Introduction
Burns represent a common type of trauma worldwide [Reference Logsetty, Shamlou, Gawaziuk, March, Doupe, Chateau, Hoppensack, Khan, Medved, Leslie, Enns, Stein, Asmundson and Sareen1]. The World Health Organization estimates the occurrence of about 11 million burns and the death of 300,000 burn patients annually worldwide, resulting in the loss of about 18 million disability-adjusted life years [Reference Rybarczyk, Schafer, Elm, Sarvepalli, Vaswani, Balhara, Carlson and Jacquet2, Reference Li, Yao, Tan, Zhou, Li, Wu and Luo3]. Approximately 90% of burn injuries were documented in low- and middle-income regions, including China [Reference Rybarczyk, Schafer, Elm, Sarvepalli, Vaswani, Balhara, Carlson and Jacquet2], and remain a major public health concern. Central venous catheters (CVCs) are indispensable for burn treatment as they provide stable vascular access for fluid resuscitation, safe infusion of antibacterial and vasoactive drugs [Reference Gotchac, Poullenot, Guimber, Ecochard-Dugelay, Schneider, Peretti, Billiauws, Borderon, Breton, Chaillou Legault, Chambrier, Comte, Coste, Djeddi, Dubern, Dupont, Espeso, Fayemendy, Flori, Fotsing, Gastineau, Goulet, Guiot, Jirka, Languepin, Layec, Quilliot, Rebouissoux, Seguy, Talon, Turquet, Vallee, Willot, Lamireau and Enaud4], parenteral nutritional support therapy [Reference Allon, Brouwer-Maier, Abreo, Baskin, Bregel, Chand, Easom, Mermel, Mokrzycki, Patel, Roy-Chaudhury, Shenoy, Valentini and Wasse5], continuous hemodialysis therapy [Reference Karagiannidou, Zaoutis, Maniadakis, Papaevangelou and Kourlaba6], and continuous hemodynamic monitoring [Reference Chovanec, Arsene, Gomez, Brixey, Tolles, Galliers, Kopaniasz, Bobash and Goodwin7].
Catheter-related infection (CRI) and thrombosis are the two major, sometimes lethal, complications of CVCs, with central line–associated bloodstream infection (CLABSI) being the most serious presentation, and characterised by a significantly higher incidence – two- to three-fold – in burn patients (15.5–29.1 per 1,000 catheter days) than in other patients (4.80–8.64 per 1,000 catheter days) [Reference Garcia, Spitzer, Beaudry, Beck, Diblasi, Gilleeny-Blabac, Haugaard, Heuschneider, Kranz, McLean, Morales, Owens, Paciella and Torregrosa8-Reference Magiorakos, Srinivasan, Carey, Carmeli, Falagas, Giske, Harbarth, Hindler, Kahlmeter, Olsson-Liljequist, Paterson, Rice, Stelling, Struelens, Vatopoulos, Weber and Monnet11].
CLABSI not only prolongs the patient’s hospital stay and increases the medical burden [Reference Garcia, Spitzer, Beaudry, Beck, Diblasi, Gilleeny-Blabac, Haugaard, Heuschneider, Kranz, McLean, Morales, Owens, Paciella and Torregrosa8] but also may affect clinical outcomes and increase morbidity and mortality [Reference Wang, Lu, Song, Zhou, Liu and Zhang9, Reference Zhang, Brusasco, Anile, Corradi, Mariyaselvam, Young, Almog, du, Yu, Zhu, Zhang, Cao and Hong10]. It has become one of the key indicators of nosocomial infections worldwide, with serious complications ranging from sepsis syndromes, endocarditis, and haematogenous transmission, which leads to additional healthcare, estimated in one study to be increased over two-fold [Reference Magiorakos, Srinivasan, Carey, Carmeli, Falagas, Giske, Harbarth, Hindler, Kahlmeter, Olsson-Liljequist, Paterson, Rice, Stelling, Struelens, Vatopoulos, Weber and Monnet11]. More importantly, CLABSI is an independent risk factor for death [Reference Zeng, Wu, Li and Jia12], and as such its prevention and treatment have become priorities in hospital infection control.
