INTRODUCTION
Asymptomatic meningococcal carriage is common, but invasive meningococcal infection is not. We studied the risk of cancer and immune-related disease in people who have had meningococcal disease for two reasons. First, the factors that govern individual susceptibility to meningococcal disease are not well understood but are influenced by genetic polymorphisms and by genetically determined characteristics of the host immune response to infection [Reference Texereau1, Reference Haralambous2]. Susceptibilities to cancer, and to immune-mediated disease, also have a strong genetic basis. Some genetic polymorphisms that are associated with an increased risk of one disease may be prevalent because they help to protect against other life-threatening infections and therefore have evolutionary survival value. It is conceivable that susceptibility to invasive meningococcal disease is associated with susceptibility and/or resistance to other diseases.
Second, we considered it useful to seek information that would enable clinicians to advise on whether patients who have had meningococcal disease were at any different risk of these groups of diseases than the general population.
For these reasons, we hypothesized that people who have meningococcal disease may differ from others in their susceptibility to cancer and to immune-mediated diseases.
METHODS
Population and data
We used data from the Oxford Record Linkage Study (ORLS) [Reference Gill3, Reference Goldacre4] and from English national Hospital Episode Statistics (HES) [Reference Goldacre and Gill5, Reference Gill and Goldacre6]. The ORLS includes brief statistical abstracts of records of all hospital admissions (including day cases) in National Health Service (NHS) hospitals, and all deaths regardless of where they occurred, in defined populations within the former Oxford NHS Region from 1963 to 1998. The hospital data were collected routinely in the NHS as the region's hospital statistics system and were similar to English national HES. The death data derive from death certificates. The data for each individual were matched and linked, as they accrued, and are now anonymized and archived. The Oxford data were matched and linked using probabilistic linkage with identifiers that included names, dates of birth, sex, and other corroborating information (such as hospital medical record number) [Reference Gill3]. The identifiers were held in a separate computer file (the ‘identifiers file’) from the rest of the data (the ‘analysis file’) on each person's record. A unique personal number for each individual patient, generated within the ORLS system, was used as the ‘bridge’ between the identifiers file and the analysis file, such that the analysis file contained a set of successive records known, from the unique personal number, to relate to the same person. The automated matching and linkage algorithms and software were developed by ORLS staff. The English data, an extract from linked national HES and death data, spanned 1999–2005. The whole English national dataset was matched and linked for the English Department of Health, by ORLS staff, using the patients' NHS number, postcode and date of birth; and using automated matching and linkage software developed by ORLS staff [Reference Gill and Goldacre6]. The data were split into an identifiers file and an analysis file, as described above for the ORLS. Both linked files are held as time-sequenced records of successive events and their dates (e.g. successive hospital admissions, admission and death) for each individual. This enables analytical staff to easily identify first and subsequent admissions for conditions of interest. The Oxford dataset has the benefit of long periods of ‘follow-up’. The English national dataset covers a much shorter period but a much larger population. For these reasons, both datasets were used.
The English NHS Central Office for Research Ethics Committees approved the current work programme of analysis using the datasets (reference number 04/Q2006/176).
Using the ORLS, a cohort of people with meningococcal disease was constructed by identifying an admission for the disease to an NHS hospital during the study period. The International Classification of Disease (ICD) codes used were 057 in ICD-7, 036 in ICD-8 and ICD-9, and A39 in ICD-10. A reference cohort was constructed by identifying the first admission for each individual with various medical and surgical conditions as a main diagnosis or operation (see Table notes). This is based on a ‘reference’ group of conditions that has been used in other studies of associations between diseases [Reference Goldacre4, Reference Goldacre7, Reference Goldacre8]. People were included in the meningococcal or reference cohort if they did not have an admission for cancer or immune-mediated disease either before or at the same time as the admission for meningococcal disease or the reference condition. We searched the database for any subsequent NHS hospital care for, or death from, cancer or immune-mediated disease in these cohorts. We considered that rates of cancer or immune-mediated disease in the reference cohort would approximate those in the general population of the region while allowing for migration in and out of it (data on migration of individuals were not available). The conditions included in the immune-mediated group are listed in a note on the table of results. We included diabetes mellitus with an age restriction of <30 years as a proxy for type 1 diabetes, i.e. diabetes with autoimmune aetiology (type is not invariably recorded in routine hospital statistics).
The same methods and selection criteria were used to construct cohorts using the English national data.
