Introduction
The Spent Fuel and Waste Disposition program of the U.S. Department of Energy (DOE) aims to investigate the design and safety function of deep geological nuclear waste repositories in a variety of host-rock settings. Proposed repository designs vary but typically include a metal canister surrounded by bentonite backfill, and emplaced within a host rock, referred to as the engineered barrier system (EBS) (Fig. 1). Potential candidate host-rock formations for the repository include crystalline (e.g. granite), sedimentary (e.g. argillite/clay rock), and salt. This experimental study is focused on an argillite-hosted repository, and the interaction of Opalinus Clay (argillite wall rock) and Wyoming bentonite (clay barrier backfill).

Fig. 1. Schematic of a generic engineered barrier system concept in argillite host rock. Bentonite blocks surround a waste canister emplaced in a horizontal tunnel.
The proposed components of the repository all have favorable properties for isolating radioactive material at ambient temperatures and pressures and under dry conditions. The U.S., however, is exploring disposing of spent nuclear fuel (SNF) assemblies (e.g. pressurized water reactors, PWRs) in configurations that may result in significant increases in repository temperatures. Modeling of the thermal evolution of a 32-PWR (60 gigawatt-days per metric ton burnup) SNF waste package emplaced within a shale host rock (25 year ventilation; 15 m package spacing) indicates the potential for canister surface temperatures to reach 299°C after 85 y (Greenberg et al. Reference Greenberg, Wen and Buscheck2013). At these elevated temperatures and in the presence of water, irreversible mineralogical changes within the bentonite backfill and at the wall rock–bentonite interface will occur during peak thermal conditions.
Many studies have focused on the long-term stability of candidate geologic materials used in disposal concepts including several large-scale, multi-decade, in situ heater tests (e.g. FEBEX at the Grimsel Test Site (Martin et al. Reference Martin, Barcala and Huertas2006) and Prototype Repository at the Äspö Hard Rock Laboratory (Johannesson et al. Reference Johannesson, Börgesson, Goudarzi, Sandén, Gunnarsson and Svemar2007)). Full-scale heater tests provide valuable information about post-closure physical, geochemical, and mineralogical changes in the repository system (e.g. Dohrmann et al. Reference Dohrmann, Kaufhold and Lundqvist2013; Dohrmann and Kaufhold Reference Dohrmann and Kaufhold2014; Fernandez et al. Reference Fernández, Kaufhold, Sánchez-Ledesma, Rey, Melón, Robredo, Fernández, Labajo and Clavero2018; Hadi et al. Reference Hadi, Wersin and Serneels2019). The completed full-scale studies to date have focused on temperatures <150°C, limiting comparison to disposal associated with a higher thermal load. Modeling studies characterize the mineralogical evolution of the repository system, but may not fully capture complex, coupled, mineral reactions in the engineered system (e.g. Zheng et al. Reference Zheng, Rutqvist, Birkholzer and Liu2015). Experimental studies, including the present study, can provide insight into key mineralogical and geochemical changes in a simplified system that can be utilized in models to understand long-term repository function and introduce hypotheses that can be tested in full-scale experiments.
The overall objective of the present study was to assess the long-term performance of engineered barrier materials in a high-temperature, argillite-hosted nuclear waste repository. Material transformations at temperatures of 200–300°C and pressures of 150 bar were tested in seven long-term (6-week to 6-month) hydrothermal experiments using Opalinus Clay from Mont Terri, Switzerland, and Wyoming bentonite from Colony, Wyoming. Further objectives were to select experimental conditions (e.g. fully saturated conditions) that encouraged mineral reactions to reach near steady state over the experiment duration, to characterize the reaction product mineralogy and geochemistry in order to identify clay-mineral and zeolite-forming reactions that are of concern for bentonite stability, and to uncover any links between aqueous geochemical changes and the mineral reactions.
BACKGROUND
Wyoming Bentonite
Bentonite has long been proposed as an EBS material for nuclear waste due to its availability and large clay mineral content (e.g. Pusch Reference Pusch1979). Clay minerals provide a chemical barrier that can attenuate radionuclide migration and a physical barrier due to the ability of expansive clay to swell, seal cracks, and preclude groundwater infiltration (Pusch Reference Pusch1979; Dohrmann et al. Reference Dohrmann, Kaufhold and Lundqvist2013; Sellin and Leupin Reference Sellin and Leupin2014). The bentonite used in the present study was provided by Bentonite Performance Minerals LLC from Colony, Wyoming, U.S.A. and is composed dominantly of Na-montmorillonite (general composition: Na0.33(Al,Mg)2(Si4O10)(OH)2·nH2O), lesser clinoptilolite and feldspar, and minor biotite, pyrite, quartz, and opal. Under dry conditions, the bentonite mineral assemblage may be stable to over 350°C (Wersin et al. Reference Wersin, Johnson and McKinley2007); however, moisture is likely to be present in the natural geologic environment. Under water-saturated conditions and temperatures >100°C, alteration of clay minerals may occur (e.g. Mosser-Ruck et al. Reference Mosser-Ruck, Cathelineau, Guillaume, Charpentier, Rousset and Barres2010; Ferrage et al. Reference Ferrage, Vidal, Mosser-Ruck, Cathelineau and Cuadros2011; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014). Both laboratory and in situ (full-scale) experiments can provide insight into the stability of bentonite at repository conditions and are, therefore, important in assessing the long-term function of a nuclear waste disposal site.
Mineralogical changes within the bentonite EBS materials that affect the ability of smectite to expand are a primary concern for the long-term function of a nuclear waste repository. The reduction of swelling capacity of smectite, due to the formation of non-swelling clays, cementation of smectite lamellae caused by silica precipitation, and/or recrystallization to other mineral phases (e.g. zeolites), is believed to be one of the greatest risks to the repository stability and isolation capability compared to other mineral reactions (Pusch et al. Reference Pusch, Takase and Benbow1998; Pusch and Kasbohm Reference Pusch and Kasbohm2002). Previous laboratory-scale investigations have investigated clay mineral transformations relevant to EBS systems over a wide range of repository temperatures (i.e. ~25 to 300°C), alone and in contact with metals that approximate potential canister materials (Madsen Reference Madsen1998; Meunier et al. Reference Meunier, Velde and Griffault1998; Guillaume et al. Reference Guillaume, Neaman, Cathelineau, Mosser-Ruck, Peiffert and Abdelmoula2003; Hofmann et al. Reference Hofmann, Bauer and Warr2004; Wersin et al. Reference Wersin, Johnson and McKinley2007; Mosser-Ruck et al. Reference Mosser-Ruck, Cathelineau, Guillaume, Charpentier, Rousset and Barres2010; Ferrage et al. Reference Ferrage, Vidal, Mosser-Ruck, Cathelineau and Cuadros2011; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014).
The reduction of swelling capacity of montmorillonite may be due dominantly to the formation of non-swelling clays (e.g. illite, K(Al,Mg,Fe)2(Si,Al)4O10[(OH)2]) (Wersin et al. Reference Wersin, Johnson and McKinley2007). For example, in experimental systems where K+ was reacted with bentonite, the formation of non-swelling K+ rich, collapsed layer smectite and/or illite was observed (e.g. Mosser-Ruck et al. Reference Mosser-Ruck, Cathelineau, Baronnet and Trouiller1999; Kaufhold and Dohrmann Reference Kaufhold and Dohrmann2010; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014). In the alteration of montmorillonite to illite, silica is liberated through the generalized reaction:
The low availability of K+ and silica saturation in the system may limit illitization (Pusch and Madsen Reference Pusch and Madsen1995; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014; Savage et al. Reference Savage, Wilson, Benbow, Sasamoto, Oda and Walker2019). For example, in bentonite systems reacted with NaCl solutions, montmorillonite structural alteration is not observed (Kaufhold and Dohrmann Reference Kaufhold and Dohrmann2009; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) in comparison to experiments in a K+ rich environment (Kaufhold and Dohrmann Reference Kaufhold and Dohrmann2010).
The influence of bulk chemistry on clay-mineral reactions is observed in full-scale experiments. In the full-scale Prospective Repository experiment at the Äspö Hard Rock Laboratory in Sweden, cation exchange was observed in the smectite, but no structural changes were observed in the bentonite blocks after a period of eight years at temperatures between 60 and 85°C (Dohrmann and Kaufhold Reference Dohrmann and Kaufhold2014). In the FEBEX experiment, smectite alteration was observed only close to the heater surface (100°C) and included recrystallization to saponite and chlorite and a decrease in cation exchange capacity and surface area (Fernandez et al. Reference Fernández, Kaufhold, Sánchez-Ledesma, Rey, Melón, Robredo, Fernández, Labajo and Clavero2018).
