Tinea infections caused by zoophilic dermatophytes are predominant in Southern Europe and the Mediterranean [Reference Terragni, Lasagni and Oriani1]. Infections are grouped according to the site of infection, such as tinea corporis (body) or tinea capitis (scalp) [Reference Weitzman and Summerbell2] and a common aetiological agent of tinea infection is Microsporum canis. This infection usually arises after a contact with infected cats or dogs. Although possible, human-to-human transmission is not the most effective means of transmission, and is generally self-limiting [Reference Snider, Landers and Levy3]. Clinically, M. canis infection elicits an intense host reaction and produces inflammatory, eczematous lesions with erythema and scaling. These lesions occur at sites of minor trauma, often in children aged <10 years. The incubation period is usually 10–14 days [Reference Ginter-Hanselmayer4].
Because of the broad differential diagnosis for dermatophytoses, the identification and laboratory confirmation of M. canis infection is made by light microscopic examination of skin scrapings in 10–15% potassium hydroxide (KOH), and fungal culture on Sabouraud's dextrose agar (SDA). M. canis strains exhibit a brilliant yellow-green fluorescence under ultraviolet light. Similar fluorescence can also be seen in infections with M. audouinii, M. rivalieri, and M. ferrugineum strains. However, not all M. canis strains produce fluorescence and so its absence does not exclude a diagnosis of dermatophytosis [Reference Snider, Landers and Levy3].
Tinea corporis and tinea cruris respond satisfactorily to topical therapies with azoles, allylamines, benzilamine derivatives, and hydroxypyridones. Systemic antifungals are required for tinea capitis when the infected areas are large, when present in a mixed skin infection, and in immunocompromised individuals [Reference Gupta, Chaudhry and Elewski5]. In Slovenia, the first two cases of M. canis infection were reported in 1966 and there were no more cases until 1977; subsequently the incidence increased steeply in the 1980s [Reference Lunder6]. Notification of M. canis infection to the National Institute of Public Health of Slovenia (NIPH) is mandatory for all physicians. In the last 20 years its incidence has continued to increase in absolute numbers and as a proportion of zoonotic dermatophytes to become one of the most common species isolated from patients with dermatophyte infections [Reference Lunder and Lunder7]. The absolute numbers and incidence per 100 000 inhabitants increased from 924 (47/100 000) in 1998 to 3444 (168/100 000) in 2011, respectively. Children are predominantly affected, but a considerable increase has also been noted in adults.
Outbreak data have been collected systematically at the national level since 1995. During that period, three M. canis outbreaks in the Ljubljana region were reported to the NIPH. The first outbreak of tinea capitis involved two 2-year-old children in kindergarten in October 1998 in Ljubljana. In September 1999, M. canis infection occurred in 23 pupils aged 7–14 years in an elementary school in Hrastnik. The last reported outbreak occurred in August 2000, when 21 individuals aged 5–39 years were affected after swimming in a public swimming pool in Ljubljana and four cases required hospitalization [8].
We describe here the first outbreak of M. canis infection to occur in the Ljubljana region in 12 years. The outbreak occurred in two elementary schools in Domžale municipality (population 11 600), situated in the north-eastern part of the Ljubljana region with 3200 children attending eight primary schools with 115–741 pupils and 14–76 teachers in each. The first elementary school notified the Regional Institute of Public Health (RIPH) Ljubljana on 12 November 2012 of five pupils with skin symptoms, presumed to be tinea infection; the first reported case had symptom onset on 4 October 2012. The five cases occurred in pupils that attended a birthday party on 24 September 2012, where a recently adopted stray kitten was present. On 13 November 2012, the second elementary school in the same municipality notified the RIPH Ljubljana of suspected tinea infection in three of their pupils; the first reported case had symptom onset on 15 November 2012. On 14 November 2012, an outbreak control team consisting of two epidemiologists from RIPH Ljubljana and the NIPH initiated an outbreak investigation.
We report the epidemiological investigation of the outbreak at the two schools to identify the source of infection and to apply control measures. Cases were actively searched for using in-depth questionnaires distributed to 135 pupils and employees from the classes where the cases occurred at both schools. Data collected included demographic information (age, gender, occupation, place of residence), clinical details (date of onset, signs and symptoms, their duration, treatment, outcome), and exposures and activities performed during the observed period (travel history, contact with patient, contact with domestic or stray animals).
