Introduction
Almost everywhere around the world, the development of wind energy stands as one of the pillars of the energy transition (Teske et al. Reference Teske, Giurco, Morris, Nagrath, Mey and Briggs2019). However, this mode of energy production is also a source of negative impacts on biodiversity, particularly for birds and bats (Drewitt & Langston Reference Drewitt and Langston2006, Schuster et al. Reference Schuster, Bulling and Köppel2015, Barclay et al. Reference Barclay, Baerwald, Rydell and Perrow2017, Thaxter et al. Reference Thaxter, Buchanan, Carr, Butchart, Newbold and Green2017, Serrano et al. Reference Serrano, Margalida, Pérez-García, Juste, Traba and Valera2020). Wind power plants can have two types of negative effects on these volant animals. First, birds and bats are susceptible to direct mortalities caused by collisions with wind turbines or by barotrauma (Barclay et al. Reference Barclay, Baerwald, Rydell and Perrow2017, De Lucas & Perrow Reference De Lucas, Perrow and Perrow2017). Second, like all large artificial infrastructures, wind power plants are responsible for indirect impacts, such as habitat loss, disturbance and barrier effects (Drewitt & Langston Reference Drewitt and Langston2006, Schuster et al. Reference Schuster, Bulling and Köppel2015, Fox & Petersen Reference Fox and Petersen2019). Wind energy is currently developing very rapidly around the world, and this rate is projected to continue accelerating in the near future (Teske et al. Reference Teske, Giurco, Morris, Nagrath, Mey and Briggs2019). In this context, assessing and mitigating the harmful impacts that this development will have on wildlife has become a primary concern for biodiversity conservation (Fox & Petersen Reference Fox and Petersen2019, Serrano et al. Reference Serrano, Margalida, Pérez-García, Juste, Traba and Valera2020, Durá-Alemañ et al. Reference Durá-Alemañ, Moleón, Pérez-García, Serrano and Sánchez-Zapata2023).
In many countries around the globe, the construction of wind power plants is regulated by environmental protection laws, which usually require pre- and post-construction impact studies to assess the extent of negative effects on wildlife, notably protected species (Saidur et al. Reference Saidur, Islam, Rahim and Solangi2010). Regarding the risk of direct mortality of birds and bats, environmental impact assessments (EIAs) have historically focused on estimating fatality risk at the individual level (May et al. Reference May, Masden, Bennet and Perron2019) by simply addressing the question as to how many individuals of a given species are at risk of dying from collision. However, for species conservation purposes, it is crucial to assess the consequences that such mortality risk might have at the population level (May et al. Reference May, Masden, Bennet and Perron2019). So far, the few EIA studies that have attempted to quantitatively assess population-level impacts have usually relied on one of two approaches. Some studies have applied a decision rule called ‘potential biological removal’ (PBR) in an effort to calculate the quantitative limits of ‘sustainable’ collision fatalities (e.g., Poot et al. Reference Poot, van Horssen, Collier, Lensink and Dirksen2011, Leopold et al. Reference Leopold, Boonman, Collier, Davaasuren, Jongbloed and Lagerveld2014, Busch & Garthe Reference Busch and Garthe2016, NIRAS 2016). Other studies have focused on simulating population trajectories to predict the fate of populations exposed to collisions with wind turbines (e.g., Carrete et al. Reference Carrete, Sánchez-Zapata, Benítez, Lobón and Donázar2009, Masden Reference Masden2010, García-Ripollés & López-López Reference García-Ripollés and López-López2011, Poot et al. Reference Poot, van Horssen, Collier, Lensink and Dirksen2011, Rydell et al. Reference Rydell, Engström, Hedenström, Larsen, Pettersson and Green2012, Schaub Reference Schaub2012, Sanz-Aguilar et al. Reference Sanz-Aguilar, Sánchez-Zapata, Carrete, Benítez, Ávila, Arenas and Donázar2015, Grünkorn et al. Reference Grünkorn, von Rönn, Blew, Nehls, Weitekamp and Timmermann2016, Korner-Nievergelt et al. Reference Korner-Nievergelt, Brossard, Filliger, Gremaud, Lugon and Mermoud2016).
