1. Introduction
It is considered that the current epidemic of gonorrhoea mainly affects men, particularly gay, bisexual and other men who have sex with men (MSM) in Australia [Reference Chow, Grulich and Fairley1, Reference Jasek2]. Gonorrhoea is deemed to be uncommon among women in major Australian cities (<1% in the population); however rises have been observed in the 2010s [Reference Misson3–Reference Chow5]. This increase has also been reported among women in other high-income countries such as England, Spain or the USA [6–Reference Savage10]. The rise in gonorrhoea cases has also been observed in MSM and heterosexual men [Reference Phillips11]. Overall, there was an 80% increase in the notification rate of gonorrhoea (from 65.5 to 118.0 per 100 000) between 2013 and 2017 in the total Australian population.
The reasons underlying the rise in gonorrhoea among women remain unclear. While the Australian Study of Health and Relationships surveys reported there was no change in condomless penile-vaginal sex nor in the number of sexual partners among women between 2001 and 2013 [Reference Rissel12, Reference De Visser13], trend data on sexual practices among Australian women thereafter is scarce and other factors (e.g. sex overseas, use of smartphone dating applications) may have changed over time and contributed to the rise of gonorrhoea [Reference Misson3, Reference Aung14–Reference Sundbeck, Agardh and Östergren16]. Other reasons besides sexual practices may include sociodemographic changes or changes in health services use. The increase in health care demand from the rising rates of sexually transmitted infections (STIs) may be making it harder to access health care, and consequently increasing the duration of untreated infection which would lead to ongoing transmission [Reference Needleman17–Reference Foley19].
Genital gonorrhoea can be asymptomatic in women and, hence individuals may not be aware of their infection for long periods. Untreated gonorrhoea in women can lead to pelvic inflammatory diseases and other serious complications including ectopic pregnancy or infertility [Reference Walker and Sweet20], making prompt identification and treatment especially important. However, recent studies on genital gonorrhoea symptomatology in women are very limited and most studies were conducted in the 1970s and culture was used for gonorrhoea diagnosis in these studies [Reference Pedersen and Bonin21–Reference Wallin23].
Accordingly, the primary aim of this study was to estimate the proportion of genital gonorrhoea cases that were asymptomatic and symptomatic among women attending a sexual health clinic in Melbourne in Australia using nucleic acid amplification test (NAAT). The secondary aim was to describe the time from the last sexual contact to the onset of symptoms and the time to attend health care following the onset of symptoms to determine if these offered any insights into reasons for the recent increases in the infection rates and consequently, to potential public health interventions.
2. Methods
2.1 Study population
This was a cross-sectional study including all women aged 16 years or above attending the Melbourne Sexual Health Centre (MSHC), Australia, between August 2017 and August 2018. MSHC is a public HIV/STI clinic that offers a range of free clinical services regarding sexual health. MSHC is the largest sexual health clinic in Victoria and provides more than 50 000 clinical consultations a year. For the purposes of this study, only cisgender women were included.
Prior to August 2017, as genital gonorrhoea was considered very uncommon in female attendees, testing was only recommended among women who reported genital symptoms (i.e. presence of discharge or dysuria) or sexual contact with someone diagnosed with gonorrhoea infection as per the Australian STI Management Guidelines [24]. However, MSHC implemented an in-house screening policy in August 2017, and all women attending MSHC, regardless of the presence of symptoms, were offered genital gonorrhoea and chlamydia screening by NAAT using Aptima Combo (AC2) assay (Hologic Panther system; Hologic, San Diego, CA, USA) [Reference Johnson25, 26]. All clients attending MSHC were asked if they had any symptoms by the triage nurse and then by the treating clinician. Symptomatic women were examined by the clinician and an endocervical swab was obtained for NAAT and/or culture for Neisseria gonorrhoeae. No examination was performed among asymptomatic women [Reference Lee27], but they were asked to self-collect a vaginal swab or first pass urine (FPU) and the specimens were tested by NAAT for N. gonorrhoeae. If a swab was collected for culture, it was plated immediately onto modified Thayer-Martin medium for gonorrhoea culture and a smear prepared for Gram stain and microscopy to look for Gram negative diplococci. For individuals who tested positive by NAAT but had no culture performed at the screening visit, a culture test was performed on the subsequent visit before gonorrhoea treatment was administered, to determine the antimicrobial susceptibility profile.
