Introduction
Clearing tropical forest for agriculture is a growing problem worldwide, and Sumatra (Indonesia), which contains c. 10% of the world’s remaining tropical rainforest (Hammond Reference Hammond1997), is not exempt. Although approximately a quarter of the island is part of a protected area network (Gaveau et al. Reference Gaveau, Curran, Paoli, Carlson, Wells, Besse-Rimba, Ratnasari and Leader-Williams2012), deforestation still occurs within protected area boundaries and the surrounding landscape. Agriculture has contributed to an estimated loss of 17.6% of primary tropical forest across the island between 2000 and 2012, and with demand for products such as palm oil, coffee and timber continuing to rise, the loss will continue (Laumonier et al. Reference Laumonier, Uryu, Stu, Budiman, Setiabudi and Hadian2010, Margono et al. Reference Margono, Potapov, Turubanova, Stolle and Hansen2014). This habitat loss has been linked to population declines in many mammals, including flagship species such as Sumatran tiger (Panthera tigris sumatrae), Sumatran rhinoceros (Dicerorhinus sumatrensis) and Asian elephant (Elephas maximus sumatrensis) (Kinnaird et al. Reference Kinnaird, Sanderson, Brien, Wibisono and Woolmer2003, Linkie et al. Reference Linkie, Martyr, Holden, Yanuar, Hartana, Sugardjito and Leader-Williams2003, Hedges et al. Reference Hedges, Tyson, Sitompul, Kinnaird, Gunaryadi and Aslan2005, Isnan et al. Reference Isnan, Subrata and van Strien2006).
In the face of substantial habitat loss, there have been conflicting ideas regarding how to most effectively conserve species in a human-dominated landscape. Contiguous, intact habitat is necessary for wildlife conservation in Southeast Asia. While further fragmenting of primary forest should be discouraged, small remnant forests located within agricultural landscapes are commonly disregarded as suitable wildlife habitat and are rarely considered during habitat assessments or when developing species conservation initiatives. However, there may be benefits to preserving remnant forest fragments (hereafter fragments) already present in the landscape. For example, low-intensity tropical agriculture that maintains fragments can support high levels of biodiversity, and fragments provide added ecosystem services such as biological pest control and pollination (Gilroy et al. Reference Gilroy, Edwards, Medina Uribe, Haugaasen and Edwards2014). Although forest patches are often too small to contain the home ranges of large animals, these patches may act as steppingstones during dispersal through a matrix of degraded habitat or as refugia for resting and possibly breeding (Dunning et al. Reference Dunning, Danielson and Pulliam1992, Rajaratnam et al. Reference Rajaratnam, Sunquist, Rajaratnam and Ambu2007, Lees & Peres, Reference Lees and Peres2009, Mohamed et al. Reference Mohamed, Sollmann, Bernard, Ambu, Lagan, Mannan, Hofer and Wilting2013). In Indonesia and Malaysia, sun bears (Helarctos malayanus; Linkie et al. Reference Linkie, Dinata, Nofrianto and Leader-Williams2007a, Reference Linkie, Dinata, Nugroho and Haidir2007b), Sumatran tigers (Linkie et al. Reference Linkie, Haidir, Nugroho and Dinata2008, Wibisono et al. Reference Wibisono, Linkie, Guillera-Arroita, Smith and Sunarto2011, Sunarto et al. Reference Sunarto, Kelly, Parakkasi, Klenzendorf, Septayuda and Kurniawan2012), leopard cats (Prionailurus bengalensis; Rajaratnam et al. Reference Rajaratnam, Sunquist, Rajaratnam and Ambu2007) and Asiatic golden cats (Pardofelis temminckii; McCarthy Reference McCarthy2013) use forest fragments or degraded forest. Maintaining fragments near plantations may also be important for the life cycles of birds and butterflies that inhabit the plantations, and they may act as population sources for these agricultural areas (Koh Reference Koh2008). However, fragments surrounded by oil palm plantations in Borneo had lower bird diversity and abundance than contiguous forest (Edwards et al. Reference Edwards, Hodgson, Hamer, Mitchell, Ahmad, Cornell and Wilcove2010).
