Introduction
Emerging from the literature is a consistent observation that cognitive dysfunction, a transdiagnostic psychopathological domain in psychiatry, is a principal determinant of a person’s general function and other patient-reported outcomes (PROs).Reference McIntyre 1 , Reference Millan 2 During the past two decades, it has been amply documented that neuropsychiatric disorders (NPDs) disproportionately account for burden of illness attributable to chronic noncommunicable medical disorders globally.Reference Murray and Lopez 3 It is also likely that human capital costs attributable to NPDs will disproportionately increase as a consequence of population aging and beneficial risk factor modification of other common and chronic medical disorders (e.g., cardiovascular disease).Reference Razzouk 4 The early age at onset, the relatively high incidence and prevalence, and the chronic and unfavorable illness trajectory, as well as the absence of scalable, preventable, disease-modifying and/or curative therapies for NPDs account for the staggering human, societal, and economic costs.Reference Insel 5 , 6
The ignominious psychosocial and human capital consequences of NPDs have provided the impetus to identify the dimensions/domains of psychopathology that primarily mediate health outcomes among affected individuals. Whether NPDs are typologized according to severity (i.e., common, severe), age of onset of initial observable characteristics (i.e., childhood, adulthood, late life), and/or conceptual pathophysiological processes (i.e., neurodevelopmental, neurodegenerative), deficits in cognitive function account for more variance in PROs and costs attributable to NPDs than any other psychopathological domain.Reference McIntyre 1 , Reference Millan 2 , Reference Sokratis, Ion and Alexandra 7
For example, major depressive disorder (MDD) debases human capital more than any other brain-based disorder among younger populations (i.e., 18–45 years).Reference Greenberg, FournierA-A and Sisitsky 8 Epidemiological and clinical studies provide results that are in accordance with the assertion that cognitive deficits (self-rated, objectively measured) account for more variability in interpersonal adjustments and/or workplace performance (i.e., absenteeism, presenteeism) than total depression symptom severity and/or other domains (i.e., factors) in persons with mood disorders.Reference McIntyre 9 – Reference Baune and Renger 12 It is additionally noted that cognitive deficits in MDD may predate the onset of “mood symptoms” in MDD and may progress in overall magnitude of deficits as a function of episode frequency/illness duration.Reference Vinberg, Miskowiak and Kessing 13 , Reference Miskowiak 14
Notwithstanding the availability of multiple modalities of antidepressant treatment, relatively few studies in psychiatry have primarily sought to determine whether improving cognitive function in MDD improves PROs and/or is cost effective.Reference Lee 10 , Reference Papakostas and Culpepper 15 The mediational relevance of cognition in MDD potentially extrapolates to all NPDs, indicating that screening for, measuring, preventing, and treating cognitive deficits in psychiatry is not only a primary therapeutic target, but also should be conceptualized as a transdiagnostic domain to be considered regardless of patient age and/or differential diagnosis.Reference McIntyre, Lee and Mansur 16 , Reference Culpepper 17
Transdiagnostic domains
In keeping with the view that disturbances in cognitive functions are a transdiagnostic phenomenon, the National Institutes of Health has proffered the Research Domain Criteria (RDoC), which broadly aims to provide a biobehavioral mechanistic matrix of NPDs.Reference Insel 18 Among NPDs, deficits in general cognitive function, social cognition (i.e., theory of mind), negative cognitive valence systems (e.g., perceived threat), and positive cognitive valence systems (e.g., motivation and reward) are distributed across four of the current five RDoC domains.Reference McTeague, Huemer and Carreon 19 – Reference Lin, McCormick and Dhe-Paganon 21 The RDoC framework is supported by animal and human cognitive neuroscience data indicating that domain-based psychopathology (i.e., self-report, observable characteristics) is subserved by discrete multilevel and multimodal substrates. It is expected that future discovery and development of psychiatric treatments are more likely to adopt a “domain-based” rather than a “disease-based” (e.g., bipolar disorder, schizophrenia) approach.Reference Insel 22 – Reference McIntyre 24 For example, a treatment capable of ameliorating abnormalities in reward or cognition domain would not only have transdiagnostic application, but also would mimic the strategic framework of developing treatments in other chronic diseases (i.e., disease-agnostically targeting dimensions/domains).