Zeolite formation within bentonite EBS material has also been reported (e.g. Mosser-Ruck et al. Reference Mosser-Ruck, Cathelineau, Guillaume, Charpentier, Rousset and Barres2010; Ferrage et al. Reference Ferrage, Vidal, Mosser-Ruck, Cathelineau and Cuadros2011; Cheshire et al. Reference Cheshire, Caporuscio, Jové-Colón and McCarney2013, Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014; Mosser-Ruck et al. Reference Mosser-Ruck, Pignatelli, Bourdelle, Abdelmoula, Barres and Guillaume2016). Zeolites may form as a result of clinoptilolite dissolution under silica-saturated conditions (Cheshire et al. Reference Cheshire, Caporuscio, Jové-Colón and McCarney2013, Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) or clay-mineral reactions (Mosser-Ruck et al. Reference Mosser-Ruck, Cathelineau, Guillaume, Charpentier, Rousset and Barres2010). Dissolution of clinoptilolite, which makes up roughly 13% of the Wyoming bentonite used in this study, and precipitation of analcime may result in a slight volume loss within the bentonite buffer (Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014).
Opalinus Clay
Opalinus Clay used in the present study was sourced from the Mont Terri Underground Rock Laboratory in Canton Jura, northern Switzerland. The rock used was from the shaley facies of Opalinus Clay at Mont Terri (drill core BFE-A10) and was exposed to air (i.e. oxidizing conditions) before the experiment. Opalinus Clay is considered to be a favorable medium for a repository based on its high clay content, low permeability, high sorption potential for radionuclides, and crack-sealing properties (NAGRA 2002; Bossart and Thury Reference Bossart and Thury2008; Bossart and Milnes Reference Bossart and Milnes2017). Several experimental studies have examined the mineralogical and chemical evolution of Opalinus Clay at temperatures below 200°C. The interaction of high-pH fluids and Opalinus Clay had been evaluated at ambient (e.g. Adler et al. Reference Adler, Mäder and Waber1999; Taubald et al. Reference Taubald, Bauer, Schäfer, Geckeis, Satir and Kim2000) and elevated (e.g. 90–200°C; Honty et al. Reference Honty, Wang, Osacký, Uhlík, Czímerová and Madejová2012; Chermak Reference Chermak1992) temperatures. The presence of cement and/or high-pH solutions at ambient temperatures (25°C) results in the dissolution of the precursor chlorite in the Opalinus Clay (Taubald et al. Reference Taubald, Bauer, Schäfer, Geckeis, Satir and Kim2000) and the formation of Ca-zeolites and calcium aluminum silicate hydrate minerals (Alder et al. 1999). At higher temperature (150–200°C) and similar pH, the formation of analcime, vermiculite, and Na-rectorite was observed within powdered Opalinus Clay (Chermak Reference Chermak1992). In situ EBS experiments at the Mont Terri underground research laboratory in Opalinus Clay are in progress (HE-E, up to 140°C; Wieczorek et al. Reference Wieczorek, Gaus, Mayor, Schuster, García-Siñeriz and Sakaki2017; FE, up to 150°C; Müller et al. Reference Müller, Bossart and Milnes2018).
METHODS
Experimental
No previous experiments have been performed on the interaction of Opalinus Clay and Wyoming bentonite at elevated temperatures and pressures. Three systems were tested under water-saturated conditions: (1) Opalinus Clay only, (2) 80 wt.% bentonite with 20 wt.% Opalinus Clay, and (3) 50 wt.% all on the same line Opalinus Clay and 50 wt.% Wyoming bentonite (Table 1). The Opalinus Clay-only experiments provide a baseline for mineralogical reactions that occur at 300°C. The 20% and 50% Opalinus Clay experiments aimed to represent the interface zone between repository host rock and bentonite in order to characterize any host-rock–backfill interactions (Fig. 2). Some experiments included metal coupons to mimic the presence of a spent fuel waste canister. These experiments build on previous work by Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) that included Wyoming bentonite-only hydrothermal experiments. Experiments were conducted at either 200 or 300°C to characterize a range of systems based on various maximum temperatures in generic repository designs. Experiment duration was varied to observe kinetic effects and/or to evaluate the attainment of steady state under the experimental conditions.
Table 1. Experiment run components and conditions.
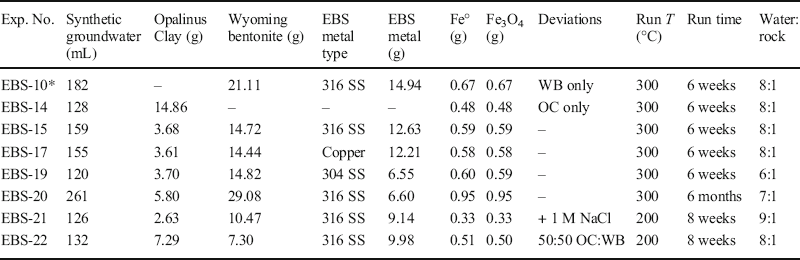
*EBS-10 is from Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) and reported here for comparison

Fig. 2. Schematic of the experimental set up (not to scale).
The Opalinus Clay and Wyoming bentonite were pulverized to <5 mm granules and sieved to <2 mm and >2 mm fractions. A synthetic groundwater solution was prepared to mimic the groundwater composition in equilibrium with Opalinus Clay (Pearson Reference Pearson2002; NAGRA 2002) using reagent-grade salts dissolved in double deionized water. This synthetic groundwater had a pH of ~7.5 and the initial chemistry is reported in Table 2. Additional 1 M (~22.9 g/L) NaCl solution (see below) was added to the Opalinus Clay solution in experiment EBS-21 to observe the influence of a saline brine.
Table 2. Brine compositions including the type solution for Opalinus Clay porewater from the Mont Terri site (Pearson Reference Pearson2002) and the composition of the synthetic solution created and used in the hydrothermal experiments.

Solutions were added at between 6:1 and 9:1 water:rock mass ratios. The redox conditions for each experiment were buffered using a 1:1 mixture (by mass) of Fe3O4 and Fe° filings that comprised ~0.5–0.9% of the total mass of the solid and liquid reactants. Coupons of 304 >stainless steel (NIST SRM 101g), 316 stainless steel (NIST SRM 160b), low-carbon steel (provided by Sandia National Laboratory), or Cu-foil (experiment EBS-17) were added to all experiments, except EBS-14. The mass of the metal pieces comprised ~4–7 wt.% of all reactants and were added in order to observe any mineral alteration and corrosion at the bentonite–coupon surface interface. Reactants (Table 1) were loaded into a flexible gold reaction cell and fixed into a 500 mL gasket-sealed reactor (Seyfried Reference Seyfried1987) (Fig. 2). Experiments were pressurized to ~150 bar (mimicking hydrostatic pressures for a shallow repository) and were heated to either 200°C or 300°C for various lengths of time. Four 300°C experiments (EBS-14, EBS-15, EBS-17, and EBS-19) were run for 6 weeks and two 200°C experiments (EBS-21 and EBS-22) were run for eight weeks. One 300°C experiment (EBS-20) was run for 6 months.
Solid reaction products were extracted post experiment and dried at room temperature under oxidizing conditions. Metal coupons were extracted from the clay and rock components. Corrosion and metal alteration phases were observed locally on the coupon surfaces, but did not extend more than ~50 μm perpendicular to the coupon surface. Corrosion and steel alteration results will be discussed in a separate article.
Characterization
Aqueous chemistry. Reaction liquids were sampled (4–5 g per sampling) weekly during the 6–8 week experiments and bi-monthly during the 6 month experiments. Fluid samples from the experiments were extracted in airtight syringes and often included gas phases. The gas phases were likely CO2-dominated based on the lack of sulfur smell and the experimental components. In contact with ambient laboratory conditions, the reaction fluids quickly reached 25°C; salt precipitation was not observed during fluid cooling. An aliquot of the reaction fluids was used to measure immediately the pH at 25°C using a Thermo Orion 4 Star pH probe (Thermo, Waltham, Massachusetts, USA). The reaction liquids were divided into aliquots analyzed for unfiltered anion, unfiltered cation, and filtered (0.45 mm syringe filter) cation determination. All aliquots were stored in polytetrafluoroethylene vials and stored at 1°C before analysis.