2.2 Data collection
We collected data regarding demographic characteristics, recent sexual practices, sex work, self-reported genital symptoms and duration, sexual contact with a person diagnosed with gonorrhoea, investigations performed and laboratory results. For the purpose of this study, we categorised gonorrhoea cases among women into two groups based on their reported symptoms: (i) symptomatic; or (ii) asymptomatic. Symptomatic cases were defined as women who had any genital symptoms on the day of testing. Symptomatic cases included not only women with a characteristic discharge for gonorrhoea (yellow, green or pus-like vaginal discharge) but also reporting other genital symptoms such as dysuria, urethral discomfort, vulvar or vaginal itch, clear abnormal vaginal discharge or lower abdominal pain. Asymptomatic cases were women who reported no genitourinary symptoms on the day of testing.
2.3 Data analysis
Descriptive analyses were conducted to summarise the key parameters. The 95% confidence intervals (CIs) for the sample proportions were calculated using Agresti-Coull (adjusted Wald) method. All analyses were conducted using SPSS (version 25, Armonk, NY: IBM Corp). Ethical approval was granted by the Alfred Hospital Ethics Committee, Melbourne, Australia (450/18).
3. Results
There were 17 258 clinic visits by 9302 individual women attending MSHC during the study period. Genitourinary specimens were obtained (FPU, endocervical or vaginal specimens) in 12 869 (74.6%) clinic visits from 7350 individual women. Genital gonorrhoea was detected in 1.1% (142/12 869) of the clinic visits where women were tested for genital gonorrhoea, corresponding to 142 different gonorrhoea cases in 140 individual women. The