Importance of cognition as a transdiagnostic domain
The relevance of cognitive domain disturbances as principal mediators of health outcomes across NPDs is expected to only amplify as the global economy and workforce adapt to the “human capital” or “digital” economy.Reference McIntyre and Lee 25 , Reference Doran and Kinchin 26 Moreover, quality of care initiatives across multiple jurisdictions, as well as greater emphasis on cost effectiveness and containment, provide the impetus for the health-care ecosystem, and its stakeholders, to place greater emphasis on prevention, early intervention, risk factor modification, and specific targeting of critical determinants of health outcomes.Reference Patel 27 Tacit to this reprioritization is the requirement for health-care providers to be familiar with a systematic approach to assessing cognitive functions as key determinants of proximal as well as distal health outcomes, agnostic of age and any preliminary differential diagnostic considerations. Hitherto, health-care providers have received extensive education in the approach to screening for (e.g., Mini Mental Status Exam [MMSE]) and diagnosing cognitive deficits in elderly populations.Reference Paddick, Gray and McGuire 28 Notwithstanding this, there has been relatively less attention (and consequently, deficiencies in best practices) given to a systematic screening/assessment of cognitive function deficits (i.e., subjective and/or objective) in pediatric and/or nongeriatric populations presenting to health-care providers, wherein cognitive disturbances are the primary focus of clinical attention.
Against this background, we aim herein to provide health-care providers with a meta-guideline for cognitive dysfunction (i.e., subjective, objective) in clinical practice with an emphasis on screening and differential diagnosis. We purposefully avoid an approach to cognitive dysfunction that is disease specific and/or gives priority to age. Instead, we approach cognitive function as informed by cognitive neuroscience: cognition is a transdiagnostic domain that should be assessed systematically regardless of differential diagnoses.Reference Sheffield 29 It is recognized that cognitive dysfunctions vary across NPDs and individuals as well as within individuals as a function of illness progression. It is also well established that multiple sociodemographic, clinical, and treatment factors moderate overall cognitive function as well as domain-specific performance.
It is our view that approaching psychopathology with a domain/dimensional-based approach (i.e., rather than a disease-specific approach) reflects clinical practice and is not fundamentally different than the approach taken to other targets in chronic disease regardless of etiology or diagnosis (e.g., hypertension, peripheral blood glucose).Reference McIntyre, Cha and Soczynska 30 , Reference McCoy, Castro and Rosenfield 31 It is anticipated that a systematic approach to screening and measuring cognitive dysfunction across NPDs will streamline assessment, diagnosis, and care pathways, and it is hoped that this tactic will presage greater precision, consistency, appropriateness, and cost effectiveness of care.
