Introduction
Interoception, the perception of signals from inside the body, plays an important role in health and disease. Altered interoception can be observed in mental disorders with physical symptoms, such as major depressive disorder (MDD) (Avery et al., Reference Avery, Drevets, Moseman, Bodurka, Barcalow and Simmons2014; Dunn, Dalgleish, Ogilvie, & Lawrence, Reference Dunn, Dalgleish, Ogilvie and Lawrence2007; Terhaar, Viola, Bar, & Debener, Reference Terhaar, Viola, Bar and Debener2012) or somatic symptom disorders (Pollatos et al., Reference Pollatos, Herbert, Wankner, Dietel, Wachsmuth, Henningsen and Sack2011; Schaefer, Egloff, Gerlach, & Witthoft, Reference Schaefer, Egloff, Gerlach and Witthoft2014). One important factor for altered interoception and, therefore, the generation of physical symptoms in these disorders is an allostatic disturbance of regulatory circuitry of bodily systems (Harshaw, Reference Harshaw2015; Khalsa et al., Reference Khalsa, Adolphs, Cameron, Critchley, Davenport, Feinstein and Interoception Summit2018; Schulz & Vögele, Reference Schulz and Vögele2015). Two prominent examples of these regulatory circuitries concern both physiological stress axes, that is the sympatho-adreno-medullary (SAM) axis and hypothalamic–pituitary–adrenocortical (HPA) axis (Chrousos & Gold, Reference Chrousos and Gold1992; McEwen, Reference McEwen2007). One way to understand symptom generation in mental disorders may thus be elucidating the impact of both stress axes on interoception.
Interoception is considered a multifaceted construct and can be subdivided into (Garfinkel, Seth, Barrett, Suzuki, & Critchley, Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015): (a) Interoceptive accuracy (IAcc), that is the correspondence between objectively occurring and perceived bodily signals (e.g., heartbeats), which is typically assessed using heartbeat perception tasks (e.g., “heartbeat counting task” [HCT]: Schandry, Reference Schandry1981). (b) Interoceptive sensibility (IS), which refers to the tendency to focus on signals from inside the body. This facet is based on self-reports, such as confidence ratings about one's accuracy or specific questionnaires. (c) Metacognitive interoceptive awareness, representing the correspondence between IAcc and IS, which is estimated with intra-individual correlations between both measures, thus requiring a large number of trials to produce interpretable data (Garfinkel et al., Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). IAcc is interpreted as the most basic indicator of interoceptive abilities as it shows a stable relationship with both other facets, whereas the other facets remain partially unrelated (Forkmann et al., Reference Forkmann, Scherer, Meessen, Michal, Schachinger, Vogele and Schulz2016; Garfinkel et al., Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015, Reference Garfinkel, Manassei, Hamilton-Fletcher, In den Bosch, Critchley and Engels2016).
IAcc increases after a strong laboratory stressor when attention is only focused on heartbeats (Schandry & Specht, Reference Schandry and Specht1981; Schulz, Lass-Hennemann, Sutterlin, Schächinger, & Vögele, Reference Schulz, Lass-Hennemann, Sutterlin, Schächinger and Vögele2013). Both physiological stress axes might be involved, as both cortisol (Maeda, Ogishima, & Shimada, Reference Maeda, Ogishima and Shimada2019; Schulz, Strelzyk, et al., Reference Schulz, Strelzyk, Ferreira de Sa, Naumann, Vögele and Schächinger2013) and beta-adrenergic activation (Eichler & Katkin, Reference Eichler and Katkin1994; Herbert, Pollatos, Flor, Enck, & Schandry, Reference Herbert, Pollatos, Flor, Enck and Schandry2010; Moor et al., Reference Moor, Mundorff, Bohringer, Philippsen, Langewitz, Reino and Schachinger2005) may increase IAcc. It remains unclear, however, whether one of these axes was responsible for the increasing effect after a laboratory stressor (Schulz, Lass-Hennemann, et al., Reference Schulz, Lass-Hennemann, Sutterlin, Schächinger and Vögele2013). A limitation of one previous study was that only the peripheral branch of the SAM axis was activated (e.g., stimulation of beta1-adrenergic receptors by adrenaline and beta1-adrenergic blockade by esmolol infusion) (Moor et al., Reference Moor, Mundorff, Bohringer, Philippsen, Langewitz, Reino and Schachinger2005), whereas the role of the central branch (e.g., central noradrenergic system) remained unclear. The first aim of the current study was, therefore, to elucidate the impact of an activation of the entire SAM axis by a pharmacological intervention on IAcc.
Central alpha2-adrenergic receptors have their highest density within the locus coeruleus (LC) (Coull, Reference Coull1994) and the nucleus tractus solitarius (NTS) (Rockhold & Caldwell, Reference Rockhold and Caldwell1980) and, as autoreceptors, mediate a negative feedback mechanism for central noradrenergic and sympathetic activity. Alpha2-antagonists have been shown to induce increased alertness, vigilance (Berridge & Foote, Reference Berridge and Foote1991) and sympathetic cardiovascular activation as indicated by increased heart rate (HR), systolic (SAP), and diastolic arterial blood pressure (DAP) (Philippsen et al., Reference Philippsen, Hahn, Schwabe, Richter, Drewe and Schachinger2007). In summary, the blockade of alpha2-adrenergic receptors can be used to activate both central and peripheral components of the SAM axis.
Previous studies of acute stress on interoception have mainly investigated the role of normally functioning physiological stress axes of healthy individuals for IAcc. In contrast, the relationship between physiological stress axes and IAcc in chronic stress or stress-related disorders, such as MDD, remains unclear. Environmental stress and adverse life events, such as adverse childhood experiences (ACE), play an important role in the development and clinical course of MDD (Brown, Harris, & Hepworth, Reference Brown, Harris and Hepworth1994; Brown, Schulberg, Madonia, Shear, & Houck, Reference Brown, Schulberg, Madonia, Shear and Houck1996; Kessler, Reference Kessler1997; Paykel, Reference Paykel2001). Therefore, it is not surprising that changes of stress hormones and neurotransmitter regulation related to the physiological stress axes have been associated with MDD. Noradrenaline has even been suggested to play a key role in the pathophysiology of MDD (Maletic, Eramo, Gwin, Offord, & Duffy, Reference Maletic, Eramo, Gwin, Offord and Duffy2017). Especially regarding alpha2-adrenergic receptors, there is evidence suggesting increased affinity and density in the LC and the prefrontal cortex in MDD patients (Cottingham & Wang, Reference Cottingham and Wang2012; Garcia-Sevilla et al., Reference Garcia-Sevilla, Escriba, Ozaita, La Harpe, Walzer, Eytan and Guimon1999; Ordway, Schenk, Stockmeier, May, & Klimek, Reference Ordway, Schenk, Stockmeier, May and Klimek2003; Rivero et al., Reference Rivero, Gabilondo, Garcia-Sevilla, La Harpe, Callado and Meana2014). Interestingly, chronic social stress can affect alpha2-receptor regulation depending on receptor subtypes, timing, and brain region (Flugge, Reference Flugge1996, Reference Flugge1999; Flugge, van Kampen, Meyer, & Fuchs, Reference Flugge, van Kampen, Meyer and Fuchs2003). ACE, especially as defined in this study as repeated physical or sexual abuse, constitute a severe chronic stress condition and an important risk factor for mental disorders including MDD. Furthermore, ACE affect the stress regulation systems (Heim, Ehlert, & Hellhammer, Reference Heim, Ehlert and Hellhammer2000; Orr, Metzger, & Pitman, Reference Orr, Metzger and Pitman2002; Otte et al., Reference Otte, Gold, Penninx, Pariante, Etkin, Fava and Schatzberg2016) and may therefore be one important reason for alterations in the stress systems in MDD (Heim et al., Reference Heim, Ehlert and Hellhammer2000; Heim, Newport, Mletzko, Miller, & Nemeroff, Reference Heim, Newport, Mletzko, Miller and Nemeroff2008; Otte et al., Reference Otte, Neylan, Pole, Metzler, Best, Henn-Haase and Marmar2005).