In this paper, we argue that PBR, as currently used in the context of EIA, is ill-adapted to the task of assessing population-level impacts of wind energy infrastructures. On the other hand, the use of population projections, in combination with metrics of relative impact as a decision rule, is much better suited to this task.
Potential biological removal
What is PBR?
PBR is a ‘harvest’ control rule that was originally developed as a means to define sustainable limits of cetacean incidental catches by commercial fishing vessels in data-poor situations (Wade Reference Wade1998, Moore et al. Reference Moore, Curtis, Lewison, Dillingham, Cope and Fordham2013). The harvest quota, expressed as the number of individuals removed each year, is based on a simple formula (Equation 1; Wade Reference Wade1998):
where R max corresponds to the theoretical maximum growth rate of the population (i.e., when it is at low density and in the absence of anthropogenic mortalities), N min is a conservative estimate of the population size and F R is a coefficient between 0.1 and 1.0, often referred to as the ‘recovery factor’ (Wade Reference Wade1998, Dillingham & Fletcher Reference Dillingham and Fletcher2008). R max and N min are biological parameters that must be estimated for the studied population. Parameter N min is usually estimated from field data, and, for birds, R max is often approximated using allometric relationships that only require knowing the species’ average adult survival and its age at first reproduction (Niel & Lebreton Reference Niel and Lebreton2005). The recovery factor F R is not a biological parameter but an adjustment parameter that must be tuned to ensure that the PBR quota fulfils a predetermined conservation objective even in the presence of uncertainties. The tuning of F R requires simulating population trajectories under a realistic demographic model, often called the ‘operational model’ (Moore et al. Reference Moore, Curtis, Lewison, Dillingham, Cope and Fordham2013), and testing the influence of a range of F R values on the population’s fate (Wade Reference Wade1998, Dillingham & Fletcher Reference Dillingham and Fletcher2008). Based on these simulation results, an F R value is chosen to ensure that, when the associated PBR harvest rule is implemented, the population will have a high probability of stabilizing at a level that is equal to or greater than the predefined long-term conservation objective (Fig. 1). This tuning and assessment procedure, the purpose of which is to test the robustness of the PBR decision rule in a specific context, is called a management strategy evaluation (MSE; Bunnefeld et al. Reference Bunnefeld, Hoshino and Milner-Gulland2011). Implementing such a MSE not only requires building an operational model for the species being targeted but also implies that a quantitative conservation objective has been clearly defined beforehand (e.g., Richard & Abraham Reference Richard and Abraham2013, Haider et al. Reference Haider, Oldfield, Tu, Moreno, Diffendorfer, Eager and Erickson2017). This means setting a population size threshold, often expressed as a fraction of the carrying capacity (Wade Reference Wade1998), with the intention of maintaining the population above this threshold to in the long run (Moore et al. Reference Moore, Curtis, Lewison, Dillingham, Cope and Fordham2013). When correctly implemented, the PBR approach can be effective at preventing or reversing population collapses (Cooke et al. Reference Cooke, Leaper, Wade, Lavigne and Taylor2012, Moore et al. Reference Moore, Curtis, Lewison, Dillingham, Cope and Fordham2013).

Figure 1. Theoretical trajectories of two populations with two different initial states (red, green) suffering the same rate of annual mortality, which is equal to the potential biological removal (here, with F R = 1). Independently from their initial state, each population tends towards the same equilibrium, which is equal to half the carrying capacity (K/2, blue horizontal line). The black horizontal line represents the full carrying capacity K.
Why is PBR not suited to the context of EIA?
First, there is a fundamental difference of scope between EIA and PBR. EIA is a disturbance-centred endeavour, in the sense that it focuses on assessing the impact of a given infrastructure development project. This means that the entry point of the impact analysis is necessarily the source of disturbance itself, not the population. On the other hand, PBR is a population-centred approach, in which every source of disturbance affecting a given population must be considered (Dillingham & Fletcher Reference Dillingham and Fletcher2011). Indeed, the rationale for the PBR approach is to find the total amount of non-natural mortalities (removals) that a population can sustain (Wade Reference Wade1998). Using the PBR decision rule in an EIA context is thus a gross oversimplification because it considers a single source of anthropic mortality, ignoring all others that populations suffer (Green et al. Reference Green, Langston, McCluskie, Sutherland and Wilson2016, O’Brien et al. Reference O’Brien, Cook and Robinson2017).