Systematic Approach to Assessing Cognition
(Figure 1) For patients presenting with cognitive complaints as a focus of clinical concern or for whom cognitive deficits are suspected based on change in patient function, the initial step begins with ascertaining whether the cognitive deficits are subjective and/or objective. Across NPDs, it is well established that subjective and objective cognitive complaints exhibit minimal correlation.Reference Pranckeviciene, Deltuva, Tamasauskas and Bunevicius 32 – Reference Demant, Vinberg, Kessing and Miskowiak 37 A separate body of literature indicates that changes in subjective and objective cognitive measures over time may correlate to a greater extent than pretreatment cross-sectional cognitive measures.Reference Kinsinger, Lattie and Mohr 38 , Reference Ott 39
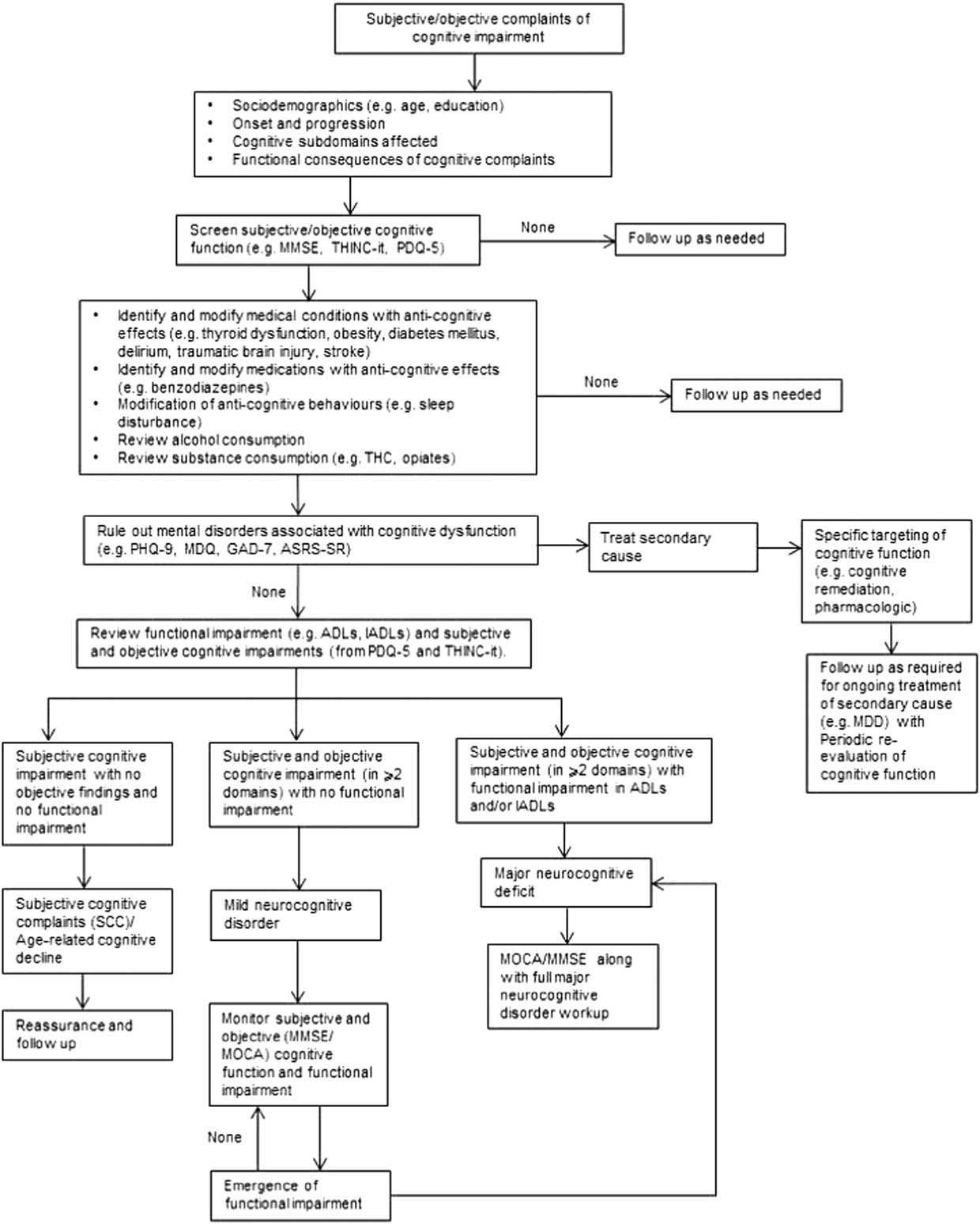
Figure 1 Algorithm for cognitive screening and assessment in the clinical setting.
The dissociation between subjective and objective cognitive performance is a consequence of multiple moderating factors (e.g., depressive symptoms).Reference Shi 40 Implicit is the need for health-care practitioners to provide reassurance when cognitive deficits are solely subjective and are not verified objectively and/or associated with meaningful functional impairment. Notwithstanding this, vigilance for the possibility of objective cognitive impairment should remain in circumstances in which a patient or caregiver reports functional deficits in the absence of subjective cognitive complaints.