In essence, CLABSI is a diagnosis of exclusion, and requires other infections (wound, respiratory, and urinary tracts) to be ruled out first. Consequently, its diagnosis may be delayed and may impact negatively on appropriate treatment [Reference Aldea13]. However, as most current studies focus on the screening of risk factors for CLABSI, a quantitative method to predict the infection remains lacking in clinical practice. A nomogram is a common presentation of a prediction model, which can quantitatively integrate multiple risk factors with different ratios [Reference Fochtmann-Frana14]. In practice, a total risk score can be calculated by the actual value of several variables and give the risk probability of a specific infection in a patient or population, with an obvious benefit for their prevention and treatment.
In this study, we analysed retrospectively the clinical features, infection profiles, and related risk factors of CLABSI in burn patients through the construction of a novel nomogram with the aim to provide a simple, practical, and quantitative support system for the timely diagnosis, and treatment of affected patients.
Methods
Study design
A retrospective study was conducted at the Institute of Burn Research, Southwest Hospital, Third Military Medical University, between January 2018 and December 2021. This is one of the largest burn centres in the world and has 126 inpatient beds (including 18 beds in a burn intensive care unit), and annually admits approximately 1,300 patients from southwest China. The study was approved by the ethical committee of the Southwest Hospital (No. KY202245). In accordance with national legislation and institutional requirements, written informed consent from the participants’ legal guardian / next of kin was not required to participate in this study.
Data collection
Patients with CVCs were primarily identified through a search of the hospital’s burn database. Only burn patients were included; others with CVCs inserted before admission, or no documented bacterial cultures or incomplete data were excluded, along with patients admitted later than 1 month after burn injury. In total, 222 patients with 630 CVC events were included. The following data were extracted from medical records: demographic data (gender, age, admission date, injury date, BMI), clinical features (burn aetiology, area and location, inhalation injury, infection, transfusion, in-bed days, operation numbers), CVC information (insertion date, puncturing times, specific veins and sides, tip location, extubation date, and others), as well as CLABSI details (pathogen name, detecting time, drug resistance), and outcomes (death, length of hospital stay).
CVCs insertion and management
The application of CVC was determined by doctors, mainly based on the following considerations: fluid resuscitation, long-term parenteral nutrient supply, infusion of antimicrobials, vasoactive and other stimulus agents, repeated blood tests, and continuous blood purification treatment. All CVCs were inserted by an experienced team according to practice guidelines [Reference Castelli, Pognani, Stuani, Cita and Paladini15]. All operators wore masks, caps, sterile gloves, and surgical gowns. Hand disinfection with 70% ethanol by volume or other hand disinfection solutions was mandatory before and after catheter placement, replacement, viewing, adjustment, or dressing change. For placement via deep burn wounds, 20 g/L tincture of iodine was used, and via superficial burn wounds, chlorhexidine or other iodine-containing disinfectants were used.
Ultrasound was used to guide the catheterisation of CVCs when venipuncture catheterisation was difficult, and if the catheter was inserted in a burn wound, gauze with povidone-iodine was applied to the catheter exit site. Dressings were changed, and the length of the outer catheter was measured daily. Dressings were replaced if they appeared damp, loose, or visibly polluted. Catheter puncture points and signs of systemic infection were observed daily, and if local inflammation at the puncture site or vascular CRI was suspected, a comprehensive evaluation was made by the medical team to determine whether extubation was necessary. If CLABSI was suspected, peripheral blood samples were collected for microbiological culture before, or immediately after, catheter removal.