Major cations and trace metals were analyzed via inductively coupled plasma-optical emission spectrometry (ICP-OES) (Optima 2100 DV, Perkin Elmer, Waltham, Massachusetts, USA) and inductively coupled plasma-mass spectrometry (ICP-MS) (Elan 6100, Perkin Elmer, Waltham, Massachusetts, USA) utilizing EPA methods 200.7 and 200.8 at Los Alamos National Laboratory. Ultra-high purity nitric acid was used in sample and calibration preparation prior to sample analysis. Internal standards (Sc for ICP-OES and Bi, In, and Y for ICP-MS) were added to samples and standards to correct for matrix effects. Standard Reference Material (SRM) 1643e Trace Elements in Water was used to check the accuracy of the multi-element calibrations. Inorganic anion samples were analyzed by ion chromatography (IC) following EPA method 300 on a Dionex DX-600 system (Thermo, Waltham, Massachusetts, USA). Aqueous chemical results are presented in Appendix 1. Typical 2σ uncertainties for the aqueous chemistry results are less than < ~5%. Geochemical evaluation of aqueous species activities and mineral saturation states were performed using The Geochemist’s Workbench v.12 and EQ3/6 (Wolery and Jarek Reference Wolery and Jarek2003) software using the V8.R6+ and a modified version of the YMP thermodynamic database (Wolery and Jove Colon Reference Wolery and Jové Colón2007). The geochemical evaluation was conducted using filtered cation and anion concentration data. Equilibrium aqueous speciation calculations were conducted by initially computing aqueous speciation of quench experimental fluids at 25°C followed by recalculation at the experimental temperatures (200–300°C). Speciation calculations assumed equilibrium with pCO2 of 10–3.5 bars and charge balancing on Cl–. Calculations for solution pH and silica activity can be found in Appendix 3.
Scanning electron microscopy. Reaction products from each experiment were imaged using a FEITM Inspect F scanning electron microscope (SEM) (FEI, Hillsboro, Oregon, USA) at Los Alamos National Laboratory. All samples were either Au- or C-coated prior to SEM analysis. Imaging with the SEM was performed using a 5.0–10.0 kV accelerating voltage and 1.5–3.0 nm spot size. Qualitative energy dispersive X-ray spectroscopy (EDX) for mineral identification was performed at 30 kV and a 3.0–5.0 nm spot size using an EDAX Octane Elite silicon drift detector (AMETEK, Berwyn, Pennsylvania, USA).
X-ray diffraction (XRD). The mineralogy of the starting materials and bulk reaction products was determined by quantitative X-ray diffraction (QXRD). Only the metal coupons (if present) were removed before the reaction product samples were analyzed — the reacted Wyoming bentonite and Opalinus Clay fractions were analyzed together for the powder XRD analysis for EBS-15 through EBS-22. Samples were ground with 20 wt.% corundum (Al2O3) for analysis of the bulk rock (Chung Reference Chung1974). Powder mounts were prepared for QXRD measurements, which were conducted using a Siemens D500 diffractometer (Siemens, Munich, Germany) with CuKα radiation. Data were collected from 2 to 70°2θ with a 0.02°2θ step size and count times of 8 to 12 s per step. QXRD data were determined using FULLPAT (Chipera and Bish Reference Chipera and Bish2002) (Table 3). Typical 2σ uncertainties of the FULLPAT method were ~3.4% (Chipera and Bish Reference Chipera and Bish2002).
Table 3. Quantitative X-ray diffraction (XRD) results from the starting materials and the bulk reaction products (only stainless steel coupons were removed before sample powdering).

*Representative compositions for the starting mixtures of Wyoming bentonite (WB) and Opalinus Clay (OC) are calculated from data at either an 80:20 or a 50:50 ratio.
The clay mineralogies of the reacted Wyoming bentonite and Opalinus Clay fractions were also characterized. Discrete Opalinus Clay shale fragments (~1–5 mm) were separated manually from the fine-grained clay matrix composed of mostly Wyoming bentonite. The clay matrix was dominantly Wyoming bentonite, but may have contained trace amounts of Opalinus Clay powder. The Opalinus Clay fragments were sonicated in DI water to remove clay groundmass material before crushing. The separate sample fractions of Wyoming bentonite and Opalinus Clay fragments were crushed gently by hand in an agate mortar and pestle. The <2 μm particles from each sample were separated via sedimentation in deionized (DI) H2O. A portion of the <2 μm suspension was dropped on a zero-background quartz plate, dried on a hot plate at 60°C to create an oriented clay mount, and X-rayed from 2 to 40°2θ angle with a step size of 0.02 at 8 to 12 s per step. The oriented clay mount was then saturated with ethylene glycol in a 60°C oven for 24 h and the XRD analysis was repeated.
Electron microprobe analyses. Electron microprobe (EMP) analyses were performed at the University of Oklahoma using a Cameca SX50 electron microprobe (CAMECA, Gennevilliers, France) equipped with five wavelength-dispersive spectrometers and a PGT PRISM 2000 energy-dispersive X-ray detector. Quantitative analysis was performed by wavelength-dispersive spectrometry using 20 kV accelerating voltage, 20 nA beam current, and 2 μm spot size. Matrix corrections employed the PAP algorithm (Pouchou and Pichoir Reference Pouchou and Pichoir1984), with oxygen content calculated by stoichiometry. Counting times were 30 s on peak for all elements, yielding minimum levels of detection (calculated at 3σ above mean background) in the range of 0.01 to 0.03 wt.% of the oxides for all components except F (0.16 wt.%). All standards for elements in the silicates were analyzed using 30 s count times on the peak, using K-alpha emissions. Mineral standard information and oxide detection limits can be found in Appendix 2. Typical uncertainties for the EMP analyses were <1% based on long-term standard reproducibility.
Results
Aqueous Chemistry
The experimental parameters that likely had the greatest effect on the evolution of the solution chemistry were salinity, temperature, and water:rock ratio. The 200 and 300°C experiments started with a near neutral pH (25°C) of 7.5 (Table 2). The solution pH for all experiments generally dropped within the first three weeks (Fig. 3). The 200°C experiments ended with pH values at 25°C between 5.5 and 6. The 300°C experiments with a water:rock ratio between 7:1 and 8:1 (EBS-14, EBS-15, EBS-17, EBS-20) reached pH (25°C) values between 5 and 5.5 (Fig. 3). Experiment EBS-19 had a water:rock ratio of ~6:1; solution chemistry evolved at slightly higher pH values (pH ~6) in comparison to the other 300°C runs (Fig. 3). The recalculated pH values at 300°C were between 4.3 and 5.7 and at 200°C values were ~5.5. Plots for cation and anion concentrations can be found in the Supplementary Materials. As expected, the addition of 1 M NaCl to the starting solution of EBS-21 resulted in much higher [Na+] and [Cl–] in comparison to the other experiments. Further, [Ca2+], [SO4 2–], [Fe2+], and [Al3+] are elevated in comparison to all other results. Aqueous silica remained below detection in EBS-21.
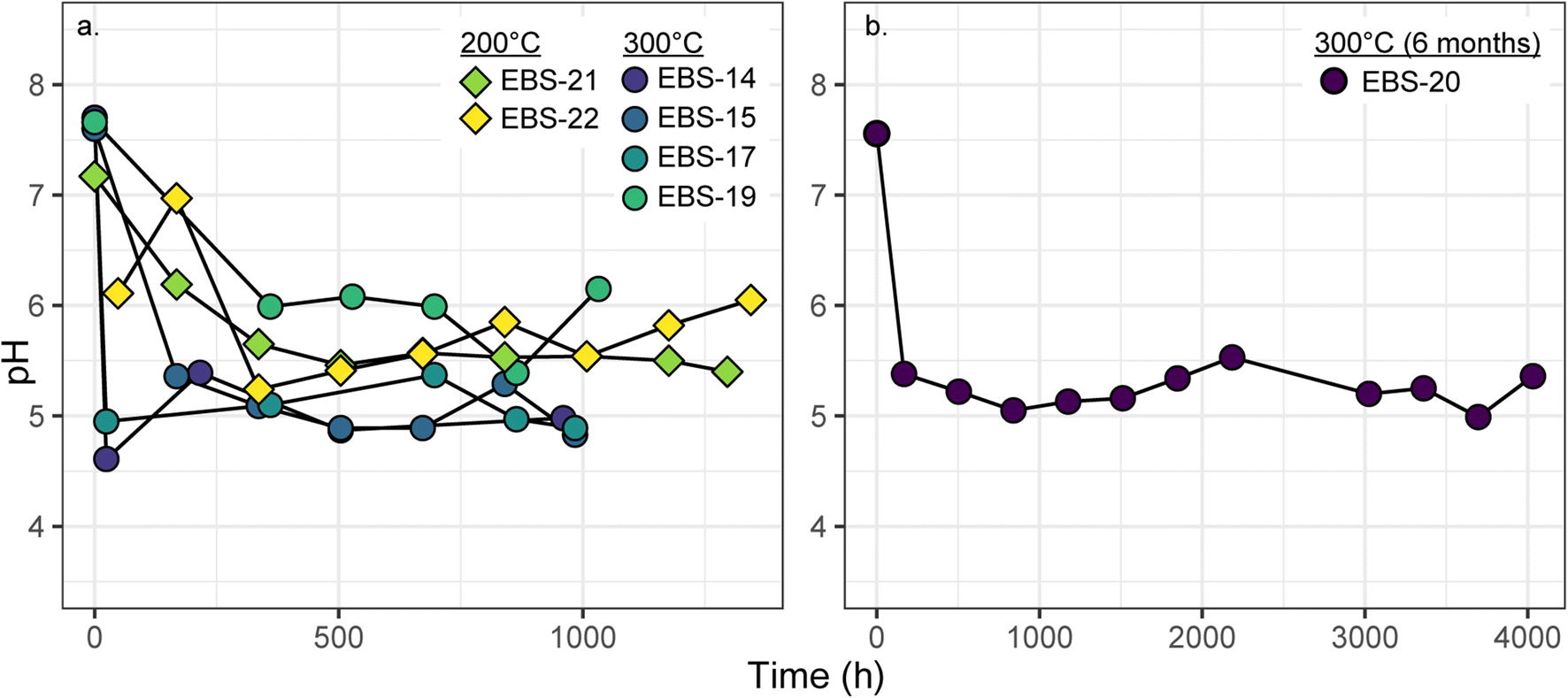
Fig. 3. pH (at 25°C) evolution of a 6–8-week and b 6-month experiments.