Thus, increased central alpha2-adrenergic receptor sensitivity characterized by enhanced affinity and density might be especially present in a subgroup of MDD patients with a history of ACE. Results of a study using a challenge test with an alpha2-receptor agonist in individuals with ACE but no MDD suggest increased alpha2 receptor sensitivity in association with childhood trauma (Lee, Fanning, & Coccaro, Reference Lee, Fanning and Coccaro2016). This highlights the necessity to disentangle potential effects of MDD and ACE on alpha2-adrenergic receptor function.
In summary, previous studies of acute stress effects on IAcc cannot reveal relevant processes underlying potential alterations of IAcc in stress-related disorders. The second aim of the current study was, therefore, to clarify the impact of an SAM axis activation on IAcc in healthy individuals with and without ACE, as well as in MDD patients with and without ACE.
We assessed MDD patients with and without ACE, and healthy individuals with and without ACE, with the aim of disentangling differential effects of MDD and ACE. Participants were tested once after intake of a yohimbine and once after intake of a placebo pill. Two indicators of interoception were measured in a common HCT: (a) IAcc, which is seen as most relevant indicator of interoception, and (b) IS, reflecting subjective beliefs about one's accuracy (Garfinkel et al., Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). In accordance with previous studies of acute stress on IAcc (Schulz, Lass-Hennemann, et al., Reference Schulz, Lass-Hennemann, Sutterlin, Schächinger and Vögele2013), we hypothesized (I) an increase of IAcc and IS after yohimbine administration, but not after placebo intake. Furthermore, we expected (II) principally lower IAcc and IS in MDD and ACE groups than in healthy participants (Dunn et al., Reference Dunn, Dalgleish, Ogilvie and Lawrence2007; Terhaar et al., Reference Terhaar, Viola, Bar and Debener2012). Finally, we expected (III) for yohimbine effects on cardiovascular activity and interoception to be stronger in individuals with potential upregulation of alpha2-adrenergic activity (MDD and ACE groups).
Methods
Participants
The study design was approved by the ethics committee of the German Society for Psychology (Deutsche Gesellschaft für Psychologie). All participants provided written informed consent. Healthy participants and outpatients received monetary reimbursement (100 €) for participation. Depressed patients and healthy participants were recruited by public postings and from our specialized affective disorder unit at the Department of Psychiatry and Psychotherapy, Campus Benjamin Franklin, Charité – Universitätsmedizin Berlin.
Depressed patients were included if they fulfilled criteria for a current episode of MDD as assessed by a trained psychologist (L.K.K. or C.E.D.) using a German version of the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) axis I (SCID-I) (Wittchen, Zaudig, & Fydrich, Reference Wittchen, Zaudig and Fydrich1997) to validate psychiatric diagnoses. In addition to the SCID-I interview, current depressive symptoms were assessed using a clinical rating scale and a questionnaire (Montgomery Asberg Depression Rating Scale [MADRS] (Montgomery & Asberg, Reference Montgomery and Asberg1979; Williams & Kobak, Reference Williams and Kobak2008) and the Beck Depression Inventory (BDI) (Beck, Steer, & Brown, Reference Beck, Steer and Brown1996).
ACE was defined as repeated physical or sexual abuse at least once a month over one year or more (Heim et al., Reference Heim, Ehlert and Hellhammer2000) before the age of 18. Results by Heim et al. (Reference Heim, Ehlert and Hellhammer2000) suggest long-lasting hyperreactivity of the autonomic nervous system after the experience of ACE. ACE was assessed by a screening interview and validated by the German version of the semistructured interview, the Early Trauma Inventory (ETI) (Bremner, Vermetten, & Mazure, Reference Bremner, Vermetten and Mazure2000; Wingenfeld et al., Reference Wingenfeld, Driessen, Mensebach, Rullkotter, Schaffrath, Spitzer and Heim2011). In addition, adverse childhood experiences were measured using a German version of the Childhood Trauma Questionnaire (CTQ) (Bernstein et al., Reference Bernstein, Stein, Newcomb, Walker, Pogge, Ahluvalia and Zule2003; Wingenfeld et al., Reference Wingenfeld, Spitzer, Mensebach, Grabe, Hill, Gast and Driessen2010).
For the MDD groups, exclusion criteria were schizophrenia, schizoaffective disorder, bipolar disorder, depressive disorder with psychotic features, dementia, abuse of alcohol or drugs, and panic disorder. Healthy participants with and without ACE were free of any current mental disorder. Further exclusion criteria for all participants were central nervous system (CNS)-relevant diseases, neurological diseases, severe physical conditions, for example diabetes Type 1 and 2, steroid diseases, hypertension, current infections, pregnancy, and the intake of psychotropic medication. Physical health criteria were checked by physical examination, clinical interview, and blood count.
Of 138 participants, data of 24 participants were excluded from analysis because participants completed only one testing day or data were incomplete due to technical malfunction or insufficient data quality. The final dataset consisted of 114 participants: 22 MDD patients with ACE (MDD+/ACE+), 24 MDD patients without ACE (MDD+/ACE−), 23 participants with ACE but no current or lifetime MDD (MDD−/ACE+), and 45 participants with no current or lifetime MDD and no childhood adversity (MDD−/ACE−).
Assessment of cardiovascular activity
Electrocardiogram (ECG) data were assessed using a Biopac MP150 amplifier system at 1 kHz sampling rate and a hardware high-pass filter of 0.5 Hz. Discrete blood pressure measurements were taken using a standard, automated pressure cuff, which was fixed around the right upper arm (Dinamap 1846 SX). ECG data of 5 min resting periods were analyzed with WinCPRS 1.160 software. Beat-to-beat HR data were calculated from semiautomatic QRS detection.
Interoceptive task
Due to the repeated measurement design of the study, we assessed IAcc based on the HCT only, as previous studies suggest that IAcc in the HCT task was increased if participants had completed a heartbeat discrimination task (HDT), an alternative method to assess cardiac IAcc, before (Phillips, Jones, Rieger, & Snell, Reference Phillips, Jones, Rieger and Snell1999; Schaefer, Egloff, & Witthoft, Reference Schaefer, Egloff and Witthoft2012). We presented four silent intervals (25, 35, 45, and 55 s) in random order. One training trial of 25 s length preceded the three experimental trials. Participants were asked to focus their attention on and to count their heartbeats without taking their pulse during each of these periods (and to indicate zero if they have not counted any), with a tone signaling their beginning and end. Participants were continuously monitored during assessment to ensure that they followed all study instructions. IAcc was calculated using the formula:

The majority of healthy individuals underestimate the number of heartbeats in the HCT (Zamariola, Maurage, Luminet, & Corneille, Reference Zamariola, Maurage, Luminet and Corneille2018), whereas within individuals with panic disorder, there is a certain proportion of people who overestimate the number of perceived heartbeats (Willem Van der Does, Antony, Ehlers, & Barsky, Reference Willem Van der Does, Antony, Ehlers and Barsky2000). To test for potential over- or underreporting biases of heartbeats, we calculated an alternative formula without absolute values, as previously introduced (Rost, Van Ryckeghem, Schulz, Crombez, & Vögele, Reference Rost, Van Ryckeghem, Schulz, Crombez and Vögele2017):

After each trial, participants were asked to indicate the number of perceived heartbeats and subsequently asked to rate their confidence on how correct they were on a scale ranging from 0 (not sure at all) to 8 (absolutely sure) as indicator of IS.