Skin samples were examined at the Mycological Laboratory of the Dermatology Clinic, University Medical Centre Ljubljana. Skin lesions were examined with Wood's UV lamp [Reference Lunder6] and skin scrapings were prepared with 10–15% KOH solution and examined under direct light microscopy. Specimens were also inoculated on SDA with added chloramphenicol and cycloheximide, and incubated at 30°C for up to 3 months. Cultures demonstrating growth were subcultured once each week. The identification of dermatophytes was based on macroscopic and microscopic colony characteristics [8]. Skin scrapings and hair plucks from the suspected kitten were mounted in KOH and examined by direct microscopy. Other diagnostic tests (i.e. Wood's lamp examination, fungal culture) were not performed.
An unmatched case-control study was performed. Cases were defined as pupils or employees in both elementary schools with skin lesions and M. canis-positive microbial culture and/or Wood's lamp examination between 1 October 2012 and 1 December, 2012. Primary cases were defined as pupils who developed M. canis symptoms within 14 days after attending a birthday party on 24 September 2012. Secondary cases were individuals that developed symptoms at least 14 days after the last primary case became ill. We excluded persons who travelled abroad at least 14 days prior to their symptom onset and individuals that had a specimen positive for aetiological agents other than M. canis. Controls were defined as pupils from both schools without skin symptoms in the observed period. For each primary and secondary case five controls were randomly selected from the classes where infected pupils originated (random number generation).
All analyses were performed using Stata v. 12.0 (Stata Corporation, USA). We performed descriptive analysis of the data by person, place and time and calculated odds ratios with 95% confidence intervals for different exposures. Fisher's exact test was used in the univariate analysis for comparing the proportions of exposures between cases and controls. The level of statistical significance was set at 5%.
We received 74 completed questionnaires, yielding a response of 55%. None of the employees (n = 90) responded to the questionnaire but a telephone conversation with the school principals revealed that no employee had reported skin symptoms in the last 2 months. In total 12 cases were identified, six primary and six secondary cases. All primary cases attended the birthday party, where a recently adopted stray kitten was present and five of these six cases attended the first elementary school. However, one primary case attended gymnasium activities in the second elementary school and presumably spread the infection there.
Of the 12 cases reported, all were female. Cases ranged in age from 6 to 13 years (median age 9·5 years). The pityriasiform scaling lesions typical of M. canis infections were most frequently present on the face (58%), hands (58%), and trunk (33%). None of the cases had lesions on the scalp. All cases responded completely to the treatment with topical antimycotics.
M. canis infection was diagnosed by microscopy and fungal culture in ten cases. In two secondary cases with skin lesions, a diagnosis of M. canis infection was considered probable on positive Wood's lamp examination. In specimens taken from the implicated kitten no fungal spores were viewed by microscopy.
Six primary cases and 30 randomly sampled controls were included in the analysis. The age range for cases was 8–11 years (median 8·5 years) and for controls 6–13 years (median 10 years). In the two groups age did not differ significantly. All of the cases and 10 (33%) of the controls were female. Contact with the recently adopted stray kitten at the birthday party was identified as the most likely source of M. canis infection for primary cases. For the six secondary cases and 30 randomly sampled controls the age range for cases was 6–13 years (median 12 years) and for controls 6–13 years (median 9 years). All secondary cases were female and 70% of controls were male. The results of the univariate analysis are presented in Table 1. Compared to controls, secondary cases were more likely to have participated in gymnastic classes with a primary case during the outbreak period. Secondary cases were also more likely to have touched an infected child.
Table 1. Selected exposures in primary cases, secondary cases, and controls; Microsporum canis outbreak linked to two elementary schools in Domžale municipality, Slovenia, 2012
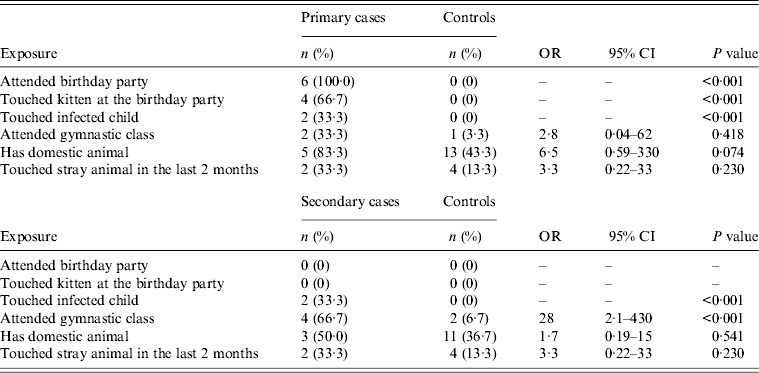
OR, Odds ratio; CI, confidence interval.