Microbiological identification and drug sensitivity test
Microbiological culture was performed by semi-quantitative or quantitative methods. The proximal tip of the extracted catheter (about 5 cm long) was rolled several times over a Columbia blood agar plate medium (Difco, Franklin Lakes, NJ, USA) in a ‘Z’ pattern, and following incubation, microbial growths were identified by standard microbiological procedures. A panel of culture agar media (blood, chocolate, etc) was used for the semi-quantitation of microbial loads in clinical samples. All media were incubated for 18–24 h in atmospheres appropriate for the species sought. Isolates were identified to species level and antimicrobial susceptibility determined using the VITEK-2 compact system analysis (BioMerieux, Saint-Vulbas, France), with reference to the Clinical and Laboratory Standards Institute for MIC breakpoint determination. Breakpoint concentrations of cefoxitin of ≤4 and ≥ 8 mg/L were used to differentiate between methicillin-susceptible and methicillin-resistant Staphylococcus aureus isolates. Multiple drug-resistant (MDR) and extensively drug-resistant (XDR) strains were defined as previously described [Reference Friedman, Mian, Mullins, Hassan, Shaver and Johnston16]. Carbapenem-resistant Acinetobacter baumannii (CRAB), carbapenem-resistant Pseudomonas aeruginosa (CRPA), and carbapenem-resistant Klebsiella pneumoniae (CRKP) were defined by resistance to imipenem or meropenem.
Definition of CLABSI
CLABSI was defined as the presence of a positive blood culture in a patient with an indwelling CVC, or within 48 hours after its removal [Reference Rickard, Marsh, Larsen, McGrail, Graves, Runnegar, Webster, Corley, McMillan, Gowardman, Long, Fraser, Gill, Young, Murgo, Alexandrou, Choudhury, Chan, Gavin, Daud, Palermo, Regli and Playford17]. The infection was diagnosed based on the following criteria: (1) the isolation of a recognised pathogen cultured from one or more blood samples; (2) a fever (>38 °C), chills, hypotension, or other signs and symptoms, as well as positive laboratory results not related to an infection at another site; and (3) isolation of the same microbial species with similar antimicrobial susceptibility profile from the blood and the catheter tip. CLABSI rates were reported as events per CVCs/1,000 line-days. For patients with multiple catheterisations, only the first positive result was diagnosed as CLABSI if consecutive positive cultures with the same microorganism occurred.
Nomogram construction and statistical analysis
Data analysis was performed using GraphPad Prism 6 (USA, GraphPad Software Inc.) and SPSS 22.0 (USA, IBM analytics). The t-test was used to compare quantitative variables with nominal distribution, and the Mann–Whitney U test was conducted to compare two categorical variables. Chi-squared test was applied to assess significant associations between two categorical variables (frequency and percentage). Multicollinearity among the included variables was analysed using collinearity diagnostics prior to regression analysis. Univariate and multivariate logistic regression (forward LR method, entry: p = 0.05; removal = (0.10)) was used to screen for factors contributing to CLABSI. Kaplan–Meier methods were used to perform survival analysis, and Cox regression models to screen out risk factors of death. Details regarding the variable assignments are shown in Table S1.
Least-absolute shrinkage and selection operator (LASSO) regression analysis, nomogram construction, and evaluation were performed in R Studio 2022.02.0 software (Prairie Trillium Release) using ‘glmnet’, ‘rms’, ‘pROC’, and ‘ggDCA’ packages. LASSO regression analysis was performed to determine associations between risk factors and CLABSI. All candidate features were entered into the analysis, and the assumption of proportional hazards was confirmed. The optimal feature combination was selected based on the LASSO regression analysis. The nomogram was constructed on the optimal feature, and evaluated by the area under the receiver operating characteristic curve (AUROC), calibration curves, and decision curve analysis. P values < 0.05 were considered statistically significant.
Results
A total of 233 patients with 678 CVCs were initially included, which was then reduced after step-by-step selection to 222 patients with 630 CVCs (Figure 1). Among 630 CVCs, a total of 118 CLABSI cases were identified, with a 1,000 line-day infection rate of 23.02. The annual incidence of CVCs with CLABSI showed an overall downward trend from 2018 to 2021 (25.42, 26.11, 18.60, and 15.51, respectively) (Figure 2a). Among the 222 patients’ cohort, 69 developed CLABSI, with a rate of 31.08%. Overall, the annual percentage of burn patients with CLABSI also gradually decreased, particularly in 2021 (Figure 2b).