Pharmacological intervention
On one testing day, participants received 10 mg of oral yohimbine (Spiegel, DESMA), whereas on the other testing day they received a placebo (P-Pills, Lichtenstein). In previous studies, yohimbine dosages of 5 mg 20 mg have been shown to affect cognitive processes (O'Carroll, Drysdale, Cahill, Shajahan, & Ebmeier, Reference O'Carroll, Drysdale, Cahill, Shajahan and Ebmeier1999; Soeter & Kindt, Reference Soeter and Kindt2011, Reference Soeter and Kindt2012; Wingenfeld et al., Reference Wingenfeld, Kuffel, Uhlmann, Terfehr, Schreiner, Kuehl and Spitzer2013).Lower dosages minimize the risk of adverse side effects. Order of drug administration was counterbalanced across participants, and drugs were administered in a double-blinded (to experimenter and participants) fashion.
Procedure
Psychological assessment took place on a separate day prior to the laboratory testing. Participants were tested in two separate laboratory sessions. At least one day elapsed between testing sessions, with an average interval between both sessions of 5 days (5.4, SD: 5.2). The experimental setup was identical on both days, except for the administration of either yohimbine or placebo. All participants were requested to refrain from physical activity and caffeine consumption on the testing days. Upon arrival at the laboratory at 09:30 h, participants were seated in a comfortable chair and underwent a first baseline ECG assessment (5 min) and discrete blood pressure measurement after a 5-minute resting period. Thereafter participants received orally either yohimbine or placebo (09:45 h), followed by a 60-minute waiting period (until 10:45 h) to allow the drug to cross the gastric passage. The HCT was part of an extended study setup, the results of which will be and has been reported elsewhere (De Punder et al., Reference De Punder, Entringer, Heim, Deuter, Otte, Wingenfeld and Kuehl2018; Deuter et al., Reference Deuter, Wingenfeld, Otte, Bustami, Kaczmarczyk and Kuehl2020; Kuehl et al., Reference Kuehl, Deuter, Hellmann-Regen, Kaczmarczyk, Otte and Wingenfeld2020). The main outcome paradigms included different cognitive paradigms in a fixed order (total duration of 60 min including breaks). As plasma peak levels of yohimbine occur approx. 90 min after oral intake (O'Carroll et al., Reference O'Carroll, Drysdale, Cahill, Shajahan and Ebmeier1999; Peskind et al., Reference Peskind, Wingerson, Murray, Pascualy, Dobie, Le Corre and Raskind1995), it is plausible that after a delay of approx. 135 min between intake and HCT, drug effects are still persisting. Prior to the HCT, ECG (5 min) and blood pressure were monitored again (11:45 h). The timeline of the experiment is presented in Figure 1. The total duration of the experimental protocol was approx. 2½ hr.
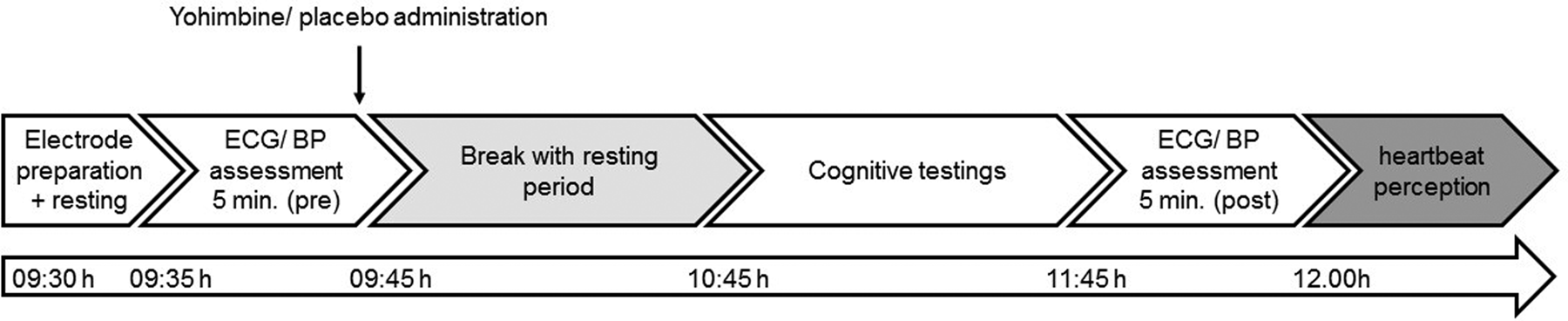
Figure 1. Timeline of experimental setup. Procedures were identical on both testing days, except for the administration of either yohimbine or a placebo substance.
Statistical analysis
Demographic and clinical characteristics, including depression and childhood trauma scores in all subscales of the BDI-II, the MADRS, the CTQ, and the ETI, were analyzed with one-way analysis of variance (ANOVA) for continuous variables or χ2 test for dichotomous variables across the four groups (MDD−/ACE−, MDD−/ACE+, MDD+/ACE−, MDD+/ACE+). If applicable, post-hoc group differences were evaluated with Tukey HSD tests. Cardiovascular data (HR, SAP, DAP) were evaluated using a 4 × 2 × 2 mixed-design ANOVA with the between-subjects factor “group,” and the within-subjects factors “drug” (yohimbine, placebo) and “time” (pre, post). Furthermore, a 4 × 2 mixed-design ANOVA with the between-subjects factor “group” and the within-subjects factor “drug” was used to evaluate effects on the dependent variables IAccHCT, IAccbias, and IS. Post-hoc tests of significant ANOVA effects were carried out using T-tests for dependent samples. To elucidate the determinants of IAccHCT and IAccbias, we calculated linear multiple regression models (enter method), one for each drug condition (Model 1: placebo, Model 2: yohimbine), separately for the criteria IAccHCT and IAccbias. Predictors of IAccHCT and IAccbias were HR, SAP, DAP (at the respective measurement occasion), depression score (BDI-II), childhood trauma score (CTQ), and IS. Critical alpha-level was set to .05 in all analyses. Analyses were carried out using SPSS version 24 (IBM Corp.).
Results
Sample characteristics
There were no significant differences between groups regarding sex, age, and body mass index (BMI), although the comparison of age and BMI across groups reached trend level, mainly caused by higher values in the MDD+/ACE+ group.
As expected, the two MDD groups (MDD+/ACE−, MDD+/ACE+) showed significantly higher total scores on the MADRS and BDI than the two healthy groups (MDD−/ACE−, MDD−/ACE+), whereas neither the two MDD groups, nor the two healthy groups differed from each other in depression scores. In addition to the diagnosis of a current MDD, 13 patients fulfilled the criteria for one or more mental comorbid disorders (MDD+/ACE− [n = 6]: four with social phobia, one somatoform pain disorder, and one avoidant personality disorder; MDD+/ACE+: [n = 7]: three with phobia [two social], one with somatoform pain disorder, one with bulimia nervosa, four with posttraumatic stress disorder [PTSD, related to ACE], and one mixed personality disorder).