Statistical methods
We calculated rates of each cancer or immune-mediated disease based on person-years at risk. We took ‘date of entry’ into each cohort as the date of first admission for meningococcal disease, or reference condition, and ‘date of exit’ for the analysis of each individual cancer or immune-mediated disease as the date of first record of cancer or immune-mediated disease, death, or the end of the data file (1998 for ORLS, 2005 for England), whichever was the earliest. In comparing the meningococcal disease cohort with the reference cohort, we first calculated rates for cancer or immune-mediated disease, standardized by age (in 5-year age groups), sex, calendar year of first recorded admission, and either district of residence (ORLS) or region of residence (England), taking the combined meningococcal disease and reference cohorts as the standard population. We then applied the overall rates to the age structure of the individual cohorts of people with meningococcal disease or with the reference conditions. We calculated the ratio of the standardized rate of occurrence of cancer or immune-mediated disease in the exposure cohorts relative to that in the reference cohort. The confidence interval for the rate ratio and χ2 statistics for its significance were calculated as previously described [Reference Breslow and Day9]. The statistical package used in this study, and in other ORLS studies of disease associations, was developed by one of the authors (D.G.R.Y.).
In comparing the meningococcal and reference cohorts, the precision of the rate ratio depends on the number of people with each subsequent disease within each cohort. The size of the meningococcal cohort is fixed by the number in the dataset with the condition. In the reference cohort, we included all the people in the dataset with the comparison conditions in each age group. We did this to maximize the numbers in each stratum in the reference cohort in order to maximize the precision of the rate ratios.
Our initial plan was to confine the analysis to people aged <40 years at the time of admission for meningococcal disease. We reasoned that the great majority of cases of meningococcal disease occur in people <40 years and that, in this age range, potential problems of comorbidity with chronic degenerative disease would be largely avoided. However, analysis of the data on people aged ⩾40 years showed very similar findings of rate ratios for cancer and immune-mediated diseases as for those aged <40 years. Accordingly, we show results for both broad age groups and all ages combined.
RESULTS
In the Oxford data, there were 1373 people in the dataset who had been admitted to hospital with meningococcal disease previously. Of these, 33% (452) were aged <1 year, in total 64% (881) were aged <15 years, 26% (350) were aged between 15 and 39 years, and 10% (142) were aged >40 years. The mean period of follow-up was 13·1 years and the median was 10·0 years. There were 493 772 people in the reference cohort. In the English national data, there were 11 665 people admitted with meningococcal disease. Of these, 15% (1777) were aged <1 year, in total 59% (6903) were aged <15 years, 26% (3053) were aged between 15 and 39 years, and 15% (1709) were aged >40 years. The mean period of follow-up was 3·7 years and the median was 4·4 years. There were 3 943 304 people in the reference cohort. Table 1 shows the age distribution of people admitted with meningococcal disease and the matching ratio, by age group, of numbers of people in the reference cohort to numbers in the meningococcal disease cohort. It shows that there were substantial numbers of ‘matched’ cases in the reference cohorts for every case in the meningococcal disease cohorts.
Table 1. Number of people admitted to hospital with meningococcal disease or in the reference cohort in each age-group stratum
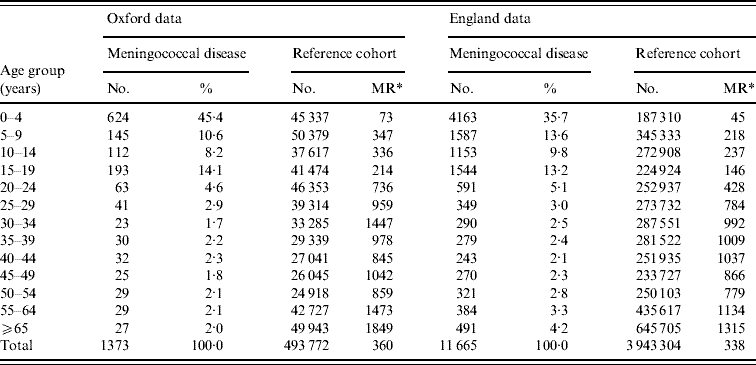
* MR, Matching ratio=the number of people in the reference cohort per person with meningococcal disease in each age stratum.
Table 2 shows the observed and expected numbers, and rate ratios, for subsequent cancer and immune-mediated disease in people who had been admitted to hospital for meningococcal disease. We found no altered risk of cancer in either the Oxford population [rate ratio (RR) 0·9, 95% confidence interval (CI) 0·4–1·6; Table 2] or the England population (RR 1, 95% CI 0·8–1·3; Table 2). The rate ratios were very similar for the people in both broad age groups (Table 2). There was no significant altered risk of immune-mediated disease in the Oxford population (RR 1·5, 95% CI 0·8–2·5; Table 2), but there was a slight and marginally significant reduction of risk in the England data (RR 0·7, 95% CI 0·5–0·9; Table 2). The rate ratios in the two age groups were very similar (Table 2).