Improved understanding of the value of fragments is essential for land-use planning, especially in biodiversity hotspots such as Indonesia. Although Indonesia contains 12% of global mammal diversity and the largest number of threatened mammal species, the majority of which depend on forest habitats, little is known regarding the effect of widespread landscape changes on Sumatran biodiversity as a whole (Sodhi et al. Reference Sodhi, Koh, Brook and Ng2004, Ministry of Environment and Forestry Indonesia 2014). This information is of the utmost importance to managers faced with prioritizing the allocation of limited conservation resources in efforts to maintain diverse ecosystems.
Bukit Barisan Selatan National Park (BBSNP; Sumatra) is highly biodiverse and has been designated as an important habitat for species of conservation concern (Wibisono Reference Wibisono2005, Dinerstein et al. Reference Dinerstein, Loucks, Heydlauff, Wikramanayake, Bryja, Forrest and Ginsberg2006, McCarthy et al. Reference McCarthy, Fuller, McCarthy, Wibisono and Livolsi2012). Despite its importance, encroachment of coffee plantations into the protected primary forest has become a serious threat to the Park (WWF – Indonesia AREAS Bukit Barisan Selatan Programme 2007), especially because coffee plantations in the area have significantly lower tree, ant and bird diversity than the primary forest (Philpott et al. Reference Philpott, Bichier, Rice and Greenberg2008). As extensive deforestation continues in and around BBSNP, many small remnant fragments of forest have been arbitrarily retained in the agricultural landscape. This is usually due to terrain that is not conducive to clearing or planting. These fragments vary in size and distance from the Park boundary and are often ephemeral. However, there have been no investigations into the mammalian presence in these fragments, and no consideration has been given to their utility in broad-scale conservation planning. In this study, we conducted camera trap surveys in BBSNP and five surrounding fragments. We compared the presence and relative abundance of species found within the Park and of those within the fragments to investigate whether forest-dwelling species used the fragments. As the fragments were small (between 0.012 and 0.152 km2), we suspected that small mammals would comprise the majority of species inhabiting the fragments, but that larger species might use them periodically for resources or as movement corridors. Determining which species utilize fragments is an important step in assessing the conservation value of the habitat.
Methods
Study area
BBSNP, encompassing 3245 km2, is the third largest protected area in Sumatra (Fig. 1). Located in southwest Sumatra between the coordinates 4°31′ to 5°57′S and 103°34′ to 104°43′E, the park covers 150 km along the Bukit Barisan Mountain Range, with topography ranging from coastline in the south to mountainous forest reaching up to 1964 m in the north (Government of the Republic of Indonesia 2004). BBSNP experiences seasonal rainfall ranging from 3000 to 4000 mm/year and temperatures of 22–35°C (O’Brien et al. Reference O’Brien, Kinnaird and Wibisono2003). It is the major watershed for the southwest region of the island. BBSNP was declared a Wildlife Sanctuary in 1935, a National Park in 1982 and a UNESCO World Heritage Site in 2004. It is part of the Tropical Forest Heritage of Sumatra and contains some of the largest tracts of lowland tropical rainforest on the island (UNESCO World Heritage Centre n.d.). It is considered an important forest area for tiger conservation and is also a critical habitat for endangered species such as the Sumatran rhino, Sumatran striped rabbit (Nesolagus netscheri) and Sumatran elephant (Wibisono Reference Wibisono2005, Dinerstein et al. Reference Dinerstein, Loucks, Heydlauff, Wikramanayake, Bryja, Forrest and Ginsberg2006, McCarthy et al. Reference McCarthy, Fuller, McCarthy, Wibisono and Livolsi2012). The park is surrounded by villages, agricultural fields and oil palm plantations. With increases in coffee prices, many small-scale coffee farmers have moved to the area, and agricultural expansion for coffee production has been the major driver of deforestation in the Park in recent years (Suyanto Reference Suyanto2007, Gaveau et al. Reference Gaveau, Linkie, Suyadi, Levang and Leader-Williams2009).