Available evidence also indicates that informant (e.g., family) ratings of an identified patient’s cognitive function are reliable, valid, and highly correlated with both subjective and objective ratings of cognition. For example, the Cognitive Assessment Interview was derived from the Schizophrenia Cognition Rating Scale and the Clinical Global Impression of Cognition in Schizophrenia with neurocognitive battery measures, functional assessment, and functional outcome.Reference Ventura 41
Sociodemographic information with a particular emphasis on age and education are essential, and these are two of the most replicated variables influencing general cognitive performance. Early childhood adversity (ECA) has been associated with a myriad of medical and/or mental disorders. Preclinical evidence, as well as emerging evidence in human studies, indicates that ECA may be associated with abnormalities in general cognitive functions and social cognition. It is well established that ECA exerts deleterious effects on cognitive emotional processing that overlap with positive and negative valence disturbances.Reference Richards and Wadsworth 42
As with all patient-reported chief complaints and/or objectively established deficits in psychosocial and/or workplace function, characterization of the onset, trajectory, progression, and course is warranted. Determining whether the cognitive complaint/deficit is enduring or progressive has diagnostic implications (e.g., intellectual disability, bipolar disorder/major neurocognitive disorder, respectively). Moreover, cognitive complaints/objective deficits that temporally precede the overt onset of an NPD may represent heterotypic continuity, vulnerability markers, early prodrome of the NPD, or a distinct and separate disease process. Conversely, cognitive deficits may become a focus of clinical concern following the onset and/or amelioration of a discrete medical/mental disorder (e.g., traumatic brain injury, major depressive disorder) or spontaneous or medication-induced remission.Reference Kumar, Gao and Juengst 43
Subdomains of cognition
Cognitive functions have been variably defined and operationalized. Delineating whether deficits in cognition are primarily in the domains of learning and memory, attention/concentration, executive functions (e.g., planning, sequencing, organizing, impulse control), processing speed, language, and/or social cognition is critical.Reference McIntyre 1 It is recognized that cognitive subdomains exhibit multilinearity, yet as discrete subdomains often are subserved by discrete yet interrelated biological substrates. A separate yet overlapping taxonomy is “hot” and “cold” cognition, referring to the presence and absence of emotional valence, respectively.Reference Roiser and Sahakian 44 Examples of “hot” cognition include, but are not limited to, rumination, negative attentional biases, affect perception and recognition, and catastrophic reactions to real and/or perceived slights.Reference McIntyre, Cha and Soczynska 45
Deficits in any subdomain are not pathognomonic of any single NPD or medical disorder. The profile of cognitive deficits may provide insights into possible disease considerations. For example, childhood onset of persisting attentional disturbances with difficulties in impulse control are characteristic features of attention-deficit hyperactivity disorder, while deficits in memory (e.g., spatial) may suggest early prodromal stages of a major neurocognitive disorder when significant deficits in multiple domains of psychosocial functioning are present.Reference Fenesy and Lee 46
Functional consequences of alterations in cognitive performance
The functional consequences of cognitive dysfunction are essential when attempting to establish clinical meaningfulness. To the extent possible, characterizing “premorbid” function is instructive, as many individuals with significant “cognitive reserve” may manifest significant subjective cognitive decline that may not be verifiable using norms whereby many cognitive screening and measuring tools have been validated. General psychosocial functioning, independence in everyday living, academic performance (where applicable), and workplace productivity/presenteeism should be evaluated. Among individuals who are working, presenteeism is essential information, as workplace productivity decreases are disproportionately accounted for by presenteeism than absenteeism.Reference Razzouk 4 A review of activities of daily living (ADLs) and instrumental ADLs (IADLs) should also be conducted. A host of medical disorders are highly associated with decreased cognitive performance in both younger and older populations.