In line with our recruitment criteria, the ETI total score and the CTQ total score clearly differentiated between groups with and without ACE, in that ETI total scores were higher in the MDD+/ACE+ and the MDD−/ACE+ groups compared to the MDD+/ACE− and MDD−/ACE− groups. There were no differences within groups with ACE and groups without ACE. For the CTQ total score, significant differences between all groups were observed, with lowest scores in the MDD−/ACE− group, followed by the MDD+/ACE− group, the MDD−/ACE+, and the MDD+/ACE+ group (in ascending order).
Group differences, ANOVA statistics and post-hoc comparisons with regard to depression scores and childhood trauma questionnaires are presented in Table 1.
Table 1. Sample characteristics with regard to demographics, depression and childhood adversity
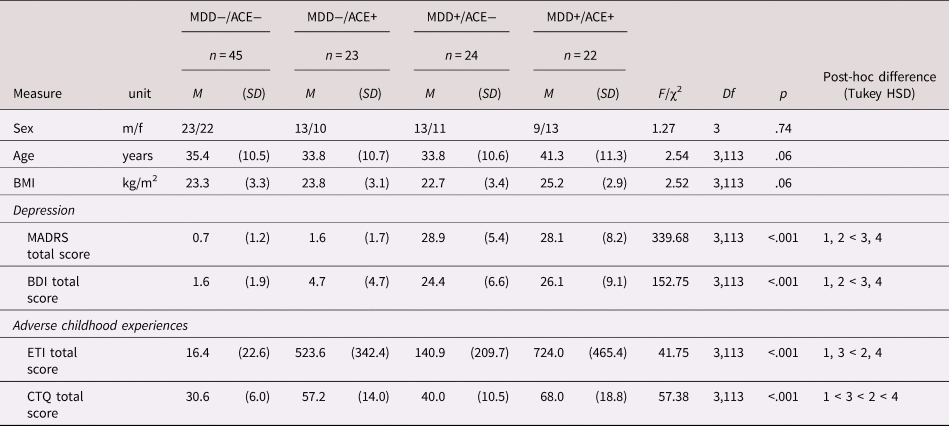
MDD = major depressive disorder, ACE = adverse childhood experiences, BMI = body mass index, MADRS = Montgomery Asberg Depression Rating Scale, BDI = Becks Depression Index, ETI = Early Trauma Interview, CTQ = childhood trauma questionnaire, M = mean, SD = standard deviation.
Cardiovascular data
Systolic arterial blood pressure (SAP)
A significant Drug × Time interaction (F[1,105] = 49.69; p < .001; ${\rm \eta }_{\rm p}^2 = .32$![]() ) indicated a stronger SAP increase from “pre” to “post” in the yohimbine (p < .001; d = 1.08) than in the placebo condition. Post-hoc analyses revealed, however, that SAP increased from “pre” to “post” also in the placebo condition, but to a lesser extent (p < .01; d = .26). Neither mean SAP, nor SAP reactivity (“pre” vs. “post”) to yohimbine differed between groups. Cardiovascular data are presented in Table 2.
) indicated a stronger SAP increase from “pre” to “post” in the yohimbine (p < .001; d = 1.08) than in the placebo condition. Post-hoc analyses revealed, however, that SAP increased from “pre” to “post” also in the placebo condition, but to a lesser extent (p < .01; d = .26). Neither mean SAP, nor SAP reactivity (“pre” vs. “post”) to yohimbine differed between groups. Cardiovascular data are presented in Table 2.
Table 2. Indicators of cardiovascular activity before and after the intake of yohimbine and a placebo substance
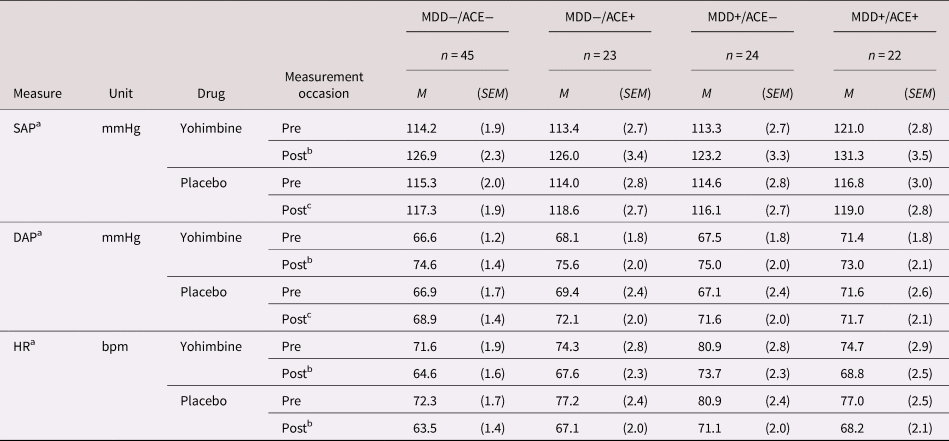
a SAP = systolic arterial pressure, DAP = diastolic arterial pressure, HR = heart rate, M = mean, SEM = standard error mean.
b Differences between “pre” and “post” significant at p < .001 (averaged over all groups).
c Differences between “pre” and “post” significant at p < .01.
Diastolic arterial blood pressure (DAP)
Comparable to SAP, we observed a significant Drug × Time interaction (F[1,105] = 16.00; p < .001; ${\rm \eta }_{\rm p}^2 = .13$![]() ). Post-hoc analyses showed that DAP increased from “pre” to “post” after placebo and yohimbine intake, but more in the yohimbine (p < .001; d = 1.01) than in the placebo condition (p < .01; d = .27). Moreover, a significant Group × Time interaction (F[3,105] = 3.77; p = .013; ${\rm \eta }_{\rm p}^2 = .10$
). Post-hoc analyses showed that DAP increased from “pre” to “post” after placebo and yohimbine intake, but more in the yohimbine (p < .001; d = 1.01) than in the placebo condition (p < .01; d = .27). Moreover, a significant Group × Time interaction (F[3,105] = 3.77; p = .013; ${\rm \eta }_{\rm p}^2 = .10$![]() ) suggests higher DAP at measurement “post” than at “pre” in the MDD−/ACE− (d = 1.05), the MDD−/ACE+ (d = .77) and the MDD+/ACE− groups (d = .89; all ps < .001), but not in the MDD+/ACE+ group (p > .10). Neither mean DAP, nor DAP reactivity (“pre” vs. “post”) to yohimbine differed between groups.
) suggests higher DAP at measurement “post” than at “pre” in the MDD−/ACE− (d = 1.05), the MDD−/ACE+ (d = .77) and the MDD+/ACE− groups (d = .89; all ps < .001), but not in the MDD+/ACE+ group (p > .10). Neither mean DAP, nor DAP reactivity (“pre” vs. “post”) to yohimbine differed between groups.
Heart rate (HR)
Mean HR was significantly higher in the MDD+/ACE− group (76.6 [2.2] bpm) than in the MDD−/ACE− group (68.03 [1.5] bpm; p = .001), whereas the other groups did not differ from each other (MDD−/ACE+: 71.6 [2.2] bpm; MDD+/ACE+: 72.2 [2.3] bpm; F[3,105] = 3.65; p = .015; ${\rm \eta }_{\rm p}^2 = .09$![]() ). Furthermore, we found a significant Drug × Time interaction (F[1,105] = 11.06; p = .001; ${\rm \eta }_{\rm p}^2 = .10$
). Furthermore, we found a significant Drug × Time interaction (F[1,105] = 11.06; p = .001; ${\rm \eta }_{\rm p}^2 = .10$![]() ). Post-hoc analyses showed that HR decreased from “pre” to “post” in both “drug” conditions (yohimbine: d = −.97; placebo: d = −1.16; all ps < .001), but the decrease was more marked after placebo than after yohimbine intake. HR reactivity to yohimbine did not differ between groups.