Sodium and potassium concentrations in the 300°C experiments and EBS-22 covered a wide range of values. Variation between experiments is not readily explainable by differing experiment materials or water:rock ratios. For example, the sodium concentrations remained near initial groundwater concentrations of 4000 mg/L in EBS-14, EBS-17, EBS-20, and EBS-22. Sodium concentrations decreased to ~2500 mg/L in EBS-15, but increased to ~7500 mg/L in EBS-19. Increased cation concentrations in EBS-19 may be due to a lower water:rock ratio (6:1); however, EBS-15 and EBS-17 had the same starting components and water:rock ratio.
Calcium concentrations decreased with respect to starting solution values (426 mg/L) in experiments with an Opalinus Clay–Wyoming bentonite mixture to values between 100 and 200 mg/L. In comparison, in the Opalinus Clay-only experiment (EBS-14), Ca2+ concentrations increased to ~550 mg/L all on the same line mg/L by the end of the experiment.
Dissolved silica concentrations increased rapidly to >100 mg/L from starting solution values of 2 mg/L by the first sample extraction in all experiments except EBS-21. Aqueous SiO2 concentrations in all the 300°C experiments remained generally constant between 100 and 200 mg/L throughout the duration of the experiment. In EBS-22, aqueous silica reached between 300 and 400 mg/L after 200 h of experiment time.
Iron and aluminum concentrations in EBS-15 through EBS-20 generally increased. Aluminum [Al3+] concentrations were below detection limits in EBS-14 and EBS-22. The 300°C Opalinus Clay and Wyoming bentonite experiments at 300°C (EBS-15 through 20) showed [Al3+] below ~0.5 mg/L. Iron concentrations generally remained below 0.5 mg/L during the experiments, with the exception of EBS-21 (saline-brine experiment). In contrast, Mg decreased during all experiments from starting concentrations of ~200 mg/L to <5 mg/L.
Quantitative X-ray Diffraction (QXRD)
The QXRD results show that unreacted Opalinus Clay was composed dominantly of illite, illite-smectite, kaolinite, calcite, quartz, chlorite, and biotite with lesser feldspar (plagioclase and potassium feldspar) and minor dolomite, pyrite, and siderite (Fig. 4, Table 3). The starting Wyoming bentonite was dominantly smectite (Na montmorillonite), with lesser clinoptilolite and plagioclase, and minor quartz, pyrite, and biotite. Powder XRD patterns for unheated Opalinus Clay and the Opalinus Clay–Wyoming bentonite mixtures are plotted in Fig. 4.

Fig. 4. Powder XRD patterns of the bulk reaction products and starting Wyoming bentonite (WB) used for calculating mineral abundances. Peaks corresponding to smectite (S), clinoptilolite (cpt), analcime (a), feldspar (feld), illite-smectite (I-S), chlorite (chl), illite (I), kaolinite (kao), quartz (Q), calcite (cal), halite (h), and corundum (c) (internal standard) are labeled.
The mineral groups present in significant amounts (>5 wt.%) in all of the reaction products included clays, feldspars, zeolites, and/or phyllosilicates, with lesser SiO2 polymorphs and/or carbonates. Other minerals identified to make up <5% in all samples included gypsum, halite, K-feldspar, magnetite, and/or pyrite. Native iron, added as a part of the iron-magnetite oxygen fugacity buffer, was observed in sample thin sections and SEM images, but was not detected in the QXRD results, likely due to its low abundance in the bulk mineralogy.
Clay minerals were the most abundant phase in all of the reaction products and were a combination of illite, smectite, mixed illite-smectite, chlorite, mica, and/or kaolinite. Illite, interstratified illite-smectite, and smectite were grouped together due to the difficulty of distinguishing between these phases in the powder XRD patterns (Table 3). Combined illite, illite-smectite, and smectite ranged between 57 and 76 wt.% in the reaction products. Mica was observed to be 1–11 wt.% and chlorite was 1–8 wt.%, whereas kaolinite was observed in EBS-14 (1 wt.%) and EBS-22 (6 wt.%).
The QXRD results indicated the presence of plagioclase and/or K-feldspar in all experiment products. For all of the experiments, the reaction products had ~1–2 wt.% K-feldspar. Plagioclase feldspar made up between ~2 and 12 wt.%. Quartz made up between 3 and 7 wt.% of the reaction products.
Zeolite minerals identified in the reaction products were dominantly clinoptilolite, with lesser analcime–wairakite. Clinoptilolite was not present in EBS-14 (pure Opalinus Clay), but comprised ~8–12 wt.% of EBS-15, EBS-17, and EBS-19 samples. Samples from EBS-20 through EBS-22 had ~3–6 wt.% clinoptilolite. Analcime–wairakite solid solution was detected in 300°C experiments (EBS-14 through EBS-20; ~0.3-2.9 wt.%), but was not observed in the 200°C reaction products (EBS-21 and EBS-22).
The powder XRD patterns highlight changes in the bulk, reaction-product mineralogy in comparison to the patterns from the starting materials (Fig. 4). The run-product mineralogy of EBS-14 (Opalinus Clay only, 300°C) compared to unheated Opalinus Clay showed the reduction in kaolinite and calcite and the appearance of analcime peaks. The illite peak in EBS-14 was also sharper and narrower in comparison to the starting Opalinus Clay, potentially indicating precursor illite recrystallization and/or new illite crystallization. The 300°C experimental products in the mixed Opalinus Clay–Wyoming bentonite systems also showed the appearance of analcime peaks, whereas the 200°C systems and starting materials (20:80 and 50:50) lacked this zeolite. Calcite peaks were reduced in intensity in the 300°C run products in the mixed systems, but were comparatively unchanged in the 200°C products (Fig. 4). The d 060 region of the XRD results did not show evidence of newly formed trioctahedral minerals; peaks remained near 1.50 Å in the run products from EBS-15 through EBS-22 (Fig. 4).
Clay Mineral X-ray Diffraction (XRD)
Oriented air-dried and ethylene glycol-saturated mounts of the < 2 μm clay fraction were analyzed in order to examine clay-mineral transformations. The XRD patterns from the reacted and unreacted materials reflected mainly clay-mineral changes under experimental conditions. The fine-grained clay groundmass of the experimental products, which included mainly Wyoming bentonite with a small amount of Opalinus Clay powder, and discrete fragments of Opalinus Clay were separated and analyzed (Figs 5 and 6).

Fig. 5. XRD results from the oriented, ethylene glycol-saturated clay fraction of the bentonite groundmass for all experiments and unreacted Wyoming bentonite. The positions of chlorite-smectie (C-S), glycolated smectite (numbered), illite (I), illite-smectite (I-S), and quartz (Q) peaks are indicated. 1Data from EBS-10 (Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) are plotted for comparison. *Clinoptilolite peak around 8.9 Å.