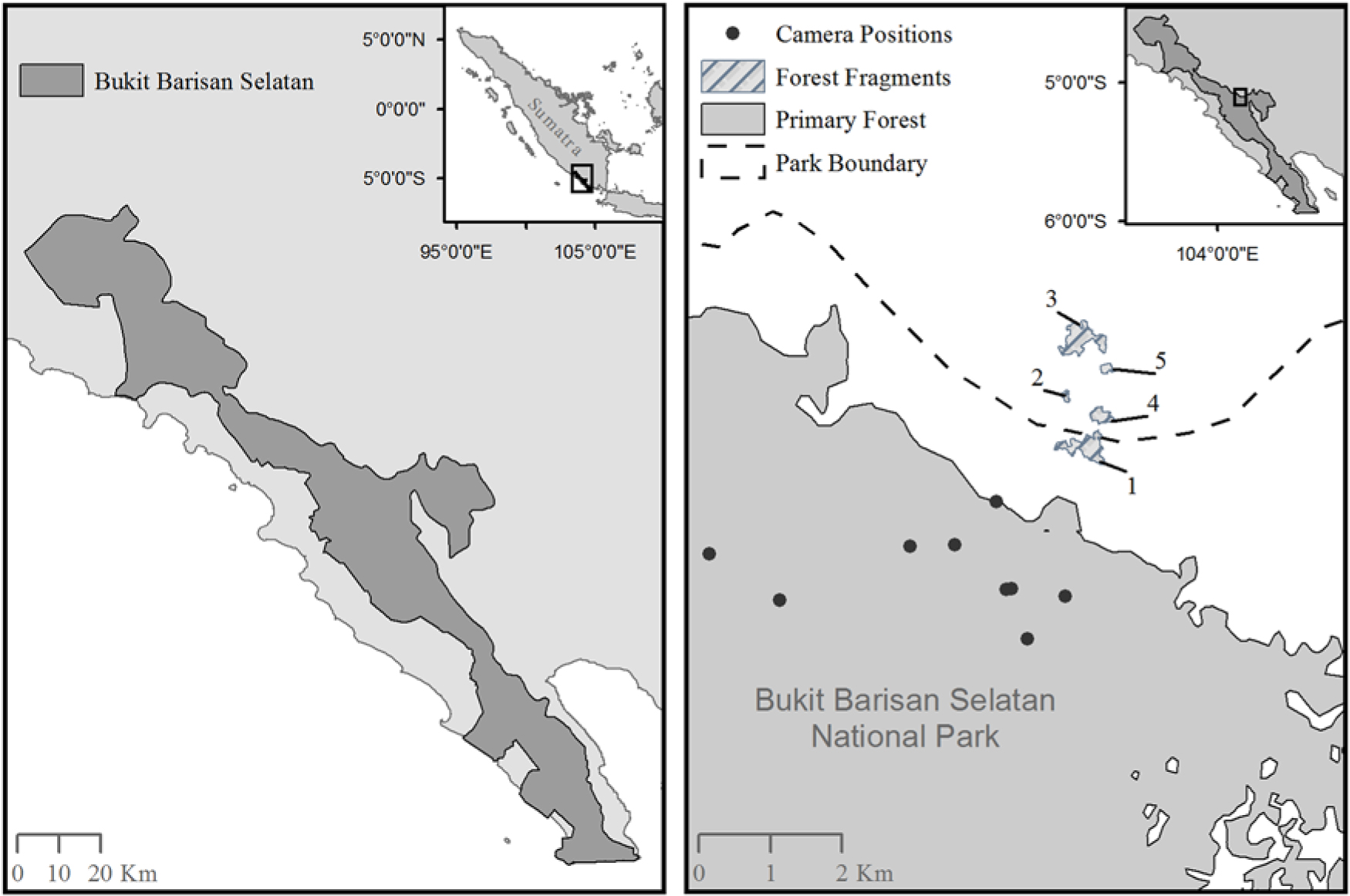
Fig. 1. A map of Bukit Barisan Selatan National Park, Sumatra (left) and a map showing the location of the camera traps inside the primary forest, as well as the five remnant forest fragments outside of Bukit Barisan Selatan National Park (right). Fragment sizes were as follows: Fragment 1 = 0.13 km2; Fragment 2 = 0.012 km2; Fragment 3 = 0.15 km2; Fragment 4 = 0.047 km2; Fragment 5 = 0.018 km2. More fragments were present in this area, but we did not have GPS locations to add them to the map. The exact locations of the cameras within the forest fragments were lost; however, there were four camera locations in Fragment 1, six camera locations in Fragment 2, six camera locations in Fragment 3, five camera locations in Fragment 4 and four camera locations in Fragment 5.