Figure 1. Flow chart of patient selection.

Figure 2. The annual incidence of CLABSI in burn patients. (a) The annual incidence of CVCs with CLABSI per 1,000-day line-days. (b) The annual percentage of burn patients with CLABSI in total burn patients.
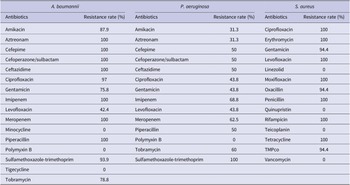
Antimicrobial resistance of bacterial isolates
As shown in supplementary Table S2, 163 bacterial isolates were identified, and over three-quarters were Gram-negative species. The three most common were A. baumannii, P. aeruginosa, and S. aureus accounting for 39.26%, 18.40%, and 12.88%, respectively.
The rate of antibacterial resistance was relatively high, with 76.09% of isolates classed as MDR, of which 18.48% were XDR. All isolates of A. baumannii were MDR (42.2% XDR), and 75.0% of P. aeruginosa were also MDR (18.7% XDR). Likewise, all S. aureus isolates were MRSA. Carbapenem resistance was universal in A. baumannii, and also evident in 62.5% of P. aeruginosa. All A. baumannii were susceptible to polymyxin B, tigecycline, and minocycline, and exhibited variable resistance to amikacin, gentamicin, levofloxacin, and tobramycin (42.4–87.9%) (Table 1). Likewise, P. aeruginosa were uniformly susceptible to polymyxin B, with varying resistance rates to meropenem and other antimicrobials, but resistant to cotrimoxazole; 62.5% were resistant to meropenem and moderately so to other antibiotics (31.3%–60.0%) (Table 1). S.aureus isolates were uniformly susceptible to vancomycin, teicoplanin, linezolid, and tigecycline, but highly resistant to other antibiotics (Table 1).
Table 1. The distribution of drug resistance of pathogens in CLABSI

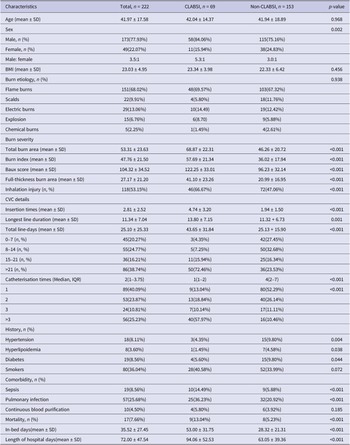
Clinical features of patients with and without CLASBI
The demographics and clinical features of the included 222 patients with and without CLABSI are shown in Table 2. CLABSI patients comprised significantly more males (5.3:1 vs. 3.0:1, p = 0.002), were of older age (42.04 ± 14.37 vs. 41.94 ± 18.89, p = 0.021), and presented with more flame burns (69.57% vs. 67.32%, p = 0.038). Burn severity was significantly higher in the CLABSI group, including total burn area (68.87 ± 22.31 vs. 46.26 ± 20.72, p < 0.001), full-thickness burn area (41.10 ± 23.26 vs. 20.99 ± 16.95, p = 0.002), revised Baux score (122.25 ± 33.01 vs. 96.23 ± 32.14, p < 0.001), burn index (57.69 ± 21.34 vs. 36.02 ± 17.94, p < 0.001), and inhalation injury (66.67% vs. 47.06%, p < 0.001). Notably, CLABSI patients had significantly more line insertion times (4.74 ± 3.20 vs. 1.94 ± 1.50, p < 0.001), longer total line-days (43.65 ± 31.84 vs. 25.13 + 15.90, p < 0.001), and the longest duration (13.80 ± 7.15 vs. 11.32 + 6.73, p < 0.001) than the non-CLABSI group. Unsurprisingly, the mortality rate (13.04% vs. 5.23%, p < 0.001), in-bed days (53.00 ± 31.75 vs. 28.32 ± 21.31, p = 0.003), and length of hospital stay (94.06 ± 52.53 vs. 63.05 ± 39.36, p < 0.001) were also higher in the CLABSI group.