). Post-hoc analyses showed that HR decreased from “pre” to “post” in both “drug” conditions (yohimbine: d = −.97; placebo: d = −1.16; all ps < .001), but the decrease was more marked after placebo than after yohimbine intake. HR reactivity to yohimbine did not differ between groups.
Interoception
Interoceptive accuracy (IAcc)
We observed a significant main effect of “drug” (F[1,110] = 5.60; p = .02; ${\rm \eta }_{\rm p}^2 = .05$![]() ), which was explained by a significant Group × Drug interaction (F[1,110] = 2.82; p = .042; ${\rm \eta }_{\rm p}^2 = .07$
), which was explained by a significant Group × Drug interaction (F[1,110] = 2.82; p = .042; ${\rm \eta }_{\rm p}^2 = .07$![]() ), indicating lower IAccHCT after yohimbine than after placebo administration in the group MDD−/ACE+ (p = .001). There were neither differences in IAccHCT between “drug” conditions in any other group (see Figure 2a) nor differences between groups. There were statistical trends suggesting difference in age and BMI between groups (mainly due to descriptively higher age and BMI in the MDD+/ACE+ group compared to the MDD+/ACE− group). As age (Khalsa, Rudrauf, & Tranel, Reference Khalsa, Rudrauf and Tranel2009) and BMI (Herbert, Blechert, Hautzinger, Matthias, & Herbert, Reference Herbert, Blechert, Hautzinger, Matthias and Herbert2013; Herbert & Pollatos, Reference Herbert and Pollatos2014) may potentially affect IAcc, we included “age” and “BMI” as covariates in the statistical model in a follow-up analysis. After controlling for both variables, the Group × Drug interaction remained significant (F[3,108] = 2.85; p = .041; ${\rm \eta }_{\rm p}^2 = .07$
), indicating lower IAccHCT after yohimbine than after placebo administration in the group MDD−/ACE+ (p = .001). There were neither differences in IAccHCT between “drug” conditions in any other group (see Figure 2a) nor differences between groups. There were statistical trends suggesting difference in age and BMI between groups (mainly due to descriptively higher age and BMI in the MDD+/ACE+ group compared to the MDD+/ACE− group). As age (Khalsa, Rudrauf, & Tranel, Reference Khalsa, Rudrauf and Tranel2009) and BMI (Herbert, Blechert, Hautzinger, Matthias, & Herbert, Reference Herbert, Blechert, Hautzinger, Matthias and Herbert2013; Herbert & Pollatos, Reference Herbert and Pollatos2014) may potentially affect IAcc, we included “age” and “BMI” as covariates in the statistical model in a follow-up analysis. After controlling for both variables, the Group × Drug interaction remained significant (F[3,108] = 2.85; p = .041; ${\rm \eta }_{\rm p}^2 = .07$![]() ). When evaluating IAccbias, there were neither significant group differences, nor any yohimbine effects (Figure 2b).
). When evaluating IAccbias, there were neither significant group differences, nor any yohimbine effects (Figure 2b).
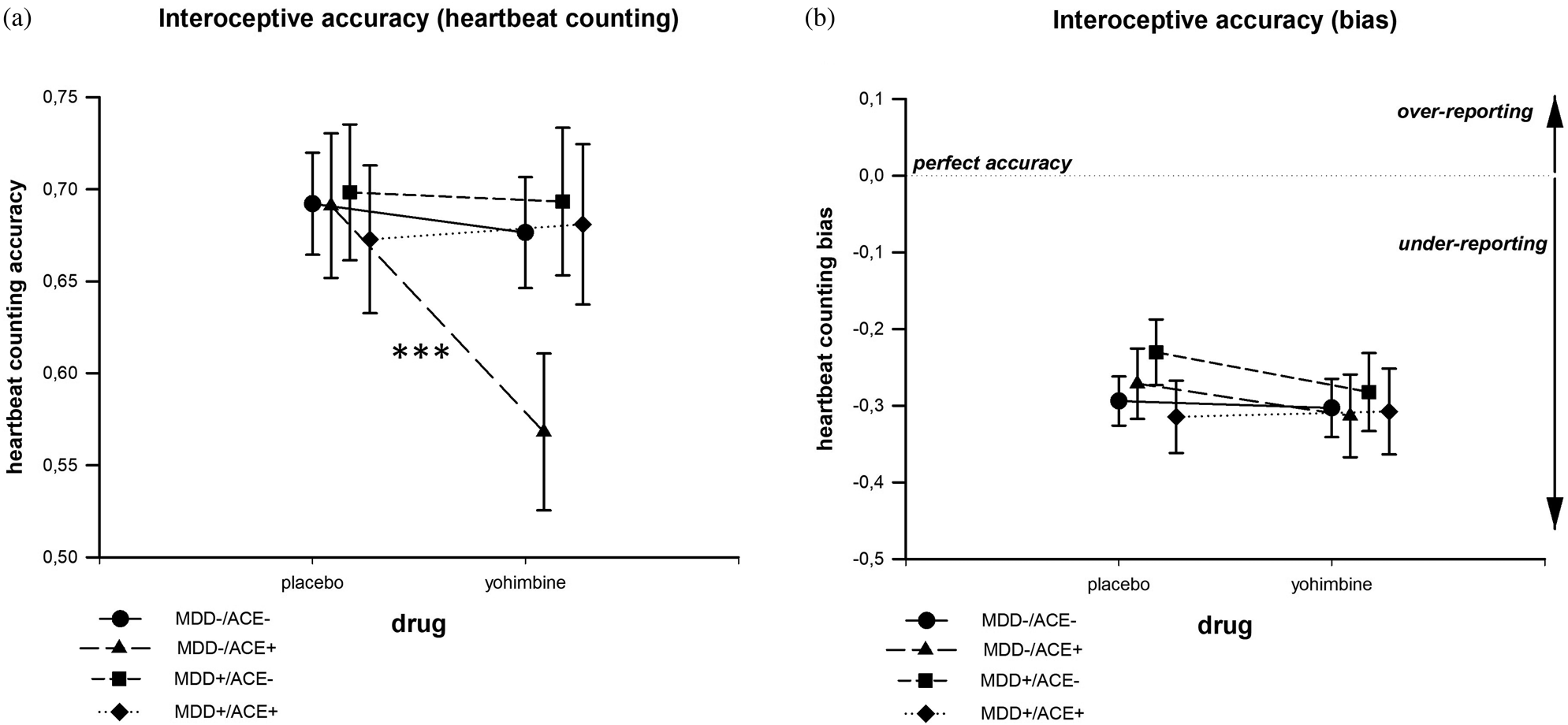
Figure 2. Interoceptive accuracy (IAcc) in the heartbeat counting task after the intake of yohimbine and a placebo substance. In the MDD−/ACE+ group, IAcc was lower after yohimbine than after placebo intake (a). When evaluating the over- versus underreporting bias of IAcc, no group difference or drug effect emerged (b).
Interoceptive sensibility (IS)
There were no significant differences between groups or between “drug” conditions (see Figure 3).