Medical illness and cognition
It is well established that thyroid dysfunction is linked to cognitive impairment and should be corrected biochemically.Reference Ritchie and Yeap 47 It has also emerged that impaired glucose tolerance, diabetes mellitus, and obesity are associated with impaired cognitive performance and mild cognitive impairment, as well as major cognitive disorders.Reference Mansur 48 Many other medical disorders with associated anticognitive effects should also be considered, including, but not limited to, delirium, cerebrovascular accidents, and traumatic brain injury. Finally, maladaptive behaviors impacting cognition, notably disruption in sleep duration, efficiency, and/or reversal of day/night schedule should be ruled out as modifiable contributors of cognitive impairment.Reference Lowe, Safati and Hall 49
Medication effects and cognition
Health-care providers should also review prescription and over-the-counter (OTC) medications, as well as complementary alternative medicines (CAMs), that may be associated with anticognitive effects. For example, mechanistically dissimilar agents like benzodiazepines, antihistamines, anticonvulsants, and corticosteroids are all associated with anticognitive effects.Reference Hindmarch 50 , Reference Qidwai, Watson and Weiler 51 The role of anticholinergics and, of more recent interest, histamine antagonism, needs to be carefully assessed. Many OTCs with sedating/somnolent effects may have anticognitive effects, and despite marketing claims, procognitive effects associated with most CAMs have not been established. The anticognitive effects of alcohol consumption are well known and need to be assessed on a personalized basis, as is the case for opiate use.Reference Ieong and Yuan 52 Moreover, the careful review of substance use is required, as multiple substances that are misused are highly associated with impaired cognitive functions (methamphetamines, ketamine).Reference Potvin, Pelletier and Grot 53 , Reference Giorgetti, Marcotulli, Tagliabracci and Schifano 54
The high and rising rates of cannabis use across jurisdictions, fueled in part by changing legal, cultural, social, and political factors, is resulting in a larger number of individuals reporting cannabis consumption during the past year.Reference Carliner, Brown, Sarvet and Hasin 55 Cannabis (notably Tetrahydrocannabinol (THC)), is established as anticognitive in both healthy and clinical populations, with the extent of reversibility variably reported.Reference Volkow 56 In addition to anticognitive effects, phytocannabinoids like THC are also associated with amotivation and incident mental disorders (e.g., schizophrenia), which are also associated with cognitive impairment.Reference Rong 57 Circumstances in which cognitive deficits persist, despite ruling out and/or managing any of the foregoing secondary causes, invite the need for additional screening for cognitive function as required. A further issue for consideration is the reporting of null findings in studies of approved antidepressant medication and its effects on cognition. As has been pointed out, clear evidence of a lack of positive effects should be considered in the context of the selected measures’ capacity to demonstrate assay sensitivity.Reference Harrison, Lam, Baune and McIntyre 58
Tools for measuring cognition
Multiple screening, diagnostic, and measurement tools for cognitive functions are available and/or published in the biomedical literature. Available instruments vary in their administration (e.g., patient-administered), interface (e.g., digital), duration, subjective and/or objective measurements, domains evaluated, proprietary and copyright properties, requirement for expert interpretation, scalability and appropriateness for point-of-care utilization, psychometric properties, validation procedures, and cultural/regional/country sensitivity. No single tool stands out as the gold standard akin to the sphygmomanometer for blood pressure evaluation. Notwithstanding, guiding principles in selecting a screening tool for cognitive dysfunction include that it is patient-administered, brief, digital and interoperable with other digital platforms, available at point-of-care, and free of cost; integrates both subjective and objective cognitive performance; has appropriate psychometric properties; and provides actionable information immediately.Reference Baune 59 , Reference Ragguett, Cha and Kakar 60
A recently validated short and feasible tool for assessment of cognition in psychotic and depressive disorders is the SCIP (Screen for Cognitive Impairment in Psychiatry).Reference Miskowiak 61 The SCIP is a brief cognitive screening tool consisting of five short objective tests of cognition that can be administered in 10–15 minutes quantifies difficulties with verbal working memory, verbal learning and memory, verbal fluency, and psychomotor speed and has high decision validity in patients with mood disorders (i.e., high sensitivity and specificity for cognitive impairment).