Table 2. Clinical features of burn patients with and without CLABSI

TBSA, total body surface area; BMI, body mass index; Baux score, age (years) + total body surface area (percent) + (17× inhalation injury).
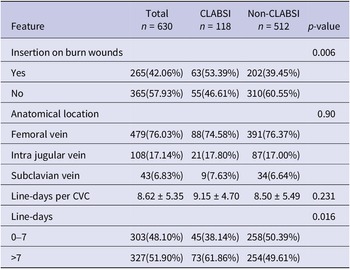
Catheter management
Details of catheter management of 630 CVCs with 5,431 line-days are shown in Table 3. CVCs were inserted more frequently on burn wounds in the CLABSI group than the non-CLABSI group (53.39% vs. 39.45%, p = 0.006) and were of longer line duration (9.15 ± 4.70 vs. 8.50 ± 5.49, p = 0.231), often exceeding 7 days (61.86% vs. 49.61%, p = 0.016).
Table 3. Catheter management of central venous catheters with and without CLASBI

Risk factors
Logistic regression analysis was used to screen 17 potential risk factors for CLABSI in burn patients. This showed that longer line-days had the greatest influence on CLABSI (OR = 2.09, p = 0.006), followed by insertion on burn wounds (OR = 1.73, p = 0.008), more catheterisation times (OR = 1.69, p = 0.032), and higher burn index (OR = 1.04, p = 0.01) (Table 4).
Table 4. Multivariate logistic regression analysis of risk factors for CLABSI in burn patients

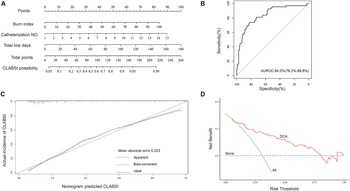
Nomogram predictive model
Consistent with the foregoing analysis, LASSO regression analysis also found that burn index, catheterisation times, and total line-days were positively related with CLABSI (Figure S1). A nomogram of predicting CLABSI was successfully constructed (Figure 3a) with a maximal AUROC value of 0.84 (95%CI 0.782–0.898) (Figure 3b), and the mean absolute error of the calibration curve was 0.023 (Figure 3c), indicating a good fit. The decision curve analysis indicated that the predictive nomogram provided a good clinical benefit (Figure 3d).

Figure 3. Construction and evaluation of nomogram for predicting CLABSI in burn patients. (a) The developed nomogram for predicting CLABSI in burn patients; (b) ROC curves of nomogram; (c) calibration curve analysis of nomogram; (d) decision curve analysis of nomogram.
CLABSI and mortality
Overall, 17 (7.6%) patients died during hospitalisation, with CLABSI patients having a significantly higher mortality rate (13.04% vs. 5.23%, p < 0.001). However, Kaplan–Meier survival analysis revealed no significant difference between both groups of patients (p = 0.163) (Figure S2). Furthermore, Cox regression analysis showed that the area of full-thickness burns was the only significant risk factor for death (OR = 2.00, p = 0.006), and having CLABSI alone was not a significant contributor to mortality (OR = 1.12, p = 0.842) (Table S3),
Discussion
CVCs have become a routine procedure in burn ICU settings, and CLABSI is the most serious and common complication arising from their use [Reference Sheridan, Neely, Castillo, Shankowsky, Fagan, Chung and Weber18]. However, confirmation of infection is difficult and resource-costing, mainly relying on local and systemic clinical signs and symptoms and confirmation by catheter and blood cultures [Reference Hajjej, Nasri, Sellami, Gharsallah, Labben and Ferjani19]. It is necessary for clinicians to show that a bacteremia is due solely to the contamination of the catheter line and exclude all other sources of infection. This requirement may result in delayed diagnosis and clinical intervention. Despite a considerable volume of research defining risk factors for CLABSI, the means to quantitatively predict the infection remain unknown. To address this, we first constructed a practical nomogram for burn patients based on screened risk factors. In addition, we investigated the association of clinical factors with mortality and recorded a number of such factors, namely, the burn index, catheterisation times, total line-days, and CVC insertion on burn wounds. A practical nomogram, based on burn index, catheterisation times, and total line-days, showed excellent predictive ability and clinical applicability.