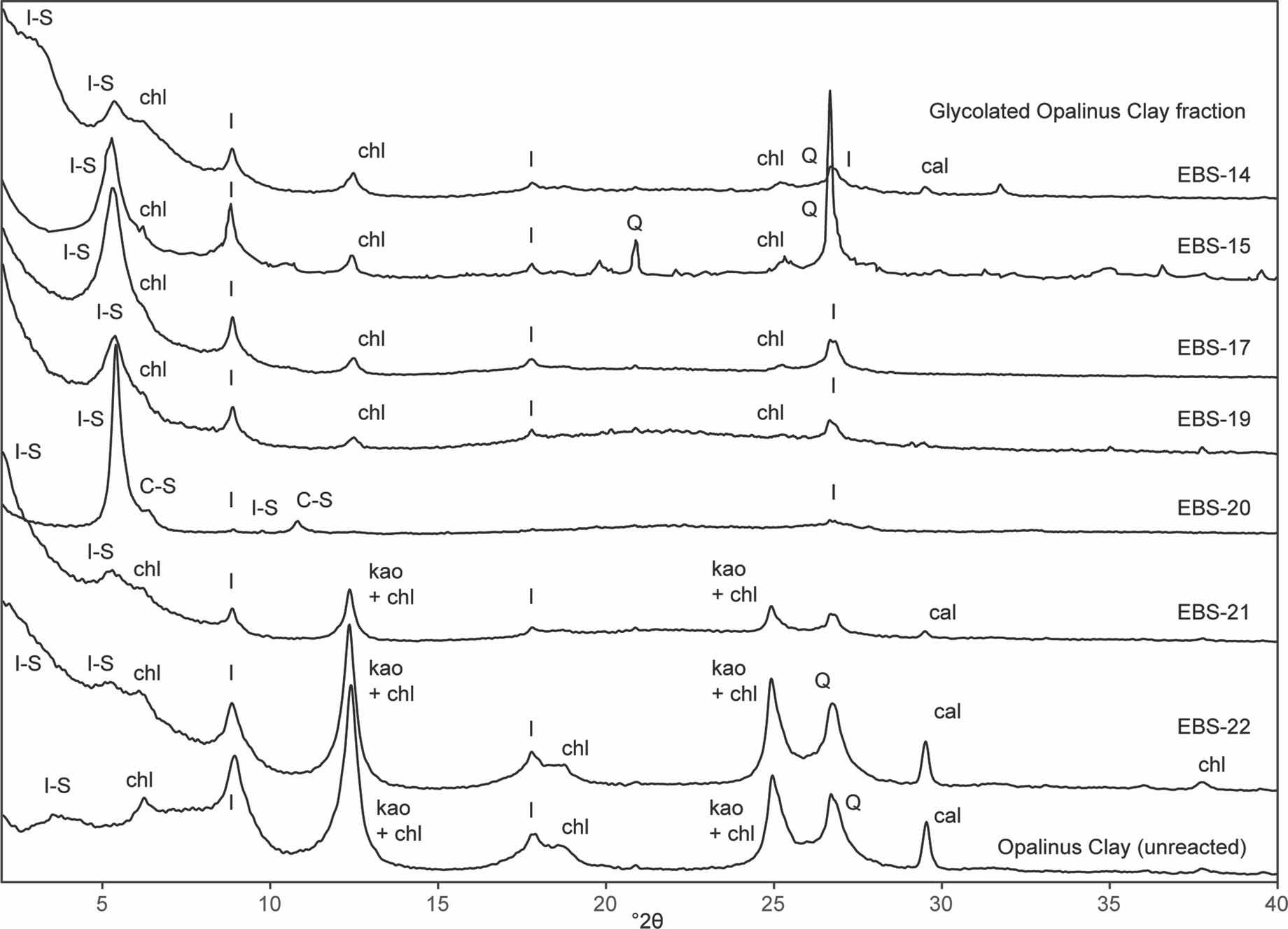
Fig. 6. XRD results from the oriented, ethylene glycol-saturated clay fraction of Opalinus Clay fragments extracted from each experiment and unreacted Opalinus Clay. Peaks corresponding to illite-smectite (I-S), chlorite (chl), illite (I), kaolinite (kao), quartz (Q), and calcite (cal) are labeled.
Clay groundmass.
Peak patterns from the ethylene-glycolated clay fraction from unreacted Wyoming bentonite and the clay fraction separated from the groundmass (mainly Wyoming bentonite, with trace amounts of Opalinus Clay) products of experiments EBS-15 through EBS-22 were characteristic of ethylene glycol-saturated montmorillonite (Fig. 5). The XRD results from unreacted Wyoming bentonite were compared to the reaction products from the present study and an example (EBS-10) from Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) (Fig. 5). Experiment EBS-10 from Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) was conducted under the same experimental conditions, but with Wyoming bentonite only. The XRD results from the bentonite and Opalinus Clay experiments showed minimal smectite alteration. Hydrothermal treatment resulted in the reduction of the glycolated-smectite d 001 values by between 0.1 and 0.3 Å. However, expandability estimates based on the position of the d 002/d 003 ethylene glycol-saturated peak positions indicated no reduction of swelling capacity of the montmorillonite in the 6week, 300°C experiments (EBS-15, EBS-17, and EBS-19) (Table 4). The 6-month experiment (EBS-20) showed a slight reduction in expandability in comparison to unreacted bentonite (~2% decrease). The experiment conducted at 200°C with a saline brine (EBS-21) showed a similar reduction in expandability to EBS-20, whereas the other 200°C experiment (EBS-22) showed no change. The difference between the d 002 and d 003 ethylene glycolated peak positions can also be used to estimate the percentage of illite in illite-smectite mixed layers. Using the calibration based on the difference in the d 003 and d 002 ethylene glycol-saturated illite-smectite peaks presented in Leupin et al. (Reference Leupin, Birgersson, Karnland, Korkeakoski, Mäderm, Sellin and Wersin2014), only the EBS-20 clay groundmass contained detectable illite-smectite mixed layers (~1%) (Table 4). Either the formation of interlayered illite or, more likely, the physical mixture of illite derived from the Opalinus Clay is responsible for the slight decrease in smectite expandability in EBS-20.
Table 4. Peak positions for ethylene glycol-saturated smectite for unheated Wyoming Bentonite and the clay fraction of the groundmass (Wyoming bentonite with trace Opalinus Clay) from the reaction products (EBS-15 through EBS-22). Expandability was calculated using three equations based on the separation between the d 002 and d 003 peak positions.
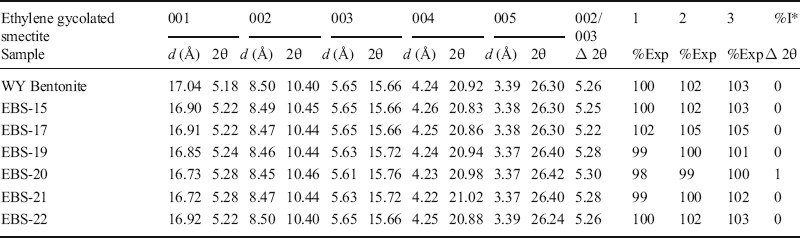
1: %Exp = 973.76 – 323.45Δ + 38.43Δ2 – 1.62Δ3 (Eberl et al. Reference Eberl, Velde and McCormick1993)
22: %Exp = 1517.8 – 548.49Δ + 68.35Δ – 2.90Δ3 (Eberl et al. Reference Eberl, Velde and McCormick1993)
3: %Exp = 766.01 – 194.10Δ + 12.924Δ2 (Moore and Reynolds Reference Moore and Reynolds1997)
*Determined based on the NEWMOD calibration curve presented by Leupin et al. (Reference Leupin, Birgersson, Karnland, Korkeakoski, Mäderm, Sellin and Wersin2014)
Opalinus Clay fragments.
Unreacted Opalinus Clay contained diverse clay and phyllosilicate minerals including illite, illite-smectite interstratified minerals, chlorite, and kaolinite (Table 3, Fig. 6). The XRD patterns of heated Opalinus Clay fragments separated from the reaction products indicated that several clay mineral transformations had occurred. In all results, mineral quantification was hindered by the presence of various mixed-layer phases.
The Opalinus Clay fragments in EBS-22 retained the same chlorite, kaolinite, and calcite peaks as the precursor materials. The results from EBS-21 showed similar XRD patterns, but with reduced intensity kaolinite and calcite peaks. In both, the main alteration signature was the shift of the low-angle reflections that indicated the presence of interstratified illite-smectite minerals (Fig. 6).
In the 300°C, 6-week experiments, the XRD patterns of Opalinus Clay fragments were characterized by mixed-layer illite-smectite, illite, and chlorite (Fig. 6). The 6-month experiment (EBS-20) showed a signature which was significantly different from the shorter 300°C experiments. Mixed-layer illite-smectite was present, likely 60% illite with 30–39% expandability based on the spacing of the d 002 and d 003 ethylene glycol-saturated peaks. The discrete illite peak around 10 Å was reduced to a minor peak. Peaks corresponding to chlorite were no longer observed. Instead, peaks corresponding to mixed-layer chlorite-smectite were present (Fig. 6).