Another tool is the THINC-it tool. The THINC-it tool is free of charge, digitalized, patient administered, and has been validated as a screening tool in adults with Major Depressive Disorder (https://thinc.progress.im/). The THINC-it tool can be used at point of care and provides the end user with an easy to translate assessment and can be used as a repeat measure across time, in adults with Major Depressive Disorder. The MMSE and the MOCA are well-known tools for dementia screening,Reference Freitas, Santana and Simoes 62 – Reference Finney, Minagar and Heilman 64 and their use, along with establishment of functional impairment in ADLs and IADLs, can be valuable when a possible major cognitive disorder is suspected. However, the MMSE and MOCA may not be sufficient to identify cognitive dysfunction in older populations with higher cognitive baseline or younger populations with diagnoses that have cognitive impairment as one of many possible symptoms (e.g., bipolar disorder, schizophrenia, diabetes mellitus, hypothyroidism). A point to be emphasized is the importance of an age-informed approach to the screening tool selected for cognitive impairment (i.e., the MMSE/MOCA is suitable for older patients, but not younger patients).Reference Baune, Miller and McAfoose 65
Ongoing research endeavors to identify new technological approaches in assessing cognitive dysfunction may provide more objective markers to supplement clinical assessment. Functional near-infrared spectroscopy (fNIRS) is a noninvasive neuroimaging technology that maps the functions of the cerebral cortex by measuring hemodynamics.Reference Lai, Ho, Lim and Ho 66 Assessment of hemodynamics by fNIRS during cognitive tasks can be a promising biomarker in personalized psychiatric practice.
If a major cognitive disorder is expected, appropriate and thorough workup is encouraged (e.g., Alzheimer’s disease).Reference Rogan and Lippa 67 If an individual exhibits deficits in two or more cognitive domains with no functional impairment, by definition, the individual has a mild cognitive disorder and should be prospectively evaluated for the possible declaration of major cognitive disorder. Subjective cognitive impairment with no objective findings and no functional impairment indicates that the individual has subjective cognitive complaints/age-related cognitive decline. Reassurance and follow-up are warranted.
Multiple screening/measurement tools for cognitive function have been validated for specific and/or select mental/medical disorders.Reference Pan 68 When major or mild cognitive disorder is not in the differential diagnosis, and age-related cognitive decline has been ruled out, it would not be unreasonable for cognitive functions to be measured with a tool with exceptional psychometric properties in healthy controls (age, sex, education-adjusted). It would be impossible to validate any tool across the plethora of medical conditions characterized by cognitive complaints. Indeed, preference is given to those screening tools validated in a specific disorder or disease state. The widespread availability of mobile phones, health-related apps, and digital literacy provides a unique opportunity for screening and surveillance of cognitive function. However, relatively few digital solutions have a rigorous and controlled evidence-based approach supporting their validity as appropriate screening and monitoring tools for cognition in the general and medical population.Reference Chan, Godwin and Gonzalez 69
When screening provides evidence that cognitive impairment is present and impacting role functioning, steps can be taken to address ways to minimize, reverse, and adapt to the dysfunction. This starts with sharing the results of the screening and enlisting interpersonal and/or professional support needed to start a treatment plan.
Conclusion
Cognitive dysfunction is a common complaint, a reason for high health-care utilization, a source of patient distress, and a principal determinant of health-care outcomes. It is also established that cognitive dysfunction is a transdiagnostic abnormality with distinct and overlapping phenotypic characteristics across mental/medical disorders. The foregoing provides the basis for health-care providers to prioritize cognitive dysfunction as a primary therapeutic target. As assessment and measurement have been demonstrated to improve health outcomes across chronic diseases, it is a testable hypothesis that screening and measuring cognitive dysfunction in the health-care ecosystem improves health outcomes among those affected. In the interim, adopting a systematic, coherent, and comprehensive approach to cognitive function evaluation that is pragmatic, patient-centric, comprehensive, and evidence-based is warranted.
Disclosures
Roger S. McIntyre reports grants from Allergan, AstraZeneca, Bristol-Myers, Janssen-Ortho, Lundbeck, Otsuka, Purdue, Pfizer, Shire, Sunovion, Neurocrine, and Takeda outside the submitted work.