Second, because EIA lacks a population-centred perspective, no quantitative conservation objective is usually defined for the impacted population. This deficiency has been consistently observed (e.g., Poot et al. Reference Poot, van Horssen, Collier, Lensink and Dirksen2011, Leopold et al. Reference Leopold, Boonman, Collier, Davaasuren, Jongbloed and Lagerveld2014, Busch & Garthe Reference Busch and Garthe2016, NIRAS 2016) in studies that employed the PBR formula as a decision-making tool within the context of wind energy’s impact on bird populations. As highlighted above, using PBR as a decision criterion first requires the establishment of such an objective because it constitutes a vital component of the MSE framework, within which the effectiveness of the decision rule can be rigorously evaluated.
Third, in the context of EIA, the PBR decision rule has been used without implementing an operational model and without conducting simulations to assess its robustness to uncertainties and to tune the value of the recovery factor F R (e.g., Poot et al. Reference Poot, van Horssen, Collier, Lensink and Dirksen2011, Leopold et al. Reference Leopold, Boonman, Collier, Davaasuren, Jongbloed and Lagerveld2014, Busch & Garthe Reference Busch and Garthe2016, NIRAS 2016). Instead, generic values of F R , which were derived in a completely different context (marine mammal bycatch in North America; Wade Reference Wade1998), have been blindly applied. Therefore, there is no guarantee that the harvest quota computed through this formula would effectively align with the conservation objective, assuming such an objective would have been defined for the population under consideration.
Finally, it is important to keep in mind that the PBR method as formulated by Wade (Reference Wade1998) implicitly assumes the existence of a compensatory density-dependence relationship, which means that the population growth rate is expected to increase in response to the removal of individuals, thus partially compensating for anthropogenic mortalities (Rose et al. Reference Rose, Cowan, Winemiller, Myers and Hilborn2001, Beverton & Holt Reference Beverton and Holt2012). This density-dependent mechanism is what allows a population to stabilize at some new equilibrium when facing a sustainable level of mortalities (Wade Reference Wade1998). In the absence of such a mechanism, a population exposed to additional mortalities will constantly decline, necessarily reaching extinction at some point. In birds, however, this type of compensatory mechanism cannot always be evidenced (Horswill et al. Reference Horswill, O’Brien and Robinson2017). Applying removal quotas based on the PBR approach in such situations could trigger or reinforce an unstoppable population decline and therefore have catastrophic consequences (O’Brien et al. Reference O’Brien, Cook and Robinson2017, Miller et al. Reference Miller, Furness, Trinder and Matthiopoulos2019).
Population projection analysis
Population projection analysis (PPA), also sometimes referred to as population viability analysis (PVA), is a simulation-based method to predict the future trajectory of a population under various scenarios (Boyce Reference Boyce1992, Beissinger & McCullough Reference Beissinger and McCullough2002). PPA relies on a demographic model that bears resemblance to the operational models utilized within the MSE framework to evaluate decision rules such as PBR.
In our opinion, the PPA method is well suited for assessing population impacts in the context of EIA because it can easily be framed as a disturbance-centred assessment exercise, in complete alignment with the EIA framework (Green et al. Reference Green, Langston, McCluskie, Sutherland and Wilson2016). To frame a PPA as a disturbance-centred analysis, the most relevant approach consists in running population projections under two alternative scenarios: (1) a baseline scenario without collisions; and (2) an impact scenario that includes additional mortalities due to collisions (Cook & Robinson Reference Cook and Robinson2017). The comparison of population trajectories under each of these two scenarios allows for calculation of various metrics of impact induced specifically by the infrastructure (Fig. 2). In the absence of a clear population conservation objective, which is the usual situation in EIA, we recommend using metrics of relative impact, such as the proportional difference in population size after a given time (e.g., 25 years) between the two scenarios (Green et al. Reference Green, Langston, McCluskie, Sutherland and Wilson2016). This comparative and relative approach, referred to as the ‘counterfactual of impacted to unimpacted’ population (CIU), has been shown to be less sensitive to uncertainties (Cook & Robinson Reference Cook and Robinson2017). Indeed, if some model parameters are inaccurate or some model assumptions happen to be violated, their influence on the final result will be limited because they apply equally to both scenarios.
