Undoubtedly vaccines are a fundamental tool to control the infectious diseases outbreak and are the most confident means to terminate the epidemic risk. Based on various evidence, it routinely needs 5 to 10 y to develop a vaccine for the infectious agents. Reference Wolf, Bruno and Eichberg1,Reference Excler, Saville and Berkley2 As we enter the year 2020, a new viral infectious agent engulfs the entire globe in a short time. By looking back at the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak, the global burden of coronavirus disease 2019 (COVID-19) infection is estimated at 229,329,042 cases and approximately 4,705,890 deaths (September 20, 2021). Reference Worldometer3
A remarkable spread for researching and developing vaccines was created by the increasing rate of transmission and death. Hence, all processes from the initial SARS-CoV-2 sequencing to an interim analysis of vaccine efficiency were performed in just 300 d. Reference Cleve4
Therefore, in the following attempts, 3 first vaccines (Pfizer, Moderna, Janssen) were introduced and approved by emergency by the United States Food and Drug Administration for use authorization publicly. Under the pressure of outbreak, several companies in different regions of world for instance Serum Institute in India for Astra-Zeneca and Novavax, Beijing Bio Institute in China for Sinopharm were licensed for manufacturing vaccines. Reference Kim5 These products have the green light from the World Health Organization (WHO) to be rolled out globally for emergency use. Although the vaccination is a very complex and challenging procedure and needs very special platforms to entirely eradicate COVID-19, it is necessary to produce, distribute, and vaccinate people worldwide in a short time. Reference van Riel and de Wit6
Iran has gone through 5 waves since the identification and spread of the Delta variant. Despite all efforts made by health-care workers, due to some circumstances, the death rate was reported high in some days of peak. Although the vaccination program was started before the Delta variant, there are some reasons that made the situation worse. This brief report describes the characteristics of death in vaccinated and unvaccinated people hospitalized after 14 d of vaccination in Fars province in the south of Iran.
Methods
Study Design
This cross-sectional study was performed to describe dead breakthrough cases in the study region from the initial time of the vaccination program until now (February 2, to 19 August 2021).
The ethical committee approved this study of Shiraz University of Medical Sciences (Ethical code: IR.SUMS.REC. 1399.022.). We selected a control group for more comparisons, which was age- and sex-matched and double the size of the patient group. The control group included unvaccinated who died in the hospitals from the same database of patients.
Data Collection
For any hospitalized patients who were confirmed with COVID-19, comprehensive information was recorded in electronic database registry data like; demographic data, symptoms of the disease at arrival, underlying chronic health problems, duration of time from onset of symptoms, vaccination status, doses, type, and date of injection and, the clinical outcome as death or discharge. Information was collected and entered into the electronic registry of Shiraz University of Medical Sciences.
Statistical Analysis
The data are described with proper descriptive statistics. The independent sample t-test or Pearson chi-squared test was compared for quantity and qualitative variables, respectively. In univariate analysis, a logistic regression model was fitted for variables with a P-value less than 0.2. All statistical analyses were performed with SPSS22, and a P-value less than 0.05 was considered the threshold of statistical significance.
Participants
Based on the Center of Disease Control (CDC) definition, a vaccine breakthrough infection is defined as detecting SARS-CoV-2 RNA or antigen in a respiratory specimen collected from a person 14 ≥ d after they have completed all recommended doses of COVID-19 vaccine. Participants in this study included 297 dead breakthrough infections, who were hospitalized in Fars, Iran.
Results
All vaccinated people were investigated from the beginning of the vaccination program. According to our findings, 368 cases became positive for SARS-CoV-2 infection, 1 wk after vaccination. Among 444,728 fully vaccinated people, 60,800 breakthrough cases were detected from the second week onward cumulatively (Figure 1). Overall, 501 patients died, of which 297 patients were hospitalized. These patients (n = 297) were described and compared with an age- and sex-matched group. The median age for breakthrough cases and the unvaccinated group was estimated 79 and 65 y, respectively. The sex distribution of dead breakthrough cases was 127 (42.8%) females and 170 (57.2%) males. Furthermore, in the unvaccinated death group, 273 (45.8%) females and 322 (54.11%) males were included. Although all signs and symptoms of COVID-19 were more frequent in the unvaccinated group, fever, muscle pain, headache, and anorexia were significantly different between the 2 groups. Decreasing O2 saturation (less than 93%) happened more often in the unvaccinated group significantly. The duration of hospital stay was impressively less in the unvaccinated dead patients. It means dying of COVID-19 within a short time. Vaccines type in breakthrough death patients consists of 66.63% Sinophram, 0.67% Sputnik, 0.67% COVIran Barekat and 31.99% AstraZeneca. Variables with a P-value less than 0.2 in the univariate analysis are included in a regression model. The factors were evaluated in association with breakthrough and unvaccinated death cases. Respiratory distress was more often present (odds ratio [OR] = 5.6; 95% confidence interval [CI]: 1.23, 8.93; P = 0.001) in the unvaccinated death population versus breakthrough death. Other variables were not statistically significant. More details are provided in Table 1.