This is the first reported outbreak of M. canis infection in Slovenia after more than a decade in two elementary schools in the Ljubljana region. The outbreak affected 12 pupils and lasted 48 days. All infected pupils made a full recovery. Our investigation suggests that contact with an adopted stray kitten at the birthday party was the most likely source of infection for primary cases. Contact with an infected cat was cited as the most likely source of infection in previously notified outbreaks in Slovenia [8] and corresponds to other reports, where M. canis infection in humans predominantly emerged after contact with infected pets, especially kittens and dogs, which can be asymptomatic carriers. Human-to-human transmission is infrequent and is described as self-limiting [Reference Lunder and Lunder7]. Since one primary case attended the gymnasium in the second elementary school, the most probable source of infection for secondary cases was contact with the infected child during joint sports activities. Transmission of M. canis arthrospores through clothes or footwear while participating in gymnastic classes may also have played a role in the secondary transmission [Reference Gupta, Chaudhry and Elewski5, Reference Hermoso de Mendoza9]. The young age and female prevalence of affected cases are in agreement with previous reports from Slovenia [Reference Lunder6, 8].
Early diagnosis and treatment of Microsporum infection is crucial to prevent its spread and requires a high level of attentiveness, especially by primary-care physicians, who are responsible for referring infected persons to a dermatologist [Reference Ginter-Hanselmayer4]. All our patients were referred to a dermatologist, where the diagnosis was established by a fungal culture in more than 80% of cases. The most commonly affected sites in primary cases were trunk, primarily chest, face, and hands, i.e. areas most readily exposed when playing with a cat, while the most commonly affected sites in secondary cases were hands, which can be explained by their exposure while performing joint sports activities [Reference Lunder6].
Because of the delayed notification we performed the investigation more than a month after the first case had occurred and when the secondary cases were already present. When the first case appeared on 4 October 2012, representatives of the school advised pupils with suspicious skin lesions to visit their paediatrician or family doctor and to cover the skin lesions. However, in the following 2 weeks when four more cases appeared at the first school and three cases at the second school, it was only then, on 12 November, that the school representatives informed the regional epidemiologist about a possible outbreak. The outbreak control team began an outbreak investigation and implemented additional infection control measures. At that time advice was given to cases on how to avoid onward transmission to reduce the occurrence of secondary cases, such as washing hands after handling animals, avoiding touching infected skin lesions on other people, practising good personal hygiene, especially when participating in activities involving physical contact with other people, avoiding sharing towels, clothing or hair accessories with infected individuals, and keeping the skin dry. Pupils with confirmed M. canis infection were advised to stay at home and to keep the lesions covered until the first negative microbiological result [Reference Gupta, Chaudhry and Elewski5]. Prompt communication between school and health representatives as soon as possible after the first case presentation is crucial to ensure rapid implementation of appropriate control measures and in this incident the delay in reporting clearly contributed to onward transmission of the infection. Although descriptive epidemiological evidence supports the stray kitten as the source of infection, this could not be confirmed by light microscopic examination of samples from the animal. Restriction of movement, hygienic measures, adequate diagnostic procedures (i.e. culture examination) and treatment of the infected animal with systemic and topical antifungals, hypochlorite disinfection of its environment, and looking for other infected animals are essential in controlling transmission, especially taking into account that veterinary control of cats is less strict than for dogs and the spread of infection in the former is more difficult to prevent [Reference Lunder6, Reference Hermoso de Mendoza9].
A limitation of the case-control study was that as there were relatively few cases we aimed to obtain information from five controls per case to maximize power. Controls originated from the same schools from which the cases were drawn, so there was probably a low discordance between cases and controls in terms of exposures and we might have overestimated exposure in controls. Another possible limitation was the potential presence of recall bias, as interviews were held more than a month after symptom onset in the first case. However, most questionnaires were completed by parents, who tended to have good recall regarding their children's health status and activities [Reference Mindlin10].
Although we failed to confirm M. canis infection in the relevant kitten, we remain persuaded that it was the most likely source of infection in primary cases. Prompt communication and control measures after identification of the primary cases would have prevented the secondary cases in the second elementary school. Consistent and integrated efforts by the medical and veterinary services, together with health education regarding transmission patterns and hygienic standards are needed to prevent the spread of M. canis infection and possible future outbreaks.
ACKNOWLEDGEMENTS
The authors thank all colleagues at RIPM in Ljubljana for the distribution and collection of questionnaires.
DECLARATION OF INTEREST
None.