Scanning Electron Microscopy (SEM)
300°C experiments. Analcime–wairakite crystals in the 300°C reaction products were observed either rimming fragments of Opalinus Clay or within the fine-grained bentonite clay matrix (Fig. 7a–c). Secondary electron images of the analcime–wairakite revealed that these crystals were commonly embedded within a recrystallized smectite matrix (Fig. 7d, e, g, and h). Recrystallization of clay minerals was evident within the SEM images. In some experiments, the analcime–wairakite was associated with feldspar grains that show dissolution textures (Fig. 7f).

Fig. 7. Representative SEM images from 300°C experiments with Opalinus Clay ± Wyoming bentonite. a–c: Backscatter-electron SEM thin-section images of 300°C experiments with (a) Opalinus Clay only (EBS-14) and (b,c) Opalinus Clay + Wyoming bentonite in EBS-15 and EBS-17. Analcime–wairakite (an–wr) crystals form either on cracks/edges of Opalinus Clay fragments and/or dispersed within the bentonite clay matrix. d–i: Secondary electron images of reaction products. (d) Analcime–wairakite crystals on the surface of an Opalinus Clay fragment. (e) Analcime–wairakite dispersed in smectite. (f) Newly formed analcime-wairakite and corroded albite (plagioclase). (g, h, i) Analcime–wairakite crystals from the 300°C, 6-week experiments.
200°C experiments. Smectite in the 200°C experiments was fine-grained and had less ordered textures than the montmorillonite reacted at 300°C (Fig. 8a, b). Pockets of pyrite were more prevalent than in the 300°C samples (Fig. 8a, c). Large calcite grains (possibly shell fragments) were observed, and, in some cases, pyrite grains were contained within calcite (Fig. 8c). Analcime–wairakite crystals were not identified.

Fig. 8. Reaction products from the 200°C hydrothermal experiments. (a) Pockets of framboidal pyrite in an Opalinus Clay fragment (secondary electron image) in EBS-21. (b) Quartz crystals with smectite in the clay groundmass of EBS-22 (secondary electron image). (c) Calcite and pyrite in an Opalinus Clay fragment from EBS-22 (back-scatter electron).
Electron Microprobe Analyses (EMPA)
Electron microprobe analyses primarily targeted zeolite minerals (clinoptilolite and analcime–wairakite) found in the reaction products. The results of the microprobe analyses are reported in Appendix 2.
In general, the zeolite minerals formed in the 300°C experiments had intermediate compositions between analcime (NaAlSi2O6·H2O) and wairakite (CaAl2Si4O6·2H2O) endmembers. The results are plotted in Fig. 9 and compared with results from Wyoming bentonite-only experiments (Cheshire et al. Reference Cheshire, Caporuscio, Jové-Colón and McCarney2013). Analcime–wairakite from experiments with Opalinus Clay and Wyoming bentonite (EBS-15 through EBS-20) had a relatively limited range of Si/Al ratios between ~2.5 and 3.0 and Na/(Na + Ca) values of 0.52–0.64 (Fig. 9). In comparison, the analcime–wairakite formed in the experiment with only Opalinus Clay (EBS-14) had the lowest Si/Al ratio (~2.2) and Na content (Na/(Na + Ca) = 0.22). Clinoptilolite observed in EBS-21 had similar compositions to clinoptilolite from earlier EBS experiments and unreacted clinoptilolite.

Fig. 9. Zeolite compositions from the present study (filled shapes, EBS-14 through EBS-21) and from Cheshire et al. (Reference Cheshire, Caporuscio, Jové-Colón and McCarney2013) (open shapes, EBS-1 through EBS-13). The numbers represent the EBS experiment identifier. The run conditions are as follows: 1–4, 6: 4- to 5-week bentonite-only experiments with a ramped thermal profile (25/100/200/300/25°C); 5, 10–13: 6-week bentonite-only at 300°C; 14: Opalinus Clay only for 6 weeks at 300°C; 15–20: Opalinus Clay + bentonite 6- to 8-week experiments at 300°C; and 21: Opalinus Clay + bentonite 200°C experiments. Bentonite-only experiments generally have higher Na/Na+Ca and Si/Al values. Experiments with Opalinus Clay shift to lower Si/Al and Na values.
Discussion
The hydrothermal interaction of Wyoming bentonite and Opalinus Clay wall rock with synthetic groundwater was examined in this set of experiments with varying temperature, water chemistry, and starting components. The diverse reaction products demonstrated the effects of these changing parameters.
Aqueous Chemistry
Aqueous cation and anion compositions largely reached steady-state conditions (i.e. constant measured values) after ~400 h of experiment time. Evolution of solution chemistry during the experiments probably reflected mineral–brine reactions. For example, [Ca2+] was elevated in EBS-14 (Opalinus Clay only) in comparison to 300°C experiments wich contained Opalinus Clay and Wyoming bentonite mixtures. The increase in Ca2+ was likely related to calcite dissolution, which was observed in all 300°C experiments in the SEM and QXRD results. The higher concentrations in EBS-14 were likely due to the higher proportion of Opalinus Clay and, therefore, larger amount of calcite in the starting materials (~16 wt.%) in comparison to the mixed system (~3 wt.%). Increases in aluminum in solution were observed in the mixed systems and likely were related to reactions with aluminosilicate mineral formation (e.g. clay, feldspar, and zeolite) in the bentonite as aqueous aluminum concentrations were below the detection limit in the Opalinus Clay-only experiment. Similarly, iron increased throughout the experiments that contained Wyoming bentonite, but not in EBS-14, in which aqueous iron was below the detection limit. Observed iron concentrations are similar in all 300°C experiments with Wyoming bentonite, indicating that iron was likely sourced from mineral dissolution (e.g. pyrite) in the bentonite and/or the iron buffer materials, rather than the stainless steel or Opalinus Clay. The steel coupons included in the experiments likely did not contribute in a significant way to aqueous iron. Experiment EBS-17 included a copper foil (no steel coupon) and observed iron concentrations were similar to those from experiments that included steel material (e.g. EBS-15, EBS-19, and EBS-20). Potential mechanisms of iron release include breakdown of chlorite and pyrite. Overall, the solution chemistries of experiments with similar components and P-T conditions show significant differences, further demonstrating the complexity of these experimental systems.
The pH of all experimental systems remained below 6.5, which is outside the carbonate buffer range. Calcite dissolution occurred based on the XRD and SEM observations and CO2 gas was generated and extracted during fluid sampling. The recalculated pH values at temperature for all solutions were between 4.3 and 5.5, assuming initial ambient pCO2 (10–3.5 bar) conditions, indicating that the solution chemistry was likely buffered by a mineral system other than carbonate (e.g. Al-Si minerals; Gailhanou et al. Reference Gailhanou, Lerouge, Debure, Gaboreau, Gaucher, Grangeon, Grenèche, Kars, Madé, Marty and Warmont2017). Dissolved carbonate in the system could also influence the pH of the hydrothermal solutions. For example, calcite solubility is expected to be high at these P-T and pH conditions; therefore, dissolution of preexisting calcite in the Opalinus Clay rock may have contributed to the low observed pH. Also, other experimental studies have also shown decreases of pH in hydrothermal systems containing montmorillonite or clay rock (e.g. Johnston and Miller Reference Johnston and Miller1984; Heimann Reference Heimann1993; Gailhanou et al. Reference Gailhanou, Lerouge, Debure, Gaboreau, Gaucher, Grangeon, Grenèche, Kars, Madé, Marty and Warmont2017). Moreover, zeolite-forming reactions in low-temperature experiments with Opalinus Clay were also observed at lower solution pH (Adler et al. Reference Adler, Mäder and Waber1999). The EQ3/6 geochemical calculations showed that the reaction solutions were either saturated or supersaturated with respect to clays and Na-/Ca-clinoptilolite; therefore, the reactions between brine and clay and/or zeolite likely buffered the solution pH in the experimental system.
Silica saturation has been identified as a major factor in the alteration of EBS materials (Smyth Reference Smyth1982; Bish and Aronson Reference Bish and Aronson1993; Neuhoff and Ruhl Reference Neuhoff and Ruhl2006; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014). The presence of Opalinus Clay wall rock and synthetic groundwater had significant effects on the aqueous chemistry of the system, in particular, on the silica saturation state throughout each experiment. Cheshire et al. (Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) observed saturation with respect to cristobalite throughout the duration of 300°C Wyoming bentonite-only experiments (EBS-10, Fig. 10). In comparison, the aqueous chemical measurements from the 300°C Opalinus Clay ± Wyoming bentonite experiments of this study showed similar silica concentrations throughout each experiment, regardless of the proportions of starting material. That is, experiments having Opalinus Clay:Wyoming bentonite ratios of 100:0 and 20:80 showed similar SiO2(aq) concentration values (Fig. S1). The silica measurements correlated to calculated activities at the experimental temperature of 300°C to be below quartz saturation (Fig. 10).