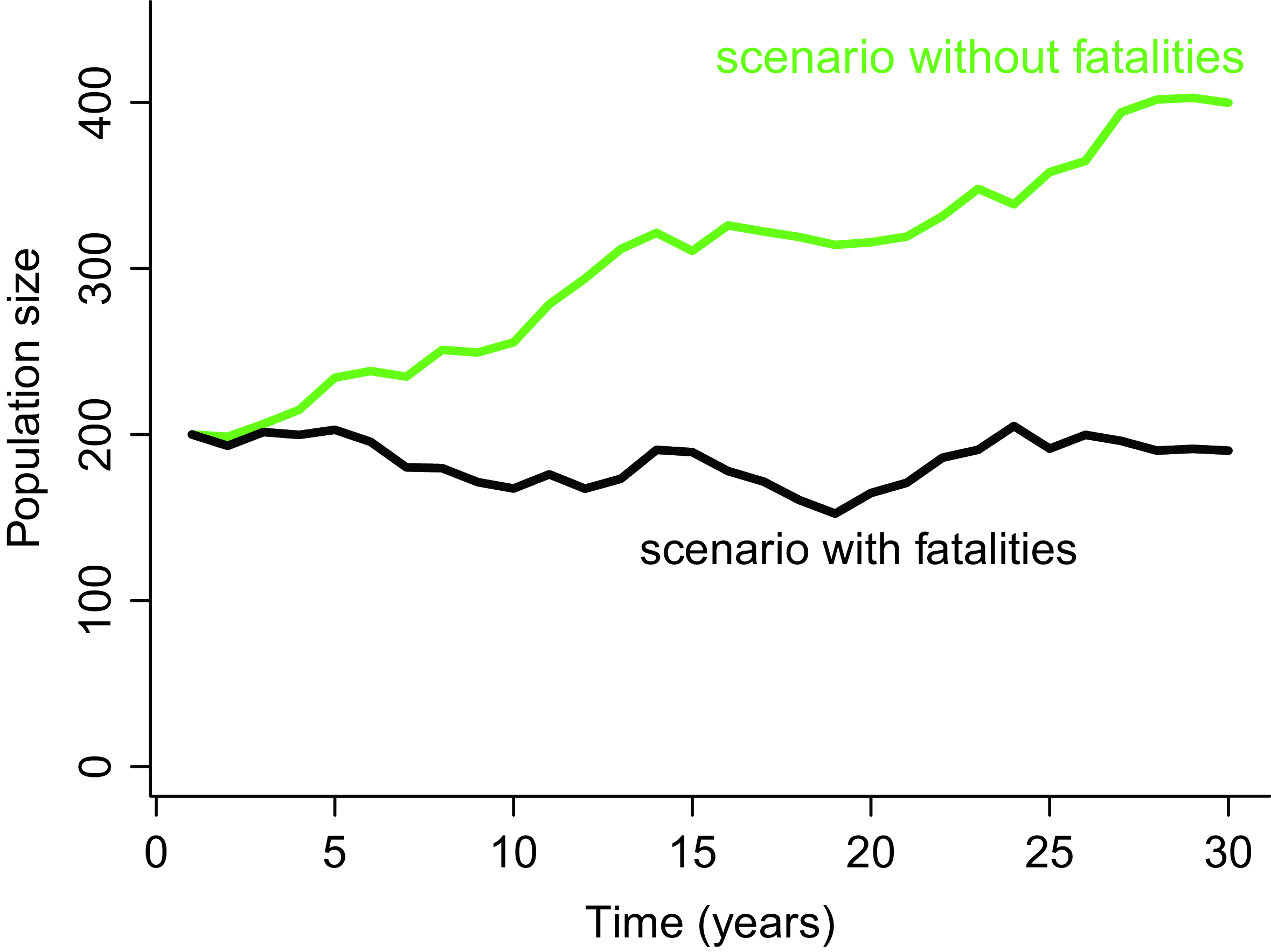
Figure 2. Example of possible population trajectories according to two scenarios: (1) without collision mortality (in green) and (2) with collision mortality due to the presence of a wind power plant (in black). The impact can be defined as the relative difference in population size between these two scenarios after some time (e.g., 30 years).