Nicole Anderson, Bernhard T. Baune, Katherine Burdick, Phillipe Fossati, Rodrigo B. Mansur, Alice Medalia, Tanya Ramey, and Joshua D. Rosenblat have nothing to disclose.
Elisa Brietzke reports personal fees from Daiichi-Sakyo outside the submitted work.
Philip Gorwood reports grants from Eli Lilly, Ethypharm, and Servier and personal fees from Janssen, Lilly, Lundbeck, Otsuka, and Servier outside the submitted work.
Catherine Harmer reports personal fees from P1vital, Lundbeck, Servier, and Pfizer, grants from UCB, and grants and personal fees from Johnson and Johnson outside the submitted work.
John Harrison reports personal fees from AbbVie, Amgen, Anavex, Astra Zeneca, Avonex, Avraham, Axon Neuroscience, Axovant, Biogen Idec, Boehringer Ingelheim, Bracket, Catenion, CRF Health, DeNDRoN, Eisai, Eli Lilly, Enzymotec, ePharmaSolutions, Forum Pharma, GfHEU, Heptares, Janssen AI, Johnson & Johnson, Kaasa Health, Kyowa Hakko Kirin, MedAvante, Merck, Mind Agilis, MyCognition, Neurim, Neurocog, personal fees and other from Neurotrack, Novartis, Nutricia, Orion Pharma, Pfizer, Pharmanet/i3, Prana Biotech, PriceSpective, Probiodrug, Prophase, Prostrakan, Regeneron, Reviva, Roche, Sanofi, Servier, Takeda, vTv Therapeutics, Velacor, Lundbeck, Compass Pathways, G4X Discovery, Cognition Therapeutics, and AlzeCure outside the submitted work.
Philip Harvey reports personal fees from Akili, Biogen, Allergan, Boehringer-Ingelheim, Forum Pharma, Genentech, Intracellular Therapies, Lundbeck, Minerva Pharma, Otsuka Digital Health, Sanofi, Sunovion (DSP), and Teva, grants and personal fees from Takeda, grants from Stanley Medical Research Foundation, and other from Neurocog Trials during the conduct of the study.
Kamilla Miskowiak reports personal fees from Lundbeck and Allergan outside the submitted work.
Carola Rong reports personal fees from EOCI Pharmacomm outside the submitted work.
Allan Young reports grants from Janssen and personal fees from Lundbeck, Livanova, and Sunovion outside the submitted work.
Stephen M. Stahl reports grants from Acadia, Alkermes, AssureX, Astra Zeneca, Arbor Pharmaceuticals, Avanir, Axovant, Biogen, Braeburn Pharmaceuticals, BristolMyer Squibb, Celgene, CeNeRx, Cephalon, Dey, Eli Lilly, EnVivo, Forest, Forum, GenOmind, Glaxo Smith Kline, Intra-Cellular Therapies, ISSWSH, Janssen, JayMac, Jazz, Lundbeck, Merck, Mylan, Neurocrine, Neuronetics, Novartis, Otsuka, PamLabs, Pfizer, Reviva, Roche, Sepracor, Servier, Shire, Sprout, Sunovion, TMS NeuroHealth Centers, Takeda, Teva, Tonix, Vanda, Valeant and Wyeth and personal fees from Acadia, Adamas, Alkermes, Allergan, Arbor Pharmaceutcials, AstraZeneca, Avanir, Axovant, Axsome, Biogen, Biomarin, Biopharma, Celgene,Concert, ClearView, DepoMed, Dey, EnVivo, Ferring, Forest, Forum, Genomind. Innovative Science Solutions, Intra-Cellular Therapies, Janssen, Jazz, Lilly, Lundbeck, Merck, Neos, Novartis, Noveida, Orexigen, Otsuka, PamLabs, Perrigo, Pfizer, Pierre Fabre, Reviva, Servier, Shire, Sprout, Sunovion, Taisho, Takeda, Taliaz, Teva, Tonix, Trius, Vanda, and Viforpharma, and other from RCT Logic and Genomind outside the submitted work.