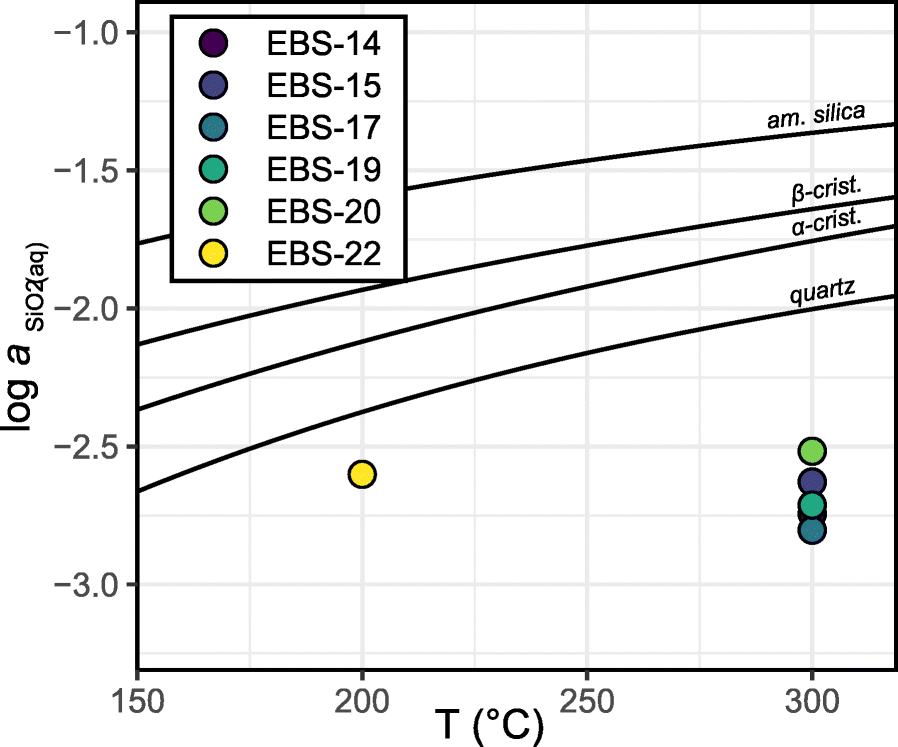
Fig. 10. Steady-state silica activity versus temperature for the 200°C (EBS-22) and 300°C (EBS-14, -15, -17, -19, and -20). Opalinus Clay + Wyoming bentonite 300°C and 200°C experiments remained undersaturated with respect to quartz. Data from EBS-21 are not shown as silica remained below the detection limit until the experiment quench. Silica saturation data are from (Rimstidt and Barnes Reference Rimstidt and Barnes1980). Am., amorphous; crist., cristobalite.
In the 200°C experiments, silica remained below the detection limit in EBS-21 until after ~1100 h, and then increased to ~100 mg/L after experiment cooling (under-saturated with respect to quartz). In comparison, the aqueous fluids from EBS-22 indicated under saturation with respect to steady-state conditions. Based on the silica concentrations and the pH of the experimental system, dissolved silica was dominantly in the form of SiO2(aq) and derived from dissolution of precursor quartz and other silicate minerals from the reactant materials. Silica saturation can exert significant effects on the mineralogical evolution of materials, including clay mineral reactions and zeolite formation, which are discussed in the following sections.
Clay Mineral Alteration
The QXRD results and powder diffraction patterns from the bulk reaction products, in comparison to the XRD characteristics of the starting mixtures of Opalinus Clay and Wyoming bentonite, overall showed increased clay content (Table 3). XRD patterns of the oriented clay fraction from the reaction product groundmass provided insight into the structure of the clay phases present, differentiating between smectite, illite-smectite, and illite. The reaction products were characterized by peaks that align with ethylene glycol-saturated montmorillonite and showed minor shifts in comparison to peak positions of unreacted Wyoming bentonite (Fig. 5). Only EBS-20, the 6-month 300°C experiment showed a slight decrease in expandability (~2%) and detectable interlayered illite in interstratified illite-smectite (~1 wt.%) based on the ethylene glycol-saturated peak positions (Fig. 5, Table 4). Observed differences between the 6-week and 6-month experiments demonstrated the effect of reaction time on the mineralogical changes. The shifts in the peak patterns in the results from the clay groundmass of EBS-20 cannot be attributed definitively to alteration of montmorillonite from Wyoming bentonite, as traces of Opalinus Clay powder and/or fragments, which contain precursor illite, may have remained. Therefore, given the very slight (~1%) estimated amount of interlayered illite in interstratified illite-smectite, the illitization of Wyoming bentonite montmorillonite over the 6-month period was likely negligible.
The clay mineral XRD results from the two 200°C experiments (EBS-21 and EBS-22) also reflect little change to the montmorillonite structure. With the addition of the highly saline brine in EBS-21, some reduction in montmorillonite expandability and kaolinite dissolution was observed. No discrete illite formation was observed in the analyzed 200°C clay fractions.
The observation of illite and illite-smectite in these experiments indicated that the presence of Opalinus Clay, which contains illite, may influence its nucleation in the EBS material. For example, discrete, newly formed illite in the clay groundmass of EBS-15 and EBS-17 likely nucleated on preexisting illite crystallites derived from the Opalinus Clay. The bulk composition of the system may have limited illitization of the Wyoming bentonite montmorillonite: cation exchange capacity measurements of bulk-rock Opalinus Clay samples reveal small values of exchangeable K+ in comparison to values for Ca2+, Na+, and Mg2+ (Pearson et al. Reference Pearson, Arcos, Boisson, Fernandez, Gäbler and Gaucher2003). These results agreed with previous investigations that found the alkali chemistry of the system (i.e. low K+) limits the formation of illite (e.g. Kaufhold and Dohrmann Reference Kaufhold and Dohrmann2009; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014). Low silica activities, however, likely favored illite stability and formation through the alteration of montmorillonite to illite, e.g. through the generalized reaction (Pusch et al. Reference Pusch, Takase and Benbow1998; Pusch Reference Pusch2001; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014):
Montmorillonite dissolution textures were not prevalent from SEM observations and interstratified illite-smectite mineral formation in the clay groundmass was not detected. However, low levels of aqueous silica indicated that the reaction above was thermodynamically favored (Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014; Savage et al. Reference Savage, Wilson, Benbow, Sasamoto, Oda and Walker2019). Montmorillonite alteration was likely kinetically slow, as demonstrated by the small difference between the expandability estimates from the 6-week and 6-month experiments. Increased solute concentration was observed to accelerate clay-mineral reactions (Kasbohm et al. Reference Kasbohm, Herbert and Henning2005), which was observed in EBS-21 having similar montmorillonite structural characteristics to the 6-month experiment.
The clay mineralogy of the Opalinus Clay fragments was altered significantly during the 300°C experiments; however, it remained comparatively unaltered in the 200°C experiments (Fig. 6). Kaolinite and calcite were reactive at 300°C, but stable during the 200°C experiments. Significant differences in the clay fraction were observed between the Significant differences in the clay fraction 6-week and 6-month experiments at 300°C. Discrete chlorite and illite dominated the pattern of EBS-15, EBS-17, and EBS-19, whereas illite-smectite and chlorite-smectite mixed-layer minerals were the main phases in EBS-20 (Fig. 6). The increased presence of clay with some expansion capacity (i.e. presence of smectite) indicates that hydrothermal reactions in the host rock may increase favorable properties. However, host-rock temperatures will likely not reach 300°C in a deep geological disposal scenario as most repository design concepts limit drift-wall temperatures to <100°C (Greenberg et al. Reference Greenberg, Wen and Buscheck2013).
The lack of montmorillonite alteration in Wyoming bentonite during interaction with Opalinus Clay rock and synthetic groundwater is a significant observation in the chemical environment of this experimental system. As mentioned above, silica under-saturation is thought to favor illite formation. The results of this set of experiments indicated that other factors, such as K+ or Al3+ availability, may control illite growth instead of aqueous silica, or that the smectite-to-illite transformation is kinetically limited. However, the experiments conducted in this study utilized a higher water:rock ratio than will likely be observed in the repository-scale environment, which may favor stability of expandable clay minerals (e.g. Whitney Reference Whitney1990). Therefore, these laboratory-scale experiments and findings should be supported by field experiments.
