Crossref Citations
This article has been cited by the following publications. This list is generated based on data provided by
Crossref.
Brown, Michael
and
Solar, Gary S.
1998.
Granite ascent and emplacement during contractional deformation in convergent orogens.
Journal of Structural Geology,
Vol. 20,
Issue. 9-10,
p.
1365.
Thompson, Alan Bruce
1999.
Some time-space relationships for crustal melting and granitic intrusion at various depths.
Geological Society, London, Special Publications,
Vol. 168,
Issue. 1,
p.
7.
Bea, Fernando
and
Montero, Pilar
1999.
Behavior of accessory phases and redistribution of Zr, REE, Y, Th, and U during metamorphism and partial melting of metapelites in the lower crust: an example from the Kinzigite Formation of Ivrea-Verbano, NW Italy.
Geochimica et Cosmochimica Acta,
Vol. 63,
Issue. 7-8,
p.
1133.
Brown, M.
and
Solar, G.S.
1999.
The mechanism of ascent and emplacement of granite magma during transpression: a syntectonic granite paradigm.
Tectonophysics,
Vol. 312,
Issue. 1,
p.
1.
Brown, M
and
Pressley, R.A
1999.
Crustal melting in nature: Prosecuting source processes.
Physics and Chemistry of the Earth, Part A: Solid Earth and Geodesy,
Vol. 24,
Issue. 3,
p.
305.
Pressley, Rachel A
and
Brown, Michael
1999.
The Phillips pluton, Maine, USA: evidence of heterogeneous crustal sources and implications for granite ascent and emplacement mechanisms in convergent orogens.
Lithos,
Vol. 46,
Issue. 3,
p.
335.
Altherr, Rainer
Holl, Albert
Hegner, Ernst
Langer, Carola
and
Kreuzer, Hans
2000.
High-potassium, calc-alkaline I-type plutonism in the European Variscides: northern Vosges (France) and northern Schwarzwald (Germany).
Lithos,
Vol. 50,
Issue. 1-3,
p.
51.
Matile, Luzius
Thompson, Alan Bruce
and
Ulmer, Peter
2000.
Physics and Chemistry of Partially Molten Rocks.
Vol. 11,
Issue. ,
p.
179.
Sawyer, E. W.
2000.
Grain-scale and outcrop-scale distribution and movement of melt in a crystallising granite.
Earth and Environmental Science Transactions of the Royal Society of Edinburgh,
Vol. 91,
Issue. 1-2,
p.
73.
GARDIEN, VÉRONIQUE
THOMPSON, ALAN BRUCE
and
ULMER, PETER
2000.
Melting of Biotite + Plagioclase + Quartz Gneisses: the Role of H2O in the Stability of Amphibole.
Journal of Petrology,
Vol. 41,
Issue. 5,
p.
651.
Nabelek, Peter I.
and
Bartlett, Cindy D.
2000.
Fertility of metapelites and metagraywackes during leucogranite generation: an example from the Black Hills, U.S.A..
Earth and Environmental Science Transactions of the Royal Society of Edinburgh,
Vol. 91,
Issue. 1-2,
p.
1.
Brown, M.
2001.
Crustal melting and granite magmatism: key issues.
Physics and Chemistry of the Earth, Part A: Solid Earth and Geodesy,
Vol. 26,
Issue. 4-5,
p.
201.
Brown, Michael
2001.
Orogeny, migmatites and leucogranites: A review.
Journal of Earth System Science,
Vol. 110,
Issue. 4,
p.
313.
Brown, M.
2001.
From microscope to mountain belt: 150 years of petrology and its contribution to understanding geodynamics, particularly the tectonics of orogens.
Journal of Geodynamics,
Vol. 32,
Issue. 1-2,
p.
115.
Thompson, Alan Bruce
2001.
Clockwise P–T paths for crustal melting and H2O recycling in granite source regions and migmatite terrains.
Lithos,
Vol. 56,
Issue. 1,
p.
33.
Kebede, T.
Koeberl, C.
and
Koller, F.
2001.
Magmatic evolution of the suqii-wagga garnet-bearing two-mica granite, wallagga area, western Ethiopia.
Journal of African Earth Sciences,
Vol. 32,
Issue. 2,
p.
193.
de Assis Janasi, Valdecir
2002.
Elemental and Sr–Nd isotope geochemistry of two Neoproterozoic mangerite suites in SE Brazil: implications for the origin of the mangerite–charnockite–granite series.
Precambrian Research,
Vol. 119,
Issue. 1-4,
p.
301.
Brown, Michael
2002.
Retrograde processes in migmatites and granulites revisited.
Journal of Metamorphic Geology,
Vol. 20,
Issue. 1,
p.
25.
Chen, Guoneng
Grapes, Rodney
and
Zhang, Ke
2003.
A Model for Mesozoic Crustal Melting and Tectonic Deformation in Southeast China.
International Geology Review,
Vol. 45,
Issue. 10,
p.
948.
Guernina, S.
and
Sawyer, E. W.
2003.
Large‐scale melt‐depletion in granulite terranes: an example from the Archean Ashuanipi Subprovince of Quebec.
Journal of Metamorphic Geology,
Vol. 21,
Issue. 2,
p.
181.
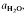 to about 30 kbar pressure. Quartzo-feldspathic melting produces steady increases in melt proportion with increasing temperature. The exact melt fraction depends on the mineral mode relative to quartz-feldspar eutectics and the temperatures of mica dehydration melting reactions. Mica melting consumes SiO2 from residual quartz during the formation of refractory Al2SiO5, orthopyroxene, garnet or cordierite.
to about 30 kbar pressure. Quartzo-feldspathic melting produces steady increases in melt proportion with increasing temperature. The exact melt fraction depends on the mineral mode relative to quartz-feldspar eutectics and the temperatures of mica dehydration melting reactions. Mica melting consumes SiO2 from residual quartz during the formation of refractory Al2SiO5, orthopyroxene, garnet or cordierite.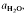 are superimposed on quartz-feldspar melting relations projected onto Ab-An-Or. Fertility to melt production varies with the mica to feldspar ratio and pressure. Pelites are more fertile than psammites at low pressures (e.g. 5 kbar), especially if they contain An40 to An50 plagioclase. At higher pressure (e.g. 10-20 kbar) and for rocks containing albitic plagioclase, psammites are more fertile than pelites. For a typical pelite (e.g. with An25 at 20 kbar), the cotectic with muscovite lies at higher
are superimposed on quartz-feldspar melting relations projected onto Ab-An-Or. Fertility to melt production varies with the mica to feldspar ratio and pressure. Pelites are more fertile than psammites at low pressures (e.g. 5 kbar), especially if they contain An40 to An50 plagioclase. At higher pressure (e.g. 10-20 kbar) and for rocks containing albitic plagioclase, psammites are more fertile than pelites. For a typical pelite (e.g. with An25 at 20 kbar), the cotectic with muscovite lies at higher 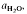 (≍·) and XAb (≍0·42) than with biotite
(≍·) and XAb (≍0·42) than with biotite 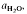 :≍0·35; XAb(≍·), thus dehydration melting of muscovite requires 10% more plagioclase for fertility than does biotite.
:≍0·35; XAb(≍·), thus dehydration melting of muscovite requires 10% more plagioclase for fertility than does biotite.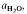 of such melts is greater than 0·3.
of such melts is greater than 0·3.