We also worked in five randomly selected fragments outside the Park (Fig. 1). Owing to the lack of mapping and other data, we chose fragments along the edge of BBSNP that represented a sample of the available fragment sizes and were varying distances from the border of BBSNP. Selected fragments were located 0.59–1.97 km from the border of the fragment to the border of the protected forest in BBSNP and were surrounded by coffee plantations. One of the fragments was partially located within the official Park boundary but was isolated from the contiguous forest due to significant illegal deforestation. Fragments ranged in size from 0.012 to 0.152 km2. There are several small villages nearby (fewer than 20 houses per village) and scattered houses throughout the area. There are some limited small-scale food crops grown nearby, but the majority of the landscape is composed of coffee plantations.
Camera trapping
We conducted camera trapping within BBSNP between June and October 2010 (two cameras) and between January and September 2011 (seven cameras) using digital remote cameras (Reconyx HC500, Holmen, WI, USA). The cameras were motion-sensored and operated constantly, using infrared photography at night. The cameras took a series of five photographs each time they were triggered with a 1–s delay between pictures, and the date and time of the pictures being taken were automatically recorded. We placed cameras within a 20–km2 sampling block (Fig. 1) that was the focus of a previous camera trapping study (McCarthy et al. Reference McCarthy, Wibisono, McCarthy, Fuller and Andayani2015). We selected a random Universal Transverse Mercator (UTM) coordinate within the block and placed a camera within 100 m of that coordinate at the location determined to be most optimal for obtaining mammal photographs. These locations were generally along animal trails that showed signs of recent activity. We secured cameras on tree trunks 25 cm above the forest floor and checked them every 30–35 days. In some cases, we moved cameras to a new UTM coordinate within the subunit to get better coverage; in other cases, we changed their batteries but did not move the cameras. Scent lures (chicken body parts, Calvin Klein One perfume (CK) or a commercial scent lure) were placed directly in front of the cameras. We used scent lures to maximize the chance that mammals would step in front of the cameras to investigate the scent, but did not expect them to draw animals from any distance. Previous studies have indicated that a wide array of scent lures do not bias density estimates generated by camera trapping, meaning that there is a low probability that scent lures would draw an individual outside of their home range (Braczkowski et al. Reference Braczkowski, Balme, Dickman, Fattebert, Johnson, Dickerson, Macdonald and Hunter2016, Jacques et al. Reference Jacques, Kapfer and Eshelman2016). In addition, more recent data have shown that the use of lures at camera trap stations does not significantly increase the detection rate of mammals in comparison to camera trap stations with no scent lures, indicating that our use of scent lures was likely ineffectual (Maxwell Reference Maxwell2018).
We also conducted camera trapping in five fragments outside of BBSNP between June and September 2013 (Fig. 1). Within fragments, cameras (Reconyx HC500, Holmen, WI, USA, and Bushnell Trophy Cam HD, Overland Park, KS, USA) were set using the same settings described above, except that the cameras took a series of three photographs every time they were triggered. Two cameras were placed within each fragment for a total of ten camera trap stations. The small size of the remnant fragments made the positioning of cameras in remnant fragments somewhat irregular. The locations were chosen to be as far apart as possible in each fragment (the smallest minimum distance between cameras was c. 10 m for the smallest fragment that was c. 12 m in size), positioned along trails or in clearings. Cameras were deployed for 30–35 days, after which they were checked so that batteries or memory cards could be replaced, and in some cases cameras were moved to different locations within the fragment to improve coverage. We used solely CK scent lures at all camera trap stations in the forest fragments, but did not expect that the use of any scent lures would affect the species present in the forest fragments (Braczkowski et al. Reference Braczkowski, Balme, Dickman, Fattebert, Johnson, Dickerson, Macdonald and Hunter2016, Jacques et al. Reference Jacques, Kapfer and Eshelman2016, Maxwell Reference Maxwell2018).