investigations performed including NAAT and culture for N. gonorrhoeae are shown in Figure 1. The median age of the 142 genital gonorrhoea cases was 28 years [interquartile range (IQR) 24–34], 79 (55.6%) were born overseas and 13 (9.2%) had a previous gonorrhoea diagnosis at the MSHC. The median number of male sexual partners in the preceding three months was 2 (IQR 1–4) and 12 (8.5%) reported having a female sexual partners in the preceding three months. Only 12 (8.5%) reported always using condoms with male partners for sex in the preceding three months, 37 (26.1%) reported having sex overseas in the preceding 12 months and 52 (36.6%) were currently working as sex workers (Table 1).
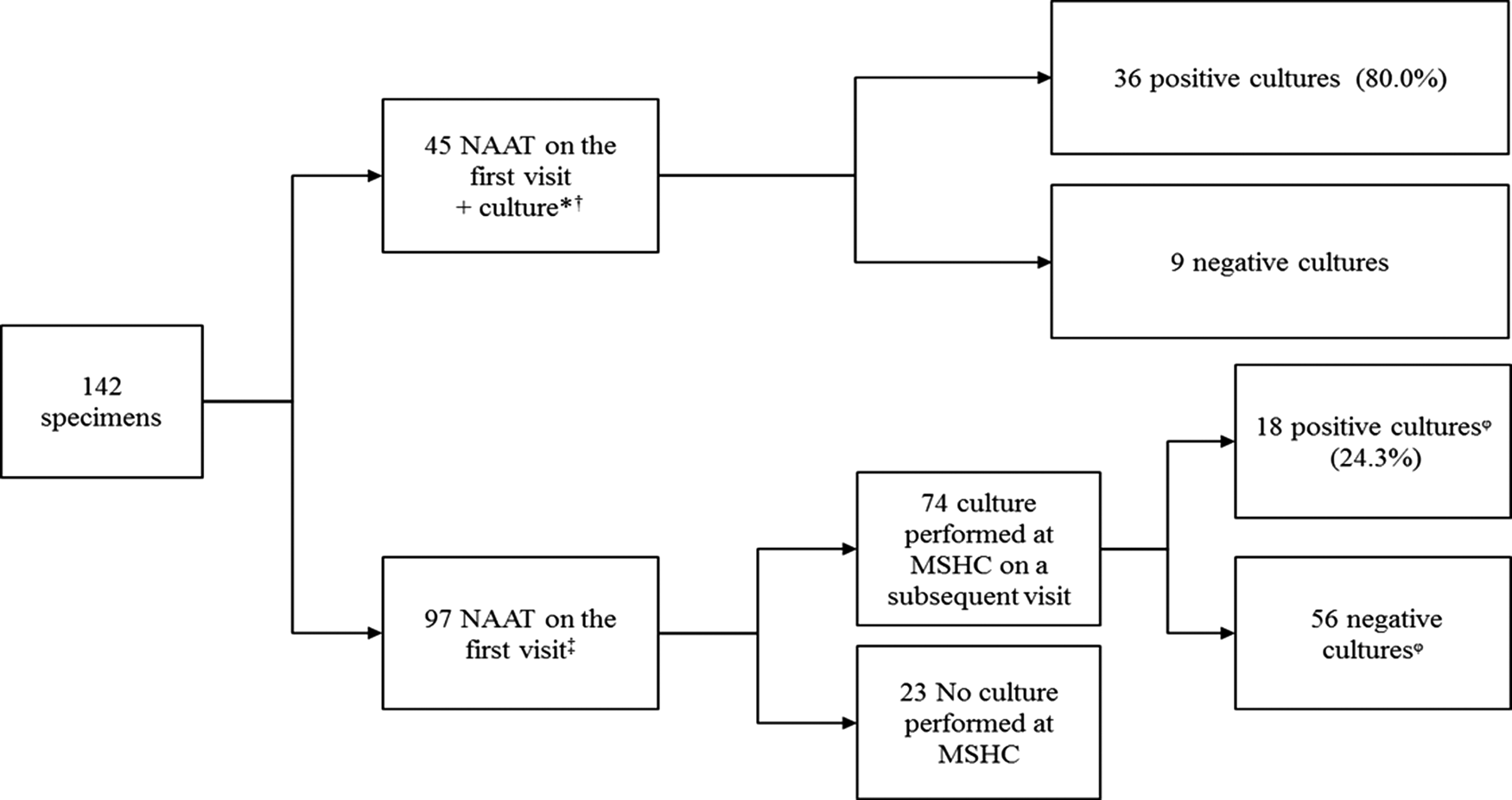
Fig. 1. Investigations performed in 142 cases of genital N. gonorrhoeae infection among women attending MSHC, 2017–2018.
*33 (73.3%) cases were symptomatic, including eight cases with yellow, green or pus-like vaginal discharge, 12 (26.7%) were asymptomatic.
†In four cases only culture was performed as it was previously diagnosed outside MSHC.
‡56 (57.7%) were asymptomatic, 41 (42.3%) had genital symptoms including eight cases with yellow, green or pus-like vaginal discharge.
ᵠOf the 56 cases with a negative culture in a subsequent visit, 10 (17.9%) had received antibiotic treatment on the first visit (five received treatment for bacterial vaginosis, two for urinary tract infection, two for chlamydia and one for both chlamydia and bacterial vaginosis); among the ones with positive cultures, four (22.2%) had received antibiotic treatment (three for bacterial vaginosis and one for urinary tract infection).
NAAT, nucleic acid amplification test; MSHC, Melbourne Sexual Health Centre.