Table 2. Occurrence of cancer or immune-mediated disease, subdivided by population and age, in people who had been admitted to hospital for meningococcal disease, compared with a reference cohortFootnote *
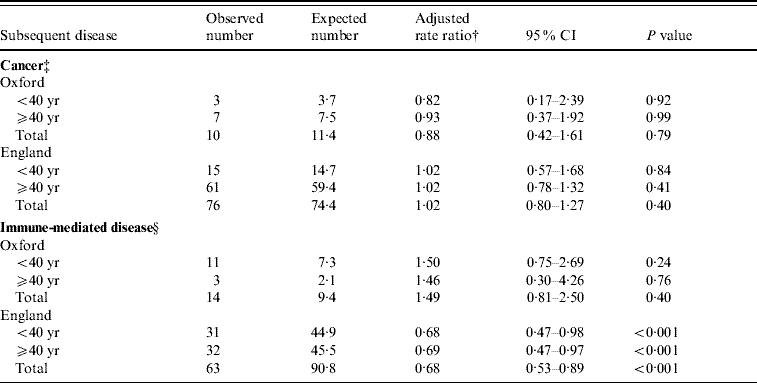
* Conditions used in reference cohort, with Office of Population, Censuses and Surveys (OPCS) code edition 3 for operations and ICD-9 code for diagnosis (with equivalent codes used for other coding editions): squint (378), otitis externa, otitis media (380–382), haemorrhoids (455), varicose veins (454), deflected septum, nasal polyp (470–471), impacted tooth and other disorders of teeth (520–521), inguinal hernia (550), ingrowing toenail and other diseases of nail (703), sebaceous cyst (706.2), internal derangement of knee (717), bunion (727.1), selected fractures (810–816, 823–826), dislocations, sprains and strains (830–839, 840–848), superficial injury and contusion (910–919, 920–924), appendicectomy (441).
† Adjusted for sex, age in 5-year bands, and time-period in single calendar years.
‡ Cancer codes included in the analysis, using ICD-9 codes (and equivalent codes in other coding editions): 140–208.
§ Immune-mediated diseases included in the analysis, with ICD ninth revision codes for each in parentheses (and equivalent codes in other coding editions): ankylosing spondylitis (720), immune-related haemolytic anaemia (283.0), chronic active hepatitis (571.4), Crohn's disease (555), coeliac disease (579.0), dermatomyositis (710.3), polymyositis (710.4), diabetes mellitus under 30 (250), Goodpasture's syndrome (446.2), Hashimoto's thyroiditis (245), idiopathic thrombocytopenia purpura (287.3), multiple sclerosis (340), myasthenia gravis (358), myxoedema (244.8, 244.9), pemphigus/pemphigoid (694.4–694.5), pernicious anaemia (281), polyarteritis nodosa (446), primary biliary cirrhosis (571.6), psoriasis (696.0, 696.1, 696.8, 696.9), rheumatoid arthritis (714), scleroderma (710.1), Sjogren's syndrome (710.2), systemic lupus erythematosus (710.0), thyrotoxicosis (242), ulcerative colitis (556).
In the Oxford dataset, there were no individual cancers or immune-mediated disease with five or more observed or expected cases and no significant rate ratios for any individual disease. In the English national dataset, cancers of the colon, lung and breast, along with multiple myeloma had five or more observed or expected cases (Table 3). There was a significantly elevated risk of multiple myeloma associated with meningococcal disease (RR 7·1, 95% CI 2·6–15·4). There were five or more observed or expected cases of Crohn's disease, ulcerative colitis, diabetes mellitus under the age of 30 years, myxoedema, psoriasis and rheumatoid arthritis (Table 3). There were significantly reduced rate ratios for Crohn's disease (RR 0·1, 95% CI 0–0·7, based on 1 observed and 7·7 expected cases), and myxoedema (RR 0·6, 95% CI 0·3–0·9, based on 16 observed and 28·1 expected cases). There was a non-significantly low risk of diabetes mellitus under the age of 30 years after admission for meningococcal disease (RR 0·7, 95% CI 0·3–1·2, based on 11 observed cases and 16·6 expected).
Table 3. Cancers or immune-mediated diseases with five or more observed or expected cases, in the England data
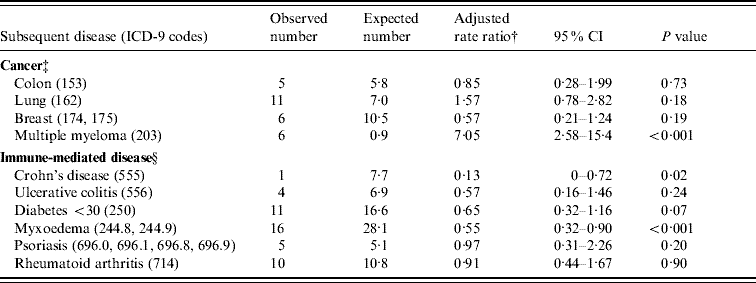
†‡§ For table notes see Table 2.