The number of trap nights for each camera was calculated as the number of days between deployment and retrieval, or the date of the last photograph captured if this was not the retrieval date. Each photograph of a mammal was identified to the species level where possible. If we were unable to determine the species but were able to identify it as a separate taxonomic unit, we included it in the analysis as a separate species (e.g., Sciuridae sp., Tragulus sp.). Poor-quality photographs where we could not identify the animal to the order level were excluded. Photographs of the same species taken within 1 hour of the first picture were considered a single detection event if we were unable to identify it as a separate individual. If multiple individuals were seen in a photograph, we counted it as multiple independent photographs; for example, if there were two macaques in a photograph, we counted it as two independent photos.
Data analysis
All analyses were conducted in R version 3.2.0 (R Core Team 2016). To compare species composition in the protected forest and fragments, we constructed species accumulation curves using the function specaccum in the vegan library using Kindt’s exact method (Oksanen et al. Reference Oksanen, Blanchet, Kindt, Legendre, Minchin, O’Hara and Simpson2015). We used the function poolaccum in the vegan library to estimate overall species richness (including observed and unobserved species) in the protected forest and fragments. We computed the estimates using Chao’s, jackknife and bootstrap methods. Chao’s method is beneficial when many individuals are only captured a few times, because jackknife and bootstrapping tend to underestimate species richness if there are a high number of rare species or too few samples. However, Chao’s method is less precise than the other two methods and may not work if average capture probability is large (Smith & van Belle Reference Smith and van Belle1984, Chao Reference Chao1987).
To compare the relative abundances of each species between the protected forest and fragments, we calculated photograph rates as the number of photographs of the species per 100 trap nights. We then compared photo rates between the protected forest and fragments using a Poisson test (stats::poisson.test function in R) with a significance level of p < 0.05.
Results
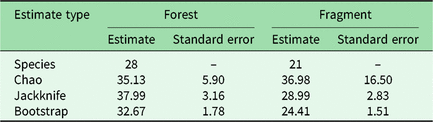
We had a total of 904 trap nights (155 for Fragment 1, 218 for Fragment 2, 214 for Fragment 3, 187 for Fragment 4 and 131 for Fragment 5) and 386 independent photographs in the fragments (42.7 independent photographs/100 trap nights), and 1381 trap nights and 247 independent photographs in the protected forest (17.9 independent photographs/100 trap nights). Twenty-eight mammal species were observed in the protected forest and 21 mammal species were observed in the fragments (Fig. 2 and Table 1), but species accumulation curves did not reach their asymptote in either location (Fig. 3). Estimates for total species richness were higher for the protected forest across most metrics (Table 2).
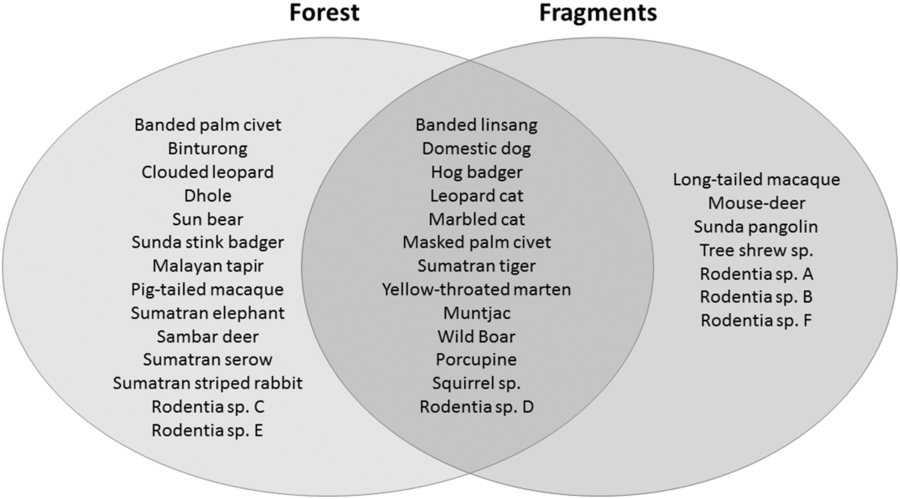
Fig. 2. A comparison of the mammalian species compositions of the primary forest of Bukit Barisan Selatan National Park (cameras deployed 2010–2011) and the five surrounding forest fragments (cameras deployed 2013).