Table 1. Demographic and sexual behaviour characteristics of 142 cases of genital N. gonorrhoeae infection among women attending MSHC, 2017–2018
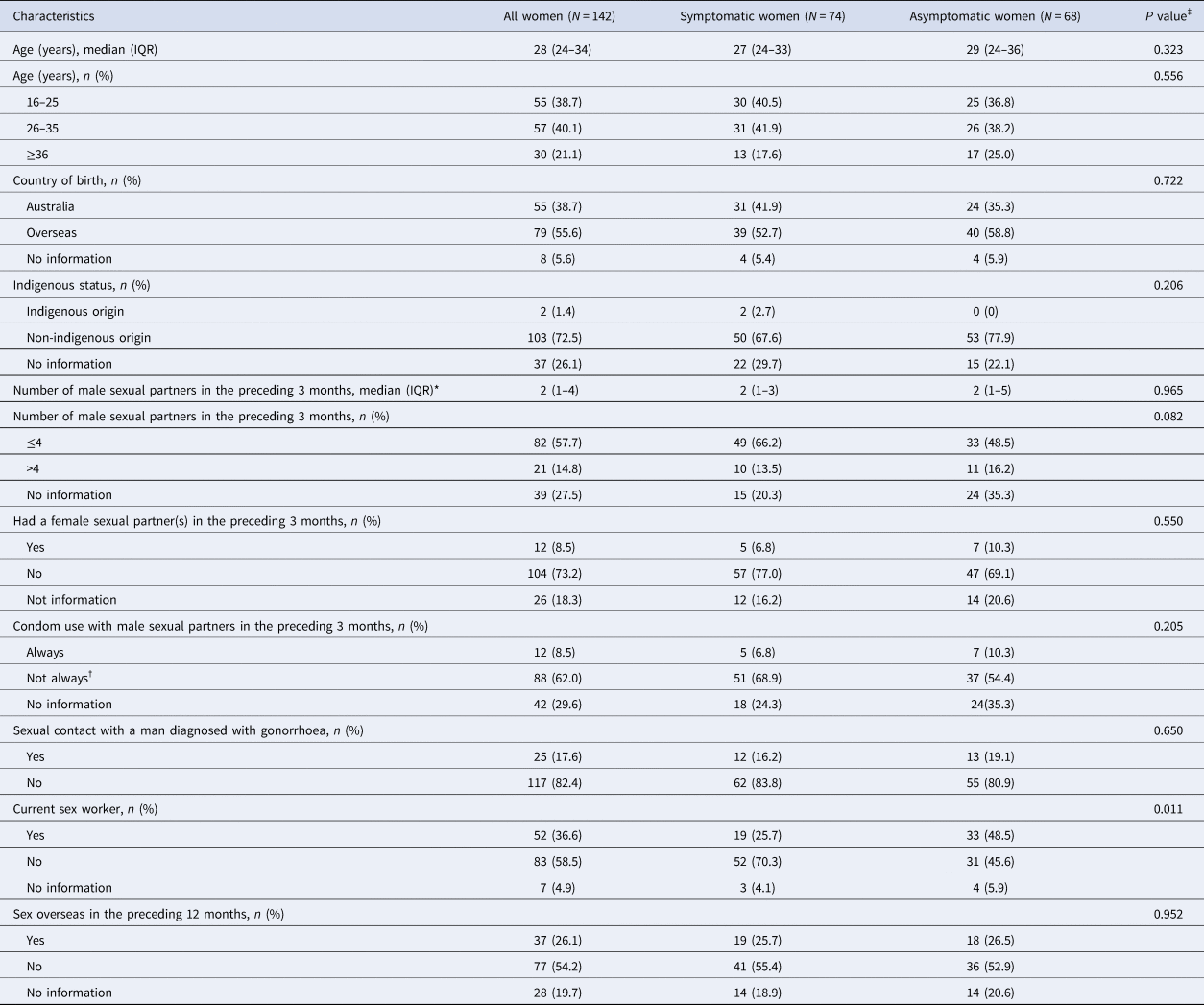
IQR: interquartile range. Data are presented as either n (%) or median (IQR).
*The median of male sex partners was calculated among 103 women who reported the number of male partners, excluding the 39 women who decided not to report the number of male partners in the preceding three months.
†‘Not always’ was defined as women who sometimes, usually or never used a condom with their male partners in the preceding 3 months.
‡Chi-squared test was used to compare categorical variables between symptomatic and asymptomatic women. Mann-Whitney U test was used to compare continuous variables between symptomatic and asymptomatic women.
Almost half of the cases (n = 68, 47.9%, 95% CI 39.8–56.1%) reported no symptoms (Table 2). Of the 74 cases (52.1%, 95% CI 44.0–60.1%) where women reported any symptom, the most common symptoms reported included a clear abnormal vaginal discharge (n = 29, 39.2%), dysuria (n = 23, 31.1%) and a change in vaginal discharge to a yellow, green or pus-like vaginal discharge (n = 16, 21.6%; 11.3% [95% CI 7.0–17.6%] over the total number of cases). A smaller proportion also reported Per Vaginal (PV)/postcoital bleeding (n = 9; 12.2%) and pelvic pain (n = 8; 10.8%) (Table 3). Among the 142 genital gonorrhoea cases, 18 (12.7%) had concurrent bacterial vaginosis and 11 (7.7%) had pelvic inflammatory disease. Of the 25 (17.6%) cases with sexual contact with a male diagnosed with gonorrhoea, 13 (52.0%) were asymptomatic, and 12 (48.0%) symptomatic, including three cases with yellow, green or pus-like vaginal discharge.