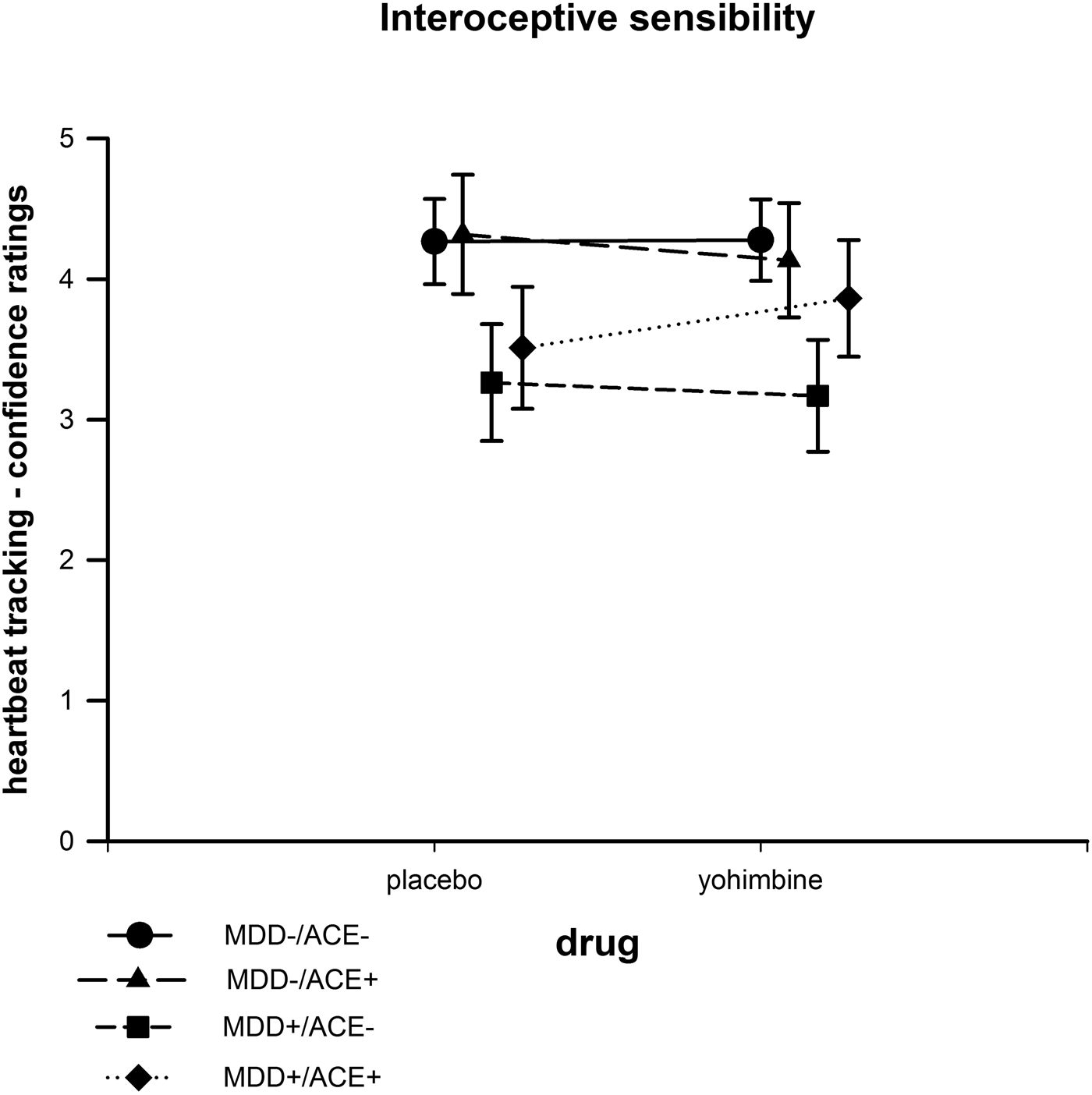
Figure 3. Interoceptive sensibility based on confidence ratings in the heartbeat counting task after the intake of yohimbine and a placebo substance.
Regression analyses
IAccHCT after placebo (Model 1) was significantly predicted by HR, SAP, DAP, BDI (measuring depression), and CTQ (measuring childhood trauma) scores and IS as indicated by a statistical significance of the overall model (see Table 3). Among all predictors, however, only HR and IS remained as a significant predictor. After yohimbine (Model 2), the overall model to predict IAccHCT was significant (Tab. 3). In this model, significant predictors were depression scores, childhood trauma scores, and IS among all predictors. With regard to the criterion IAccbias, neither the overall Models 1 and 2, nor any of the single predictors reached significance level.
Table 3. Predictors of interoceptive accuracy after placebo (regression Model 1) and yohimbine administration (regression Model 2)
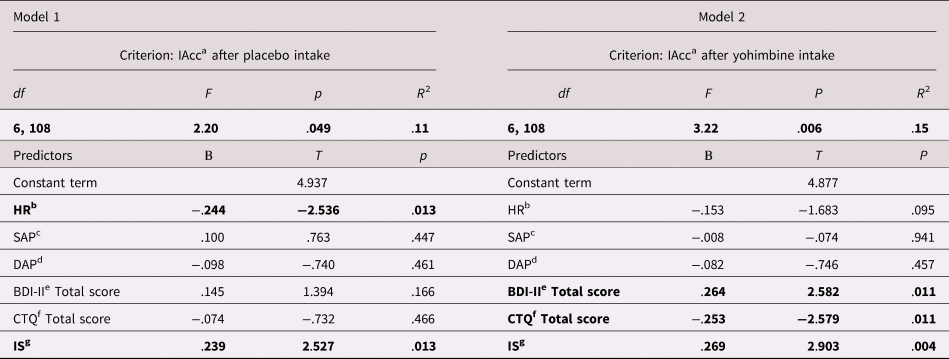
Note: Significant predictors in bold. ainteroceptive accuracy; bheart rate; csystolic arterial blood pressure; ddiastolic arterial blood pressure; eBeck's Depression Inventory II; fChildhood Trauma Questionnaire; ginteroceptive sensibility.
Discussion
The current study examined the effect of SAM axis activation by the alpha2-adrenergic antagonist yohimbine versus placebo on IAcc and IS. We investigated two healthy groups with and without ACE and two MDD groups with and without ACE, as both, MDD and ACE, may be associated with an upregulation of central alpha2-adrenoceptors. All patients and participants were free of antidepressant or cardiovascular medication. An increase of SAP and DAP, and a smaller decrease in HR in the yohimbine compared to the placebo condition suggests effective stimulation of the SAM axis by alpha2-adrenoceptor blockade. In contrast to our expectations (Hypotheses I and III), however, we observed reduced IAccHCT after yohimbine intake in the MDD−/ACE+, but not in any of the other groups. Age and BMI did not affect these results. To test if these effects were due to a selective over- or underreporting bias (Zamariola et al., Reference Zamariola, Maurage, Luminet and Corneille2018), and, therefore, the cognitive strategy to perform the HCT (Desmedt, Luminet, & Corneille, Reference Desmedt, Luminet and Corneille2018), we also evaluated the bio-polar index IAccbias which indicates positive values in overreporting and negative values in underreporting (Rost et al., Reference Rost, Van Ryckeghem, Schulz, Crombez and Vögele2017). As IAccbias did not differ between groups and did not respond to yohimbine administration, it is unlikely that the effect of yohimbine on IAccHCT is solely due to an over- or underreporting bias. In contrast to IAccHCT, IS did not respond to yohimbine intake in any group. This finding suggests that the yohimbine effects on interoception are specific for the actual perception of heartbeats, whereas this (potentially temporary) effect does not translate into a dispositional tendency to be internally self-focused (Garfinkel et al., Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015).