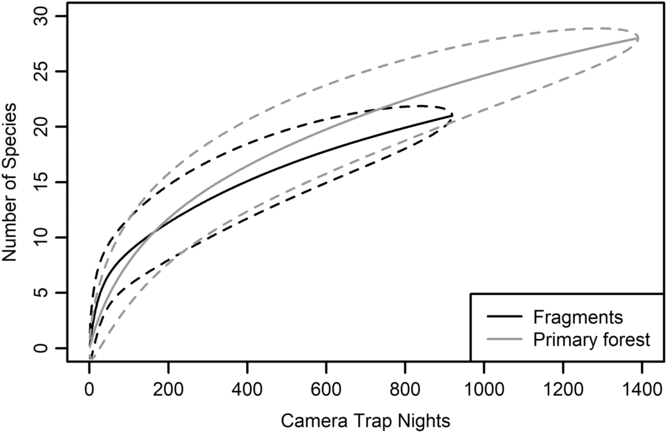
Fig. 3. Species accumulation curve for five forest fragments outside of Bukit Barisan Selatan National Park, Sumatra (black line). The curve was created using camera trap data collected in 2013. Species accumulation curve for the primary forest study site in Bukit Barisan Selatan National Park (grey line). The curve was created using camera trap data collected between 2010 and 2011. Dashed lines denote 95% confidence intervals.
Table 1. Photograph rates per 100 trap nights of mammal species photographed in Bukit Barisan Selatan National Park (cameras deployed 2010–2011) and five surrounding forest fragments (cameras deployed 2013). Significantly different rates (calculated using a Poisson test with p < 0.05) are highlighted in bold. An ‘X’ indicates which forest fragments contained the species.
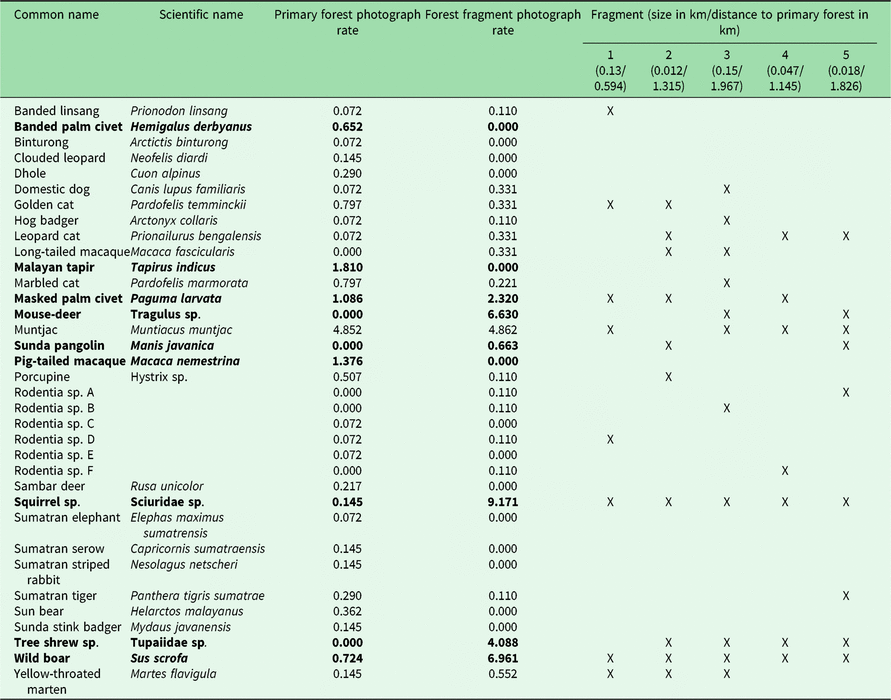
Table 2. Estimated mammal species richness in the forested study area of Bukit Barisan Selatan National Park and five forest fragments in the surrounding landscape. ‘Species’ is the observed species richness. ‘Chao’, ‘jackknife’ and ‘bootstrap’ are species richness estimators that account for species that were unobserved by the camera traps.