Table 2. Clinical characteristics of cases of genital N. gonorrhoeae infection among women attending MSHC, 2017–2018
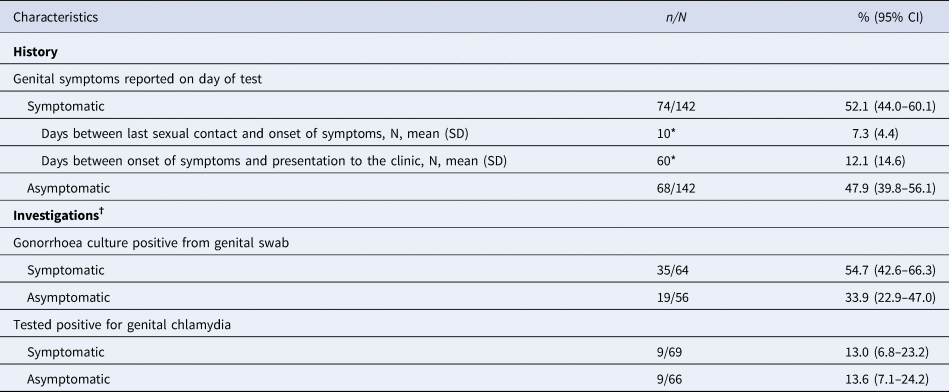
CI, confidence interval; SD, standard deviation; NAAT, nucleic acid amplification test.
*10 and 60 women reported date of last sexual contact and date of onset of symptoms, respectively. †The denominator of the percentage is the total of specimens in which the investigation was performed.
Table 3. Symptoms present on the day of testing among 74 symptomatic cases of genital N. gonorrhoeae infection among women attending MSHC, 2017–2018
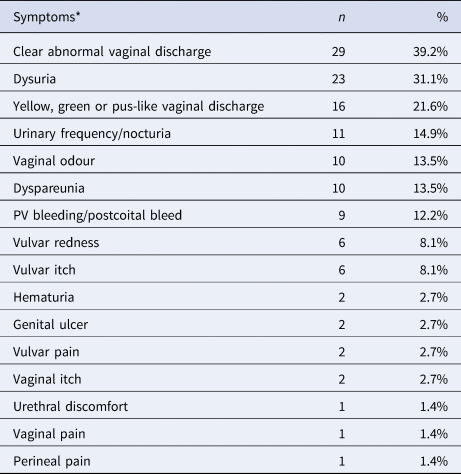
*There were cases in which more than one symptom was present
There were 10 symptomatic women who reported the date of last sexual contact, and the mean time between last sexual contact and the onset of symptoms was 7.3 days [standard deviation (SD) 4.4, ranging from 2 to 16 days]. In addition, there were 60 symptomatic women who reported the date of symptom onset, and the mean time between the onset of symptoms and presentation to the clinic was 12.1 days (SD 14.6, ranging from 1 to 90 days) (Table 2). Among these 60 symptomatic women, 22 (36.7%) presented to the clinic up to 5 days after symptoms onset, 16 (26.7%) between 6 and 10 days and 22 (36.7%) after 10 days or more.
Of the 142 cases with genital gonorrhoea, 135 were also tested for genital chlamydia and 18 (13.3%) tested positive for genital chlamydia by NAAT. Among 68 asymptomatic cases, the positivity for genital chlamydia was 13.6% and among symptomatic was 13.0% (Table 2).
4. Discussion
In this clinical audit of 142 confirmed genital gonorrhoea cases in women attending a sexual health clinic in Melbourne, we found that 48% of the cases among women did not have any reported genital symptoms on the day of testing, and only an 11% had typical N. gonorrhoeae discharge (i.e. yellow, green or pus-like vaginal discharge). This suggests that a substantial proportion of asymptomatic cases would have been missed without screening of asymptomatic women. This result also contrasts with heterosexual men where more than 90% of genital cases are symptomatic [Reference Martín-Sánchez28]. Along similar lines, time from symptoms onset to presentation to the clinic was of 12 days, which could be partially explained by the non-specific symptoms of gonorrhoea. Given the importance of the early detection of infectious cases for effective gonorrhoea control, it is possible that a public health campaign aimed at increasing awareness and knowledge on gonorrhoea, as well as, improving testing of gonorrhoea in symptomatic women and asymptomatic women at risk may be indicated.
There are some limitations in the study that should be noted. First, this study was conducted in one urban major public sexual health clinic in Melbourne that sees only 25% of the cases of gonorrhoea diagnosed in women. Our data may therefore not be representative of presentations to general practice [Reference Williamson29]. Women in our study may be biased towards to those with genital symptoms or contacts of infection. Besides that, sex workers may be overrepresented in our study for the mandatory three-monthly HIV/STI screening [Reference Chow30]. Second, recall bias might have occurred when women reported the time between the onset of symptoms and last sexual contact. Moreover, a substantial proportion of data was missing because it was not always systematically collected, hence we could only present the results of the time between last sexual contact and the onset of symptoms when the data is available. Third, sexual health services in Melbourne are quite limited and it is not always possible to see all individuals on the day that they present symptoms [Reference Needleman17]. This could have contributed to more than five days elapsed since symptoms onset to presentation to the clinic in almost two-thirds of the cases. It is therefore possible that the time between the onset of symptoms and presentation may be longer in our study than for women attending other services.