DISCUSSION
As far as we are aware, there has been no previous published hypothesis on, or data about, an altered risk of cancer in people with meningococcal disease. Howitz et al. studied the risk of autoimmune disease in people who have had meningococcal disease [Reference Howitz10]. This was done because of concerns about whether immunization against meningococcal disease, in addition to invoking a specific immune response, might alter immune function more generally. They found no association between meningococcal disease and autoimmune disease function. Their study design was similar to ours: it was based on record linkage, confined to hospital patients only, and because meningococcal disease and autoimmune diseases are both rare, their study was also based on small numbers of cases of people with both diseases.
A limitation of the datasets used in our study is that they are confined to people admitted to hospital. This should capture all those diagnosed with invasive meningococcal disease that survive long enough to reach hospital; the great majority of people with cancer; but only an unknown fraction of those in the cohorts who developed immune-mediated disease. This limitation was also noted in the study by Howitz et al. [Reference Howitz10]. However, we see no reason to think that admission thresholds for cancer or immune-mediated disease should be influenced by whether people have a prior history of meningococcal disease or not, and doubt that the use of in-patient data alone would have had much, if any, effect on our risk ratios. The diagnostic data are those as coded in the datasets according to the International Classification of Diseases. No consistent data on serogroups were available. Future studies should, if possible, include data on serogroups. Current privacy regulations preclude access to original case-notes to validate or add to the data. Accordingly, the diagnoses used in this study are those on the statistical records of the hospital admissions and on death certificates. The data on meningococcal disease in the ORLS have face validity: a publication on case-fatality rates (CFRs), using ORLS data, reported 30-day CFRs of 11·1% [Reference Goldacre11]. The authors commented [Reference Goldacre11] that this was almost identical to the CFR of 10·9% reported in an earlier study that was based on detailed clinical case-note review and diagnostic validation [Reference Goldacre12].
The age distribution of the people studied was predominantly young – more than two-thirds were aged <15 years, and nearly 90% were aged <40 years, at the time of meningococcal disease – and so the risks studied in these age groups are predominantly those of cancers and immune-mediated diseases in children and young people. The older group, aged ⩾40 years, are in an age group when cancer is, of course, more common. Thus, although only a small minority of all cases of meningococcal disease occurred in this age group, it contributed a majority of cases of subsequent cancer. We consider that our main findings are those for overall cancer risk and overall immune-mediated disease risk: the statistical power of the datasets is too low to study risks of individual cancers or immune-mediated diseases in detail. The low risk ratio for Crohn's disease and myxoedema, and the high ratio for multiple myeloma, were significant but we made multiple comparisons and would expect one or two significant associations by chance alone. Nonetheless, we note them and it would be interesting to confirm or refute the associations in other datasets. One possible source of bias is that a patient admitted for meningococcal disease may be noted to have another condition (e.g. an immune-mediated disease) that might result in a subsequent admission for investigation or stabilization after the patient has recovered from the acute life-threatening illness. The bias would go in the direction of apparently elevating the rate ratio for the other condition. We found a reduced rate ratio for Crohn's disease and myxoedema; and it is harder to envisage sources of bias that would generate a spurious negative association. If these findings are replicated elsewhere, they would raise the possibility that in some people there are immune functions that predispose to meningococcal disease but protect against Crohn's disease and myxoedema. Literature searches using PubMed showed no literature on associations between meningococcal disease and Crohn's disease, myxoedema or multiple myeloma.
Our findings should be regarded as indicative rather than definitive. Larger studies with longer follow-up through adult life, and studies large enough to assess the risks of individual diseases, would be interesting but, unless done using very large databases of routinely collected data, would not be easy to do. With these reservations, we conclude that the overall risks of cancer are neither enhanced nor reduced in people who have had invasive meningococcal disease. Our evidence suggests that immune-mediated disease is no higher than expected in people with meningococcal disease and, if anything, it may be a little lower.
ACKNOWLEDGEMENTS
The dataset of the Oxford Record Linkage Study was funded by the former Oxford Regional Health Authority and, over many years, it was built by Leicester Gill and Glenys Bettley. The Research and Development Directorate at the Department of Health funds the Unit of Health Care Epidemiology to undertake research using the Oxford Record Linkage Study and the English national linked dataset.
DECLARATION OF INTEREST
None.