The number of species observed in each fragment ranged from 7 to 11 (Table 1). All fragments contained at least one felid species, with a leopard cat and a golden cat observed in the smallest fragment, and a Sumatran tiger and a leopard cat observed in the second smallest fragment. A marbled cat was seen only in the largest fragment. Sunda pangolins (Manis javanica) were also exclusively observed in the smallest two fragments.
Discussion
This study highlights the role of fragments and indicates their possible utility in landscape-level habitat management efforts in Sumatra.
Consistent with previous studies, we observed fewer species in the fragments than the intact protected forest (Fischer & Lindenmayer Reference Fischer and Lindenmayer2007, Sampaio et al. Reference Sampaio, Lima, Magnusson and Peres2010). However, while camera trapping in the protected forest took place over multiple seasons, camera trapping in the remnant fragments only took place in June to September, and thus may have missed seasonal variation in fragment use. This could have contributed to the lower number of species observed. Interestingly, we did observe a greater photograph rate in the fragments than in the protected forest, although we note that photograph rates and other relative abundance indices do not account for differences in detectability between sites. This may have been a reflection of animals being funnelled into the fragments as they moved through limited habitat on the landscape. Our data do not allow us to substantiate this hypothesis, as we had limited camera trap coverage in the primary forest, but this observation is notable and deserves further investigation.
Because different species have different habitat size requirements, smaller habitat patches tend to support fewer species than larger ones, and the composition of smaller patches is often a subset of species in larger habitat patches (Fahrig Reference Fahrig2003). The landscape matrix surrounding the fragments can also influence the species composition by influencing movement, survival and reproduction (Fahrig Reference Fahrig2003). In some cases, species may not reside in these patches, but merely use them for food or temporary shelter. However, the lower species richness of the fragments does not necessarily indicate that fragments have low conservation value. While large mammals may be unable to survive in small remnant fragments, mid-sized generalist species were found to be more abundant in small neotropical forest fragments than in the primary forest, and small remnant patches (<100 ha) in Amazonian Brazil were found to support some forest bird and mammal species (Turner & Corlett Reference Turner and Corlett1996, Sampaio et al. Reference Sampaio, Lima, Magnusson and Peres2010). Similarly, in our study area, we found several species to have greater or non-significantly different photograph rates in the fragments than in the protected forest (Table 1).
We did not observe dhole (Cuon alpinus), Malayan tapir (Tapirus indicus), Sumatran elephant (all endangered species) or sambar deer (Rusa unicolor, vulnerable species) in the fragments. It is likely that the fragments were not large enough to provide sufficient resources for large and wide-ranging species. Hunting pressure is greater in more disturbed areas such as fragments, which could also explain the absence of hunted species such as sambar deer (O’Brien et al. Reference O’Brien, Kinnaird and Wibisono2003). It is also possible that some of these species do utilize the fragments, but were not observed during the camera trapping period, as elephant signs were found in the fragments.
Four of the five felid species observed in the protected forest (golden cat, marbled cat, Sumatran tiger and leopard cat) were observed in the fragments. Leopard cats had a greater (though not significantly greater) photograph rate in the fragments. In previous studies, leopard cats were found in greater relative abundance closer to forest edges (Azlan & Sharma Reference Azlan and Sharma2006, Pusparini et al. Reference Pusparini, Wibisono, Reddy, Tarmizi and Bharata2014, McCarthy et al. Reference McCarthy, Wibisono, McCarthy, Fuller and Andayani2015). Our results are also consistent with the conclusions of Rajaratnam et al. (Reference Rajaratnam, Sunquist, Rajaratnam and Ambu2007) that leopard cats may depend on forest fragments for survival; they are commonly found in close proximity to open areas and human settlements, and may use forest fragments as a refuge. While tigers may not depend on fragments to the same degree as leopard cats, their observation in the fragments indicates that they may use them for movement, supporting Sunarto et al.’s (Reference Sunarto, Kelly, Parakkasi, Klenzendorf, Septayuda and Kurniawan2012) conclusion that fragments may act as steppingstones between suitable habitats.