Zeolite Formation
Newly formed zeolite phases with intermediate compositions between analcime (NaAlSi2O6 (H2O)) and wairakite (Ca0.5AlSi2O6 (H2O)) formed in the 300°C experiments (EBS-14 through EBS-20) but were not identified in the 200°C runs (EBS-21 and EBS-22). Analcime–wairakite formation has been observed in other 200°C experiments, mostly at high pH (Chermak Reference Chermak1992). The absence of analcime–wairakite in the 200°C experiments agrees with previous studies that show analcime crystallization is hindered by high pressure and neutral pH (Wilkin and Barnes Reference Wilkin and Barnes1998; Benning et al. Reference Benning, Wilkin and Barnes2000). However, other factors such as the potential for sluggish reaction kinetics, aqueous silica under-saturation, and limited phase stability at lower temperatures should be considered also.
Microprobe analyses of analcime–wairakite show variation in the Si/Al vs. Na/(Na+Ca) for the different experiments from this study and from Cheshire et al. (Reference Cheshire, Caporuscio, Jové-Colón and McCarney2013) (Fig. 9). The analcime–wairakite composition range appears to be a result of the bulk composition of the system determining the zeolite-forming reactions, in agreement with Liou et al. (Reference Liou, de Capitani and Frey1991) for natural zeolite occurrence in the system CaAl2Si2O8–NaAlSi3O8–SiO2–H2O. The low levels of aqueous silica activities and Si/Al ratios observed in this set of experiments may indicate that wairakite is stable with respect to analcime (Liou et al. Reference Liou, de Capitani and Frey1991; Jove-Colon et al. Reference Jove-Colon, Hammond, Kuhlman, Zheng, Kim and Xu2016). The crystal structure of the zeolite reaction product is a topic of continued investigation (i.e. orthorhombic analcime vs. monoclinic wairakite) and the reaction products are referred to as analcime–wairakite.
Newly formed analcime–wairakite grains are observed in two contexts: within the clay matrix and rimming the Opalinus Clay fragments (Fig. 7a–c). The different context of the grains may correspond to different mineral reactions. The compositions of analcime–wairakite within the clay matrix in experiments EBS-15 through EBS-20 have Na/Na+Ca values = 0.52–0.64 and show a relatively flat trend in Si/Al. In comparison, analcime–wairakite grains rimming Opalinus Clay pieces formed in the Opalinus Clay-only experiment (EBS-14) and had the highest Ca content (Na/(Na+Ca) = 0.22) of all experiments (Fig. 9).
In Wyoming bentonite-only systems, previous workers have suggested that analcime formation under similar experimental conditions occurs due to replacement of precursor clinoptilolite dissolution-precipitation (e.g. Chipera and Bish Reference Chipera and Bish1997; Wilkin and Barnes Reference Wilkin and Barnes1998; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014) through the reaction:
The dissolution of clinoptilolite is interpreted to have contributed to silica saturation with respect to cristobalite in the Wyoming bentonite-only experiments (e.g. EBS-10; Cheshire et al. Reference Cheshire, Caporuscio, Rearick, Jove-Colon and McCarney2014). The QXRD results from experiments with Wyoming bentonite and Opalinus Clay that formed analcime–wairakite (EBS-15 through EBS-20) indicate the continued presence of clinoptilolite. In some cases (EBS-15, EBS-19), clinoptilolite abundance determined by QXRD increased with respect to an estimate of the starting clinoptilolite abundance. Silica activities remained low (below quartz saturation) in these experiments, which may also indicate that clinoptilolite dissolution was limited and did not occur over the experiment duration.
Unreacted Opalinus Clay does not contain clinoptilolite (Table 3) and, therefore, the analcime–wairakite produced in the Opalinus Clay-only experiment (EBS-14) formed through reactions involving other precursor phases. Analcime–wairakite formation from Ca-rich plagioclase feldspar has been documented (Wirsching Reference Wirsching1981); however, albite formed in the experiments (Table 3) and may reflect coupled plagioclase analcime–wairakite solid solution formation as described by Liou et al. (Reference Liou, de Capitani and Frey1991). The QXRD results from EBS-14 reveal other significant changes in major-mineral abundances between the starting Opalinus Clay and the reacted sample. Relevant QXRD observations include reduction in kaolinite (17 to 1 wt.%), calcite (16 to 8 wt.%), and quartz (14 to 7 wt.%) (Table 3).
Reactions involving kaolinite and quartz dissolution, as well as feldspar recrystallization, likely provided the aluminum and silica needed for the formation of analcime–wairakite. Levels of aqueous Al3+ remained low throughout the experiments, indicating incorporation into silicate-mineral alteration products. The availability of Na+ may come from either the synthetic groundwater (Na+ = 3800 mg/L) or from cation exchange with other clay minerals (e.g. Na-montmorillonite). Calcium was likely derived from calcite and the groundwater (Ca2+ = 426 mg/L). The formation of analcime–wairakite from kaolinite has been inferred in high-pH experiments on Opalinus Clay (e.g. Chermak Reference Chermak1992). Analcime–wairakite crystallization from Opalinus Clay materials under the lower-pH conditions of the present experiments likely occurred by the generalized reaction:
In the Wyoming bentonite and Opalinus Clay experiments, analcime–wairakite crystallization likely occurred during mineral reactions (3) and (4) described above. The larger sodium content observed in the analcime–wairakite in experiments EBS-15 through EBS-20 likely reflects the lower Ca2+ availability in the bulk aqueous composition of the system. The formation of analcime–wairakite by reaction (4) may have been the dominant reaction including aqueous silica, thus maintaining the low aqueous silica activities observed throughout the experiment.
These results show that analcime–wairakite formation is a significant mineralogical product both within the bentonite EBS material and along cracks and edges of the argillite wall rock. Further, at high temperatures (i.e. ~300°C) zeolites may form at low pH (4–7). Previous workers have observed abundant analcime formation in the presence of high pH pore fluids (Savage et al. Reference Savage, Walker, Arthur, Rochelle, Oda and Takase2007). This study highlights that zeolite formation is also significant within the EBS materials and the host-rock system (i.e. away from the cement liner) at elevated temperatures and pressures.
Conclusions
The hydrothermal interaction of Wyoming bentonite and Opalinus Clay was tested in an experimental system to gain insight into mineral-brine reactions that may occur in a high-temperature, deep geological nuclear waste repository hosted in argillaceous or clay rock. Experiments conducted at temperatures between 200 and 300°C and water:rock ratios of 6:1 to 9:1 resulted in diverse reaction products, highlighting the influence of temperature, wall- rock, and groundwater geochemistry on the alteration of EBS materials in the near-field environment. Experiments with Opalinus Clay only and brine resulted in clay mineral transformations throughout the Opalinus Clay fragments, but new zeolite formation was observed only along cracks and edges, likely due to the impermeable nature of the argillite as a result of lack of pore space or reactive surfaces. In experiments with both Wyoming bentonite and Opalinus Clay, discrete illite crystallization observed in the experiment run products likely occurred on precursor illite grains from the Opalinus Clay. Therefore, illitization of the Wyoming bentonite montmorillonite likely did not occur, supporting previous research that emphasizes the effect of bulk composition of the system on clay mineral phase stability. The formation of zeolite minerals with compositions intermediate between those of analcime and wairakite endmembers occurred at low pH through reactions involving precursor zeolite phases or clay minerals. Overall, in the experimental system, zeolite-forming reactions may have controlled the aqueous chemistry, were kinetically favored in comparison to illitization, and may be significant in a high-temperature repository scenario. These experimental results may be used to inform future modeling efforts and full-scale experiments that explore higher thermal loads for disposed spent nuclear fuel and waste than previously considered.
Electronic supplementary material
The online version of this article (https://doi.org/10.1007/s42860-020-00068-8) contains supplementary material, which is available to authorized users.
ACKNOWLEDGMENTS
The authors thank Emily Kluk at Los Alamos National Laboratory for XRD assistance and Steve Chipera at Chesapeake Energy for QXRD analyses. Scanning electron microscopy facilities were provided by the Materials Science and Technology group at Los Alamos National Laboratory. George Mason and Lindsay Hunt at the University of Oklahoma assisted with the electron microprobe analyses. Thank you to Rose Harris and Oana Marina at Los Alamos National Laboratory for the aqueous geochemical analyses. Bentonite Performance Minerals LLC provided the bentonite used in this study. Opalinus Clay was provided by Florian Kober (NAGRA). Funding was through the Department of Energy’s Spent Fuel and Waste Disposition campaign. Three anonymous reviewers, Associate Editor Reiner Dohrmann, and Editor-in-Chief Joseph W. Stucki provided helpful reviews that improved this manuscript. Los Alamos National Laboratory has assigned free release number LA-UR-19-26929 to this document.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
