The incidence of CLABSI in burn patients is significantly higher than that in other populations. In our burn patient cohort, the rate of CLABSI was 21.73 per 1,000 central line-days, which falls within the range reported (15.5–29.1) in other studies in such patients [Reference Rickard, Marsh, Larsen, McGrail, Graves, Runnegar, Webster, Corley, McMillan, Gowardman, Long, Fraser, Gill, Young, Murgo, Alexandrou, Choudhury, Chan, Gavin, Daud, Palermo, Regli and Playford24-Reference Pearse, Corley, Rickard and Marsh26] – the exception being in Tao’s study [Reference Chovanec, Arsene, Gomez, Brixey, Tolles, Galliers, Kopaniasz, Bobash and Goodwin9], which recorded a rate of 29.1 per 1,000 line-days. The main underlying reasons for the high incidence of CLABSI in burn patients are the prolonged demand for central venous access, multiple surgical treatments, and extended stays in the ICU. Other contributory factors are immunosuppression, and breakdown of the skin’s protective barrier. The presence of necrotic tissue and protein-rich exudate at the burn wound surface promotes the multiplication of bacteria on the skin surface at the insertion site which adheres to the outer wall of the catheter before entering the bloodstream. A combination of impaired skin barrier function, haemodynamic changes, and post-traumatic stress render burn patients prone to CRIs. In addition, hypoxia, ischemia, and metabolic abnormalities impact the function of immune cells [Reference Wong, Gantner, McGloughlin, Leong, Worth, Klintworth, Scheinkestel, Pilcher, Cheng and Udy20], thereby further increasing the proliferation of opportunistic pathogens.
A high burn area has been identified as a significant risk factor for CRIs [Reference Fochtmann-Frana21], but notably, patients in previous studies had significantly less burn area than those found here. Indeed, high burn areas in enrolled patients with CLABSI (68.87% ± 22.31%) appear to have been a contributory factor for its increased incidence in this study. In addition, the CDC defines CLABSI as requiring proof of catheter involvement and other possible sources of infection must be ruled out. In patients with extensive burns, the possibility of infection from the burn wound cannot generally be excluded. All patients who had a positive culture of the catheter tip with the same microbial species found in the blood culture were classified as having a central line–associated infection as opposed to simply being classified as CRI. Therefore, the frequency of CLABSI cases was higher in this study.
Risk factors for CLABSI were complicated and manifold. Our results showed that higher burn index, catheterisation through wounds, puncture times, and longer total line-days were independent risk factors for infection, which was consistent with other studies [Reference Fochtmann-Frana21-Reference Friedman, Mian, Mullins, Hassan, Shaver and Johnston23]. Apart from burn severity, proper catheter management is widely recognised as the key to preventing infection, but to date, there does not appear to be a consensus on the best strategies of catheter management for infection control. A common controversy is the duration time of central lines. This study found that the incidence of CLABSI was significantly higher in patients with catheterisation for more than 7 days, which is consistent with a recent report [Reference Rickard, Marsh, Larsen, McGrail, Graves, Runnegar, Webster, Corley, McMillan, Gowardman, Long, Fraser, Gill, Young, Murgo, Alexandrou, Choudhury, Chan, Gavin, Daud, Palermo, Regli and Playford24]. An earlier survey in the USA of national burn units found that 70% of centres had a line duration range of 3–14 days but made no recommendation or guideline for optimal duration regarding the risk of infection [Reference Sheridan, Neely, Castillo, Shankowsky, Fagan, Chung and Weber25]. However, another recent study from Australia questioned the practice of regular replacement of deep vein catheters as there was doubt that it reduced the incidence of CLABSI, and possibly even increased the opportunity of infection [Reference Pearse, Corley, Rickard and Marsh26]. Therefore, prospective randomised controlled trials are still needed to clarify the management strategy for CVCs in burn patients.