In addition to felids, the fragments also contained several species that were not observed in the protected forest, including small rodents such as tree shrews, long-tailed macaques and the Sunda pangolin, the latter of which is considered critically endangered by the International Union for Conservation of Nature (IUCN) (Challender et al. Reference Challender, Nguyen Van, Shepherd, Krishnasamy, Wang, Lee and Panjang2014). This may be due to limited sampling in BBSNP, as several of these species, including Sunda pangolins, have been observed there (O’Brien & Kinnaird Reference O’Brien and Kinnaird1996). Sunda pangolins are in serious decline throughout their range, and the biggest threat to them is illegal hunting for their meat and scales, mostly to supply the traditional medicine trade in China (Challender Reference Challender2011). Much of this supply is obtained from Indonesia and Malaysia (Challender Reference Challender2011). It is therefore noteworthy that we captured multiple photographs of pangolins in the two smallest fragments in areas completely surrounded by coffee plantations and humans.
Our fragments were close to the intact primary forest. This may increase the number of species that can be supported, because individuals in our study area can utilize both the fragments and the protected forest, assuming they can cross non-forested areas. If individuals can use a network of interconnected fragments, it may increase ecosystem resilience by providing functional diversity and connectivity (del Castillo Reference del Castillo2015). Maintaining interconnectedness in the landscape through a network of fragments surrounded by coffee plantations may be beneficial for mammal species in Sumatra, as it appears that some are able to move between them across the human-dominated matrix.
Our five small fragments outside BBSNP harboured a surprisingly high level of biodiversity, including species of conservation concern. The fact that pangolins, golden cats, marbled cats and Sumatran tigers were utilizing this habitat is significant, especially because habitat loss is such an important reason for decline in many species. Increasing habitat connectivity around BBSNP may help sustain populations of species with large home ranges and may alleviate problems associated with small populations and a lack of gene flow by connecting BBSNP to nearby primary forest (the closest being Bukit Balai Rejang Selatan, which is designated as a class II Tiger Conservation Landscape; Sanderson et al. Reference Sanderson, Forrest, Loucks, Ginsberg, Dinerstein, Seidensticker, Leimgruber, Tilson and Nyhus2006). Although studies of other forest fragments have found that smaller patches support less biodiversity and that greater distances between fragments or primary forest may decrease the number of species, these responses can be species specific and dependent on the landscape matrix (Laurance et al. Reference Laurance, Lovejoy, Vasconcelos, Bruna, Didham, Stouffer and Gascon2002). Factors such as greater human density or increased disturbance in the matrix may decrease connectivity. It appears that many species are utilizing relatively small fragments surrounded by coffee plantations around BBSNP. Fragments such as these are present throughout Sumatra and therefore should be considered as part of the conservation landscape.
Our study was small in scale, yet it provides important information on the utility of remnant forests in landscape-level conservation initiatives. Future studies should examine whether fragments in areas farther from core forests and of varying sizes in Sumatra still support high levels of biodiversity and whether species are in fact utilizing the areas as steppingstones between core habitats. If so, this would indicate that remnant forests may play an integral part in the conservation of Sumatran wildlife.
Author ORCIDs
Sarah R Weiskopf 0000-0002-5933-8191
Acknowledgements
We thank our field assistants Agus, Nedy and Teeunk, and the many others who helped us to set up our cameras in sometimes challenging conditions. We thank J Buler for his helpful comments.
Financial support
This study was supported by the Wildlife Conservation Society – Indonesia Program, the Mohammed bin Zayed Species Conservation Fund, Panthera, The Clouded Leopard Project, the United States Fish and Wildlife Service Tiger Conservation Fund, the National Fish and Wildlife Save the Tiger Fund, the University of Delaware, the Small Cat Conservation Fund, the Cat Action Treasury and Idea Wild. It was undertaken with cooperation with the University of Lampung, the staff of Bukit Barisan Selatan National Park, the Indonesian Ministry of Research and Technology and the Indonesian Ministry of Forestry.
Conflict of interest
None.
Ethical standards
None.