After placebo administration, HR was identified as a negative and IS as a positive predictor of IAccHCT, which is in line with the multifaceted model of interoception by Garfinkel et al. (Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015) and extended by Forkmann et al. (Reference Forkmann, Scherer, Meessen, Michal, Schachinger, Vogele and Schulz2016). In this model, cardiovascular activation (i.e., HR) and IS are deemed proximate levels of IAccHCT that may interact in both bottom-up and tow-down regulatory circuits (Forkmann et al., Reference Forkmann, Scherer, Meessen, Michal, Schachinger, Vogele and Schulz2016; Garfinkel et al., Reference Garfinkel, Seth, Barrett, Suzuki and Critchley2015). Therefore, the relationship of IAcc with HR and IS can be seen as support for the validity of our findings. In contrast, after yohimbine administration, depression and childhood trauma scores were identified as positive (depression) and negative (childhood trauma) predictors of IAccHCT, whereas HR was not significant. First, we conclude that alpha2-adrenoceptors play a role in mediating interoceptive signal processing. Second, we conclude that noradrenergic activation can decrease interoceptive accuracy in healthy individuals with ACE which is supported by Drug × Group interaction effect and the results of the regression analysis identifying the CTQ score as a negative predictor of IAccHCT. This speaks in favor of the hypothesis that upregulated alpha2-adrenoreceptors are associated with ACE. In contrast, we did not find such effects regarding MDD so that our results do not support the hypothesis of upregulated alpha2-adrenoreceptors in MDD. Finally, the finding that neither IS, nor any indicator of cardiovascular activation was a predictor of IAccbias suggests that cognitive biases of over- or underreporting are unrelated to the actual strength of body signals and subjective confidence about one's accuracy to report those signals.
Two important structures of the central noradrenergic system show a particularly high concentration of alpha2-adrenergic receptors: the LC and the NTS (Coull, Reference Coull1994; Rockhold & Caldwell, Reference Rockhold and Caldwell1980). The LC is involved in mediating alpha2-adrenergic effects on alertness, vigilance, and attention (Aston-Jones, Rajkowski, & Cohen, Reference Aston-Jones, Rajkowski and Cohen1999; Berridge & Foote, Reference Berridge and Foote1991; Coull, Nobre, & Frith, Reference Coull, Nobre and Frith2001; Usher, Cohen, Servan-Schreiber, Rajkowski, & Aston-Jones, Reference Usher, Cohen, Servan-Schreiber, Rajkowski and Aston-Jones1999), whereas alpha2-adrenergic effects on the NTS are responsible for cardiovascular sympathetic activation, such as an increase in HR, SAP, and DAP (Grossman, Rea, Hoffman, & Goldstein, Reference Grossman, Rea, Hoffman and Goldstein1991; Philippsen et al., Reference Philippsen, Hahn, Schwabe, Richter, Drewe and Schachinger2007). Furthermore, alpha2-adrenoreceptors mediate the processing and integration of visceral–afferent signals in the arterial baroreflex circuitries (Hayward, Riley, & Felder, Reference Hayward, Riley and Felder2002; Kubo, Goshima, Hata, & Misu, Reference Kubo, Goshima, Hata and Misu1990; Sved, Tsukamoto, & Schreihofer, Reference Sved, Tsukamoto and Schreihofer1992; Yamazaki & Ninomiya, Reference Yamazaki and Ninomiya1993).
For interoception, at least two processes of an SAM axis activation by yohimbine may be of relevance: the alertness component of attention and sympathetic activation. Both processes, however, can impact interoception in opposite ways if affected by alpha2-adrenergic blockade. On the one hand, peripheral sympathetic activation increases cardiac contractility (Scherhag, Stastny, Pfleger, Voelker, & Heene, Reference Scherhag, Stastny, Pfleger, Voelker and Heene1999), which increases IAcc (Eichler & Katkin, Reference Eichler and Katkin1994; Herbert et al., Reference Herbert, Pollatos, Flor, Enck and Schandry2010; Moor et al., Reference Moor, Mundorff, Bohringer, Philippsen, Langewitz, Reino and Schachinger2005; Schandry, Bestler, & Montoya, Reference Schandry, Bestler and Montoya1993). On the other hand, alpha2-antagonists facilitate alertness, but reduce capacity for focused and selective attention (Clark, Geffen, & Geffen, Reference Clark, Geffen and Geffen1986, Reference Clark, Geffen and Geffen1987; Hermans et al., Reference Hermans, van Marle, Ossewaarde, Henckens, Qin, van Kesteren and Fernandez2011). The dose used in the current study (10 mg) may have resulted in a predominance of the reducing effect on focused attention over an increase in sympathetic activity (at least in the MDD−/ACE+ group). Decreased IAcc in SAM axis activation may be part of an adaptive response in healthy participants with ACE. Acute stress could, therefore, result in suppression or denial of physical symptoms as survival and coping mechanism (Bernstein & Claypool, Reference Bernstein and Claypool2012; Schaan et al., Reference Schaan, Schulz, Rubel, Bernstein, Domes, Schächinger and Vögele2019) and thus promote resilience in this group.
A change of IAccHCT in response to the relatively low dose of 10 mg yohimbine in the current study suggests that an upregulation of alpha2-adrenergic receptors, such as to be expected after ACE, is necessary to result in altered IAccHCT. In the other groups, this dose may not have been sufficient to affect IAcc, as they probably do not show an upregulated sensitivity of alpha2-adrenergic receptors. Taken together with a recent study using an alpha2-receptor agonist challenge test in individuals with ACE (Lee et al., Reference Lee, Fanning and Coccaro2016), the present results support the notion of increased sensitivity of central alpha2-adrenergic receptors associated with ACE.
Afferent parasympathetic visceral–afferent signals are relayed over the cranial nerves (VII, IX, X) and the NTS, whereas sympathetic visceral–afferent signals are transmitted via the lamina1 layer of the spinal dorsal horn, before thalamic nuclei, the anterior cingulate, and insular cortices are reached (Cameron, Reference Cameron2001; Craig, Reference Craig2002; Schulz, Reference Schulz2016). While the sympathetic pathway includes the LC (Cameron, Reference Cameron2001), some sympathetic information is relayed via the NTS (Craig, Reference Craig2002), which may particularly include (alpha2-)adrenergic receptor circuitries. Both neural pathways are considered relevant for mediating visceral–afferent neural signals associated with heartbeat perception (Critchley, Wiens, Rotshtein, Ohman, & Dolan, Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004; Pollatos, Schandry, Auer, & Kaufmann, Reference Pollatos, Schandry, Auer and Kaufmann2007). On the one hand, transcutaneous vagal nerve stimulation increases of IAcc (Villani, Tsakiris, & Azevedo, Reference Villani, Tsakiris and Azevedo2019), which implies that the parasympathetic pathway is essential for cardiac interoception. On the other hand, a blockade of the alpha2-adrenergic receptor circuits in individuals with a potentially high receptor density or sensitivity decreases IAcc, suggesting that the sympathetic (neural) pathway is similarly important. One may conclude, therefore, that under homeostatic conditions, neural signal transmission from both pathways is crucial for an adequate interoception, whereas an acute or chronic allostatic condition may disrupt one or both pathways, potentially contributing to lower IAcc.
