with difficulties in their hearing (Westaway, Wittich, & Overbury, Reference Westaway, Wittich and Overbury2010). The authors suggest that this finding may reflect the fact that all participants came from a rehabilitation centre, and participants with a hearing loss were all receiving rehabilitation services. The receipt of these services would likely have benefited both the individuals and their caregivers, thus reducing the level of stress or burden experienced by these caregivers. We did not find any research looking at DSI and caregiver well-being, one of the many areas in need of further research (Wittich, Jarry, Groulx, Southall, & Gagné, Reference Wittich, Jarry, Groulx, Southall and Gagné2016).
This work has several limitations. The RAI-HC is based on subjective evaluations of vision, hearing and cognition. The assessment can draw on behavioral test results if they are included in the medical chart. The RAI-HC also captures information about functioning in these domains; however, it cannot quantify these impairments objectively. The assessment does not capture the use of assistive devices (e.g., glasses or hearing aids) or the history of rehabilitation services that have been accessed, thereby limiting our ability to understand how sensory rehabilitation and accommodation for sensory disabilities might influence the rate of change in cognition or communication. In addition, we cannot determine the temporal sequence between the onset of cognitive challenges and the onset of sensory impairments. It is possible that some individuals with a new sensory challenge had a change in their cognitive functioning that preceded the changes in sensory functioning. Given that there was a year, on average, between the initial assessment and the identification of the sensory impairment, changes in cognition may have taken place during this time, which could have influenced some of our study outcomes. We attempted to adjust for this in our multivariate models, but there remains the possibility of residual confounding based on other unmeasured factors.
Despite these limitations, a standardized assessment, like the RAI-HC, is vital to create real-time information with which clinicians can develop a person-centred care plan and respond quickly to ongoing changes for the person and their family. Approximately one third of home care clients in Ontario are likely to be identified as having new sensory challenges over time, with an associated deterioration in their communication and cognitive performance. Although our results focus on data from Ontario, multiple other provinces (e.g., Alberta, British Columbia, Nova Scotia, Manitoba) also use the RAI-HC, making our findings relevant to these other regions as well.
Clinicians within this sector are clearly working with a complex group of individuals who have multiple health conditions and unique needs. Hearing impairment is an important risk factor in the development of cognitive challenges (Lin & Albert, Reference Lin and Albert2014). Although screening for hearing and vision impairment is important, it is recognized that effective sensory interventions (e.g., assistive technologies, vision/hearing rehabilitation) and contact with health care professionals are often under-utilized strategies (Davis et al., Reference Davis, McMahon, Pichora-Fuller, Russ, Lin and Olusanya2016; Klaver, Wolfs, Vingerling, Hofman, & de Jong, Reference Klaver, Wolfs, Vingerling, Hofman and de Jong1998; Lin, Thorpe, Gordon-Salant, & Ferrucci, Reference Lin, Thorpe, Gordon-Salant and Ferrucci2011; Overbury & Wittich, Reference Overbury and Wittich2011). This may be partly explained by a lack of knowledge surrounding screening among those with cognitive challenges (McGilton et al., Reference McGilton, Hobler, Campos, Dupuis, Labreche and Guthrie2016). Consequently, there is an ongoing requirement for better training and education for home care clinicians to be able to effectively screen for these impairments and put in place a plan of care and/or referrals to maximize the person’s functional abilities and use of their residual vision and hearing. Earlier identification and care planning to accommodate sensory impairments would enable these clients to maximize their independence and quality of life.
Funding
This work was supported by the Canadian Consortium on Neurodegeneration in Aging (CCNA) (http://ccna-ccnv.ca/en) through funding to all authors. The CCNA is supported by a grant from the Canadian Institutes of Health Research (CIHR) (http://www.cihr-irsc.gc.ca/e/193.html) with funding from several partners including the Alzheimer Society of Canada, Alberta Innovates, Brain Canada Foundation, and Canadian Nurses Foundation. W.W. is supported by a Junior 1 Career Grant from the FRQ-S (# 28881 & 30620; http://www.frqs.gouv.qc.ca/en/).