In this study, the mortality of patients with CLABSI was over two-fold higher than in the non-infected group (13.04% vs. 5.23%). In America, the average reported mortality rate of patients with CLABSI was 12%–25%, and was associated with a 10–20% increase in mortality [Reference Liang and Marschall27]. Likewise, Hajjej et al. documented CLABSI mortality rates as high as 21.8%, compared to 8.3% in controls [Reference Hajjej, Nasri, Sellami, Gharsallah, Labben and Ferjani28] but found only a weak statistical association between CLABSI and survival time, partly owing to insufficient sample size. Moreover, other studies have shown a strong relationship between CLABSI and mortality [Reference Magiorakos, Srinivasan, Carey, Carmeli, Falagas, Giske, Harbarth, Hindler, Kahlmeter, Olsson-Liljequist, Paterson, Rice, Stelling, Struelens, Vatopoulos, Weber and Monnet16] and, of note, the finding that ICU-acquired CLABSI was independently associated with higher in-hospital mortality [Reference Wong, Gantner, McGloughlin, Leong, Worth, Klintworth, Scheinkestel, Pilcher, Cheng and Udy29].
This study has some limitations. First, the sample size was insufficient to generate a model containing all potential confounding factors. Nevertheless, our sample size was larger than some of the cited studies. Second, this study is intrinsically limited by its retrospective nature, and the level of evidence is possibly inferior to that of a prospective study. Likewise, burn severity and risk of CLABSI were probably higher in this study than in others due to the tertiary status of the burn centre with the referral of patients from other hospitals.
In conclusion, a practical nomogram for predicting CLABSI in burn patients showed good predictive ability and clinical applicability. Key risk factors of the nomogram included burn index, catheterisation times, and total line-days. Further studies are warranted to improve the efficacy and applicability of the nomogram in other settings.
Abbreviations
- CLABSI
-
central line–associated bloodstream infection
- CRI
-
catheter-related infection
- CVCs
-
central venous catheters
Supplementary material
The supplementary material for this article can be found at http://doi.org/10.1017/S0950268823000766.
Data availability statement
All data generated and/or analysed are available from the corresponding author on reasonable request.
Acknowledgements
We are grateful to all clinicians and laboratory specialists who collected bacterial isolates, and performed microbiological identification and drug sensitivity testing.
Author contribution
Conceptualization: Q.S., Q.L., N.L., Y.W., Z.Y., G.L., H.L.; Investigation: Q.S., M.L., Q.L., Y.W., W.S., H.L.; Methodology: Q.S., M.L., Q.L., N.L., Z.Y., G.L., W.S., H.L.; Project administration: Q.S., Q.L., N.L., Y.W., H.L.; Supervision: Q.S., M.L., Q.L., N.L., Z.Y., G.L., H.L.; Validation: Q.S., M.L., Q.L., N.L., Y.W., Z.Y., G.L., W.S., H.L.; Visualization: Q.S., M.L., Q.L., N.L., Y.W., Z.Y., W.S., H.L.; Data curation: M.L., Q.L., Y.W., W.S., H.L.; Resources: Q.L., G.L., H.L.; Software: Q.L., Y.W., H.L.; Formal analysis: Y.W.; Writing – original draft: Y.W.; Writing – review & editing: Y.W., H.L.; Funding acquisition: H.L.
Financial support
This work was supported by grants from the National Natural Science Foundation of China (8200082669) and the Innovation Group Science Foundation of Chongqing Natural Science Foundation (cstc2019jcyj-cxttX0001).
Conflict of interest
All authors report no conflict of interest relevant to this article.