There have been very limited studies estimating the proportion of gonorrhoea in women that is asymptomatic and most of these studies were conducted in the 1970s. Asymptomatic screening in women is not currently recommended in many countries such as the USA and UK. In addition, clinical diagnosis of gonorrhoea in symptomatic women is always difficult because the presence of non-specific symptoms (e.g. dysuria, dyspareunia, vulvar itch or pelvic pain) may be confounded by other infections such as bacterial vaginosis, Chlamydia trachomatis and Trichomonas vaginalis. In our study, we found that 48% of women who tested positive for N. gonorrhoeae did not report any genital symptoms on the day of screening. However, McCormack and colleagues reviewed 278 women with gonorrhoea in 1974 in Boston in a hospital setting and they found that only 19% of the cases were asymptomatic [Reference McCormack31]. Furthermore, we found that 52% (13/25) of gonorrhoea contacts that tested positive for genital gonorrhoea were asymptomatic. Our previous audit in 2017–18 showed 44% (26/59) of asymptomatic female gonorrhoea contacts tested positive by NAAT at the genital site [Reference Chow32]. However, Pariser and colleagues (1968) found that only 29% of asymptomatic female gonorrhoea contacts were culture-positive for gonorrhoea [Reference Pariser and Farmer33]. The low positivity in Pariser and colleagues’ study may be due to poor sensitivity in culture compared to NAAT. Past studies have shown that the majority of women with gonorrhoea would develop symptoms within 9–11 days after sexual exposure and this is similar to the estimate of 7.3 days in our study [Reference Platt, Rice and McCormack22, Reference Wallin23].
The increase in the rates of gonorrhoea among women in Australia has happened alongside an increase in the incidence of chlamydia and infectious syphilis since the mid-2010s [Reference Jasek2, 34, 35]. This has also led to the re-emergence of congenital syphilis in Victoria in 2017, which has not emerged in the state since 2004 [34, 35]. The Australian national surveys have revealed that there were no significant changes in the number of partners and condomless sex among women in Australia between 2001 and 2013 [Reference Phillips11, Reference Rissel12]; however, the much of the STI rise occurs after 2013, and there have been very limited behavioural studies examining sexual practices among women in Australia. The rises in gonorrhoea in women in Australia remain unclear and it may be due to other unmeasured factors (e.g. group sex, use of smartphone dating applications, transmission from higher-incidence populations) that may have changed over time. Nevertheless, given the importance of access to health care for the control of STIs, public health campaigns and sexual health educations are important to increase the awareness and recognition of STI-related symptoms. General practitioners should also be informed and trained accordingly to increase the knowledge and awareness of the current STI epidemic, testing and treatment guidelines.
In conclusion, genital gonorrhoea is largely asymptomatic or has an unspecific clinical presentation among women. Testing only women with typical N. gonorrhoeae discharge would have missed a considerable number of cases. Further efforts must be made to promote gonorrhoea screening at primary care settings in Victoria, including training on test and treat among general practitioners. Additionally, epidemiological and behavioural research is required to understand the temporal changes in sexual practices among women in Australia and how this could be related to recent increases in the incidence of gonorrhoea.
Acknowledgements
The authors acknowledge Afrizal Afrizal for his assistance with data extraction at the Melbourne Sexual Health Centre.
Funding
CKF and CSB are supported by an Australian National Health and Medical Research Council (NHMRC) Leadership Investigator Grant (GNT1172900 and GNT1173361, respectively). DAW and EPFC are supported by an Australian NHMRC Emerging Leadership Investigator Grant (GNT1174555 and GNT1172873, respectively).
Conflicts of interest/Competing interests
The authors declare that they have no conflict of interest.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the granted by the Alfred Hospital Ethics Committee, Melbourne, Australia (450/18). This study was an analysis of retrospective data collected from a clinical service. Informed consent is not required as per the ethical approval of this study.
Data Availability Statement
All relevant data generated or analysed during this study are included in this published article.