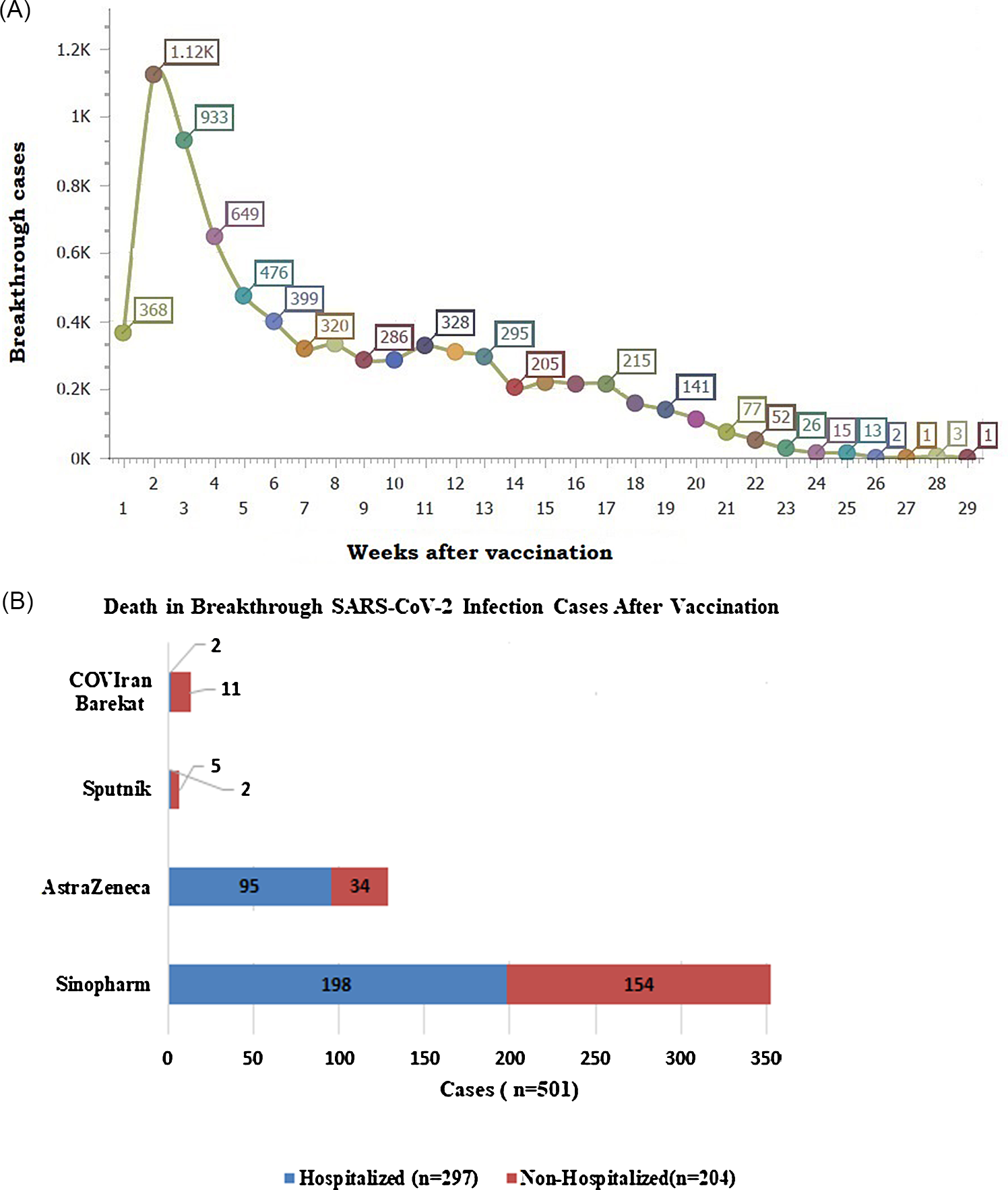
Figure 1. Cumulative breakthrough cases (A), and death count by vaccine type (B).