In the context of EIA, the use of the CIU approach also has the advantage of not requiring the definition, a priori, of a quantitative population objective – namely, a threshold of critical population size (Green et al. Reference Green, Langston, McCluskie, Sutherland and Wilson2016). The lack of a quantitative conservation objective is not an inherent feature of the PPA method but rather reflects the regulatory framework governing the EIA process (Wathern Reference Wathern2013). Indeed, in this framework, the quantification of impact is separated from the decision of what level of impact qualifies as ‘significant’ or not (Schrage Reference Schrage, Bastmeijer and Koivurova2008).
Finally, when using the CIU approach based on PPA, the impact assessment does not necessarily rely on the assumption of compensatory density dependence. The consequences of collision mortalities can thus be explored in situations in which the population would not be expected to stabilize at a new equilibrium. Overall, the PPA method offers a great deal of flexibility regarding the assumptions and the level of complexity of the demographic model being used, which allows for the right balance to be found between realism and practicality (Boyce Reference Boyce1992, Morris & Doak Reference Morris and Doak2002).
Conclusion
PBR is not simply a formula that can be applied ex nihilo to any species or situation (Moore et al. Reference Moore, Curtis, Lewison, Dillingham, Cope and Fordham2013, O’Brien et al. Reference O’Brien, Cook and Robinson2017). It must be applied from a population-centred perspective, where all sources of non-natural mortalities are being considered, and it must be embedded in a MSE framework with a clear and quantitative conservation objective (Wade Reference Wade1998, Dillingham & Fletcher Reference Dillingham and Fletcher2008, Bunnefeld et al. Reference Bunnefeld, Hoshino and Milner-Gulland2011). Our experience indicates that within the EIA process for wind energy projects, the PBR decision rule has often been utilized mechanically, without much consideration for these constraints (Poot et al. Reference Poot, van Horssen, Collier, Lensink and Dirksen2011, Leopold et al. Reference Leopold, Boonman, Collier, Davaasuren, Jongbloed and Lagerveld2014, Busch & Garthe Reference Busch and Garthe2016, NIRAS 2016). From our perspective, it appears that using PPA to quantify relative metrics of impact is better suited to the EIA process, which has a disturbance-centred perspective. With the PPA approach, one can readily keep the impact assessment and decision steps separate, as is usually done in EIA. However, this approach should not lead to neglect of the definition of clear decision-making rules, as an absence of decision is often detrimental to population conservation (Cooke et al. Reference Cooke, Leaper, Wade, Lavigne and Taylor2012).
Data accessibility
This article does not contain data.
Acknowledgements
We warmly thank Matthieu Authier for his critical and constructive comments, as well as stimulating discussions that really helped improve this paper.
Author contributions
Thierry Chambert: Conceptualization, Literature Review, Writing – Original Draft Preparation, Writing – Review & Editing. Olivier Duriez: Conceptualization, Writing – Review & Editing. Aurélien Besnard: Conceptualization, Literature Review, Writing – Review & Editing.
Financial support
We thank the MSH-SUD, as well as the funders and steering committee members of the MAPE project. OD and AB were funded by their salaries as French public servants. The post-doctoral contract of TC was funded through the MAPE project. The MAPE project was funded by the ADEME, OFB, DREAL Occitanie, LABEX CEMEB (University of Montpellier), FEE, SER, Région Occitanie, the French Ministry of Ecology (MTES/DGEC), as well as 25 wind power plant operators (the full list can be seen here: https://mape.cnrs.fr/le-projet/financeurs/).
Competing interests
The authors declare that they have no competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical standards
None.