Lower IAcc has been previously reported in a moderately depressed community sample compared to healthy individuals (Dunn et al., Reference Dunn, Dalgleish, Ogilvie and Lawrence2007), and reduced cortical representation of interoceptive signals in MDD (Terhaar et al., Reference Terhaar, Viola, Bar and Debener2012). In contrast to Hypothesis II, in the current study, there were no differences between MDD patients and healthy participants, although the present sample size was larger than in both previous studies. The inclusion of MDD patients without antidepressant medication in the current study allowed us to disentangle possible effects associated with MDD-specific psychobiological alterations (e.g., increased central alpha2-adrenergic receptor sensitivity) from pharmacodynamic effects of antidepressive medication including their impact on cardiovascular activity (Glassman, Reference Glassman1998), physiological stress axes activity (Surget et al., Reference Surget, Tanti, Leonardo, Laugeray, Rainer, Touma and Belzung2011), and attention (Constant et al., Reference Constant, Adam, Gillain, Seron, Bruyer and Seghers2005), which may all affect interoception. As in both earlier studies a substantial proportion of MDD patients were taking antidepressants (mainly selective serotonin reuptake inhibitors, SRIs) (Dunn et al., Reference Dunn, Dalgleish, Ogilvie and Lawrence2007; Terhaar et al., Reference Terhaar, Viola, Bar and Debener2012), it cannot be ruled out that the previously reported group differences in interoception may be due to pharmacological effects of medication.
We assessed ACE with two different measures: a semistructured interview (ETI) and a self-reporting questionnaire (CTQ). Group allocation was based on the ETI. However, in contrast to the ETI, which did not show higher scores individuals with MDD only, the CTQ also reflected an association with MDD diagnosis. Participants with MDD had higher CTQ scores compared to healthy controls, and this was true for both participants with and without ACE. Such a discrepancy between questionnaires and interview measures has been described in previous studies (e.g., Cisler et al., Reference Cisler, James, Tripathi, Mletzko, Heim, Hu and Kilts2013) and can be attributed to the survey methodology. The direction of causality of this effect of MDD on CTQ scores remains unknown. It is of course plausible that MDD individuals have had more negative experiences in childhood than healthy controls, even if these are not severe enough to meet the ACE inclusion criteria. Again, within the ACE+ groups, the more severely affected may also show greater vulnerability to MDD. Nevertheless, the ETI can be considered the more sensitive measure compared to questionnaires (Baldwin, Reuben, Newbury, & Danese, Reference Baldwin, Reuben, Newbury and Danese2019); and the ETI does not show these differences. An alternative explanation would, therefore, be a reporting bias in the questionnaire, which leaves no room for questions and assessments by the investigator. MDD patients might have retrospectively assessed their childhood more negatively (Colman et al., Reference Colman, Kingsbury, Garad, Zeng, Naicker, Patten and Thompson2016).
Limitations
Since we tested only patients without antidepressive medication, we cannot make any claim about how the intake of medication might affect our findings. Thus, the current findings of “normal” IAcc (placebo condition) in MDD may have to be interpreted with caution due to the limited representativeness for those MDD patients who receive antidepressant medication. We assessed IAcc only after drug intake, whereas no baseline assessment took place to reduce the possible impact of learning, as IAccHCT is subject to moderate training effects (Wittkamp, Bertsch, Vogele, & Schulz, Reference Wittkamp, Bertsch, Vogele and Schulz2018). Although the repeated-measurement design provided a within-subjects control condition, this baseline assessment would have allowed controlling for occasion-specific variance. Furthermore, IAcc was only assessed with the HCT, although the additional use of the HDT would have provided more insights on attentional processes. We decided to present only the HCT to overcome potential sequence effects if the HDT is presented before the HCT (Phillips et al., Reference Phillips, Jones, Rieger and Snell1999; Schaefer et al., Reference Schaefer, Egloff and Witthoft2012). On the one hand, recent methodological studies suggested that IAcc assessed by the HCT is potentially correlated with knowledge of one's heart rate (Murphy et al., Reference Murphy, Millgate, Geary, Ichijo, Coll, Brewer and Bird2018) or susceptibility to cognitive strategies (Desmedt et al., Reference Desmedt, Luminet and Corneille2018). On the other hand, a substantial overlap with heartbeat-evoked potentials as neurophysiological indicator of cardiac interoception (Mai, Wong, Georgiou, & Pollatos, Reference Mai, Wong, Georgiou and Pollatos2018; Pollatos & Schandry, Reference Pollatos and Schandry2004; Yuan, Yan, Xu, Han, & Yan, Reference Yuan, Yan, Xu, Han and Yan2007) and reduced IAccHCT in individuals with a degeneration of afferent autonomic nerves (Pauli, Hartl, Marquardt, Stalmann, & Strian, Reference Pauli, Hartl, Marquardt, Stalmann and Strian1991) support the validity of this task in terms of its underlying neurophysiology. One could conclude, therefore, that although IAccHCT may also be affected by potential confounding variables, it represents a well-validated method to reflect the processing of afferent signals from the cardiovascular system. Furthermore, an intravenous administration of yohimbine would have allowed a more precise timing of pharmacological effects, however, we decided for a less invasive way of yohimbine administration to avoid interference with the physiological stress axes. Finally, in the current study we focused on SAM axis activity as outcome measure, because alpha2-adrenergic receptors are seen as one component of this axis. Nevertheless, it needs to be acknowledged that activity of the HPA axis may also be affected by SAM axis activity. Potential interaction effects of both stress axes, however, as well as the role of other stress-associated mechanisms for IAcc, such as immune system activity (Khalsa et al., Reference Khalsa, Adolphs, Cameron, Critchley, Davenport, Feinstein and Interoception Summit2018; Savitz & Harrison, Reference Savitz and Harrison2018), remain to be addressed in future studies. Follow-up studies may also wish to investigate if negative findings in the MDD+/ACE+ group may be due to a specific dysregulation of HPA axis activity in MDD patients such as blunted HPA axis responses to acute stress in a subset of patients (Burke, Davis, Otte, & Mohr, Reference Burke, Davis, Otte and Mohr2005; Gold, Reference Gold2015; Gold & Chrousos, Reference Gold and Chrousos2002). In addition, the current sample size did not allow for further analyses of effects of timing and duration of ACE. As long-lasting effects of ACE are probably related to sensitive phases in development (e.g., for review: Lupien, McEwen, Gunnar, & Heim, Reference Lupien, McEwen, Gunnar and Heim2009), this aspect should also be addressed in future studies.
Conclusions
The present study shows effective activation of the SAM axis in healthy individuals with and without ACE as well as in MDD patients with and without ACE after intake of 10 mg of the alpha2-adrenergic antagonist yohimbine, as indicated by HR, SAP, and DAP levels. Only in the healthy group with ACE yohimbine intake resulted in reduced IAccHCT, which may be explained by extenuated focused attention associated with central noradrenergic activation. The underlying process may involve a persisting upregulation of alpha2-adrenoreceptors in the LC and/or suppression of physical symptoms in acute stress after ACE.
Acknowledgments
Neither the Research Support Department of the University of Luxembourg, nor the German Research Foundation had any role in study design, in the collection, analysis, and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Funding Statement
Linn Kuehl (PI), Katja Wingenfeld, and Christian Otte were supported by the grant “Effects of increased noradrenergic activity by yohimbine administration on learning and attention in patients with major depression disorder” (project KU 3106/2-1), funded by the German Research Foundation (Deutsche Forschungsgemeinschaft/DFG). André Schulz (PI), Ion-Hideo Breden, and Claus Vögele were supported by the grant “Interoception and chronic stress,” funded by the Research Support Department of the University of Luxembourg.