Discussion
This is the first report comparing breakthrough and unvaccinated deaths in Fars, Iran. Vaccines are the most effective tool for controlling this infectious outbreak in the current crisis. Despite the high level of vaccine efficiency (> 85%) for preventing severe forms of diseases, but various reports published about breakthrough SARS-CoV-2 infections in fully vaccinated individuals. Reference Bergwerk, Gonen and Lustig7–Reference Sankary, Sippel and Eberhart9 Based on these reports and current results, it was found that vaccines have conferred widespread protection against SARS-CoV-2 infection. As we see in the currents results, hospitalization was significantly more in breakthrough cases. Also, fully vaccinated individuals who experienced breakthrough infections reported fewer symptoms than unvaccinated at the time of study This means vaccines remain a critical tool for providing protection against COVID-19, especially against severe illness and hospitalization. This result supports earlier research demonstrating that vaccination offers considerable protection against the most severe COVID-19 disease signs as well as general infection protection. Reference Reynolds, Xie and Knuth10 To evaluate vaccine safety and surveillance, it is important to follow the fully vaccinated individuals to diagnose any serious complications or infections especially severe cases or dead people due to COVID-19 disease. According to the CDC report, a total of 10,262 SARS-CoV-2 vaccine breakthrough infections were diagnosed in 46 states of the United States until April 30, 2021; of those 160 (2%) cases have died. 11 Compared with our results, our death rate (501/60800; 0.82%) was lower than published reports. The median age of dead cases in the United States was 82 y old, which is more than our results. We have started the vaccination program simultaneously for the elderly individuals with especial illness in other ages (19-100 y old). This is while in the United States the age range was 71 to 89. Poor immune response, co-existence of multiple comorbidities at older ages, and overall frailty may be the main reasons why they are at higher risk. 11 Furthermore, in various reports, it was seen that 85% of breakthrough cases were infected with the B.1.1.7 variant. Reference Haas, Angulo and McLaughlin12–Reference Jacobson, Pinsky and Montez Rath15
Similar to our results, Pre-existing illness and age greater than 60 y were found to be predictors of death in unvaccinated patients in a retrospective study on predictors of death in COVID-19 vaccine breakthrough infections in Brazil. Reference Estofolete, Fares and Banho16
Critically, some reasons may increase the breakthrough infection rate or related death. The most important reason may be in terms of the failure to comply with expected health protocols after vaccination. Reference Schaffer DeRoo, Pudalov and Fu17 Various evidence shows people with special medical conditions and lower immunity due to underlying disease may not obtain optimal protection from established vaccines. Moreover, the combined effect of waning immunity and the increased prevalence of the delta variant decreases the efficacy of a vaccine and the booster (third) dose would be helpful in this circumstance. So far, in the countries with high rates of vaccination, booster doses have been started for those who are 60 y of age or older or have an immunodeficiency disease. Reference Bar-On, Goldberg and Mandel18 Another issue that should be pointed out in Iran, is waiting for the home-grown crops of vaccines. Sanctions have caused a lot of difficulties in providing initial equipment and materials. Trying local solutions and modifying the method causes an unexpected delay in manufacturing vaccines. Reference Akbarialiabad, Rastegar and Bastani19 Despite the fact that independent researchers have demonstrated vaccine efficacy, there is evidence of increased SARS-CoV-2 breakthrough rates in vaccinated individuals. Reference Kustin, Harel and Finkel13,Reference Hacisuleyman, Hale and Saito20,Reference Philomina, Jolly and John21 The main point about the strength of the current study is reporting about death breakthrough cases and comparison with the unvaccinated groups. As a suggestion, it is better to design experimental studies and evaluate the immune responses in different groups of patients and following them. Future studies will help determine the long-term effectiveness of vaccination and even the booster dose against current and emerging variants.
Table 1. Comparison of breakthrough and unvaccinated death cases

Conclusions
The findings of this study indicated that vaccination was associated with a reduction in the risk of SARS-CoV-2 infection; a severe form of disease and death rate. Equitable access to safe and effective vaccines is critical to ending the COVID-19 pandemic. As vaccine uptake increases, we anticipate a decrease in mortality and increased protection from severe forms of the disease.




