In many parts of the world, pathogens that cross the livestock/wildlife interface pose problems for natural resource managers due to the need to balance the demands of wildlife conservation with the control of disease in livestock. Brucellosis is a clear example of this type of pathogen, which, despite intensive control programmes focused on controlling this disease in livestock, has been difficult to eradicate in some populations of wild ungulates [Reference Cross1, Reference Cheville, McCullough and Paulson2].
Spain has one of the highest prevalences of brucellosis in Europe in both domestic [Reference Godfroid and Käsbohrer3] and wild ungulates [Reference Muñoz4], thus cooperation between wildlife managers, livestock owners and agricultural agencies is critical to the success of efforts to control brucellosis in this country. In Spain, Brucella melitensis is the most prevalent pathogen, e.g. in 2008 the herd prevalence of B. abortus in cattle was 0·4% whereas that of B. melitensis was 2·11% in goats and sheep [5]. Brucellosis in cattle is usually caused by B. abortus; however, when cattle are kept in close association with sheep and goats (e.g. as in several rural communities of Spain), infection can also be caused by B. melitensis [Reference Godfroid and Käsbohrer3]. For this kind of pathogen that infects a number of different hosts, the process of determining the relative importance of each host species to the persistence of the pathogen is often complex. Removal of a host species is a potential but rarely possible way of discovering the importance of that species to the dynamics of the disease.
In this work we evaluated whether the increase in brucellosis control efforts in livestock grazing in the Boumort National Game Reserve (BNGR) (42° 14′ 6″ N, 1° 8′ 4·9″ E, NE Spain) influenced the prevalence of Brucella spp. in red deer. We analysed serological data from red deer, cattle, sheep and goats from seven neighbouring municipalities from BNGR (Fig. 1). Livestock from these municipalities are brought into the BNGR to graze from early spring to late autumn every year, where they are potentially able to mingle with red deer. Red deer densities in BNGR were estimated annually during the summer using distance sampling procedures [Reference Buckland6]. In brief, for 3 days a team of rangers walked transects (n=21) of about 5 km each covering the 13·097 ha of BNGR recording the perpendicular distances to all the detected deer. About 17–20% of the BNGR red deer population are hunted annually and since 1991 every hunter in BNGR is accompanied by a game ranger who is responsible for identifying the gender and age of the shot deer. Since 1998, 1047 blood samples have been taken from harvested deer (Table 1) and we assumed that the samples taken from these deer were representative of the red deer population in BNGR as a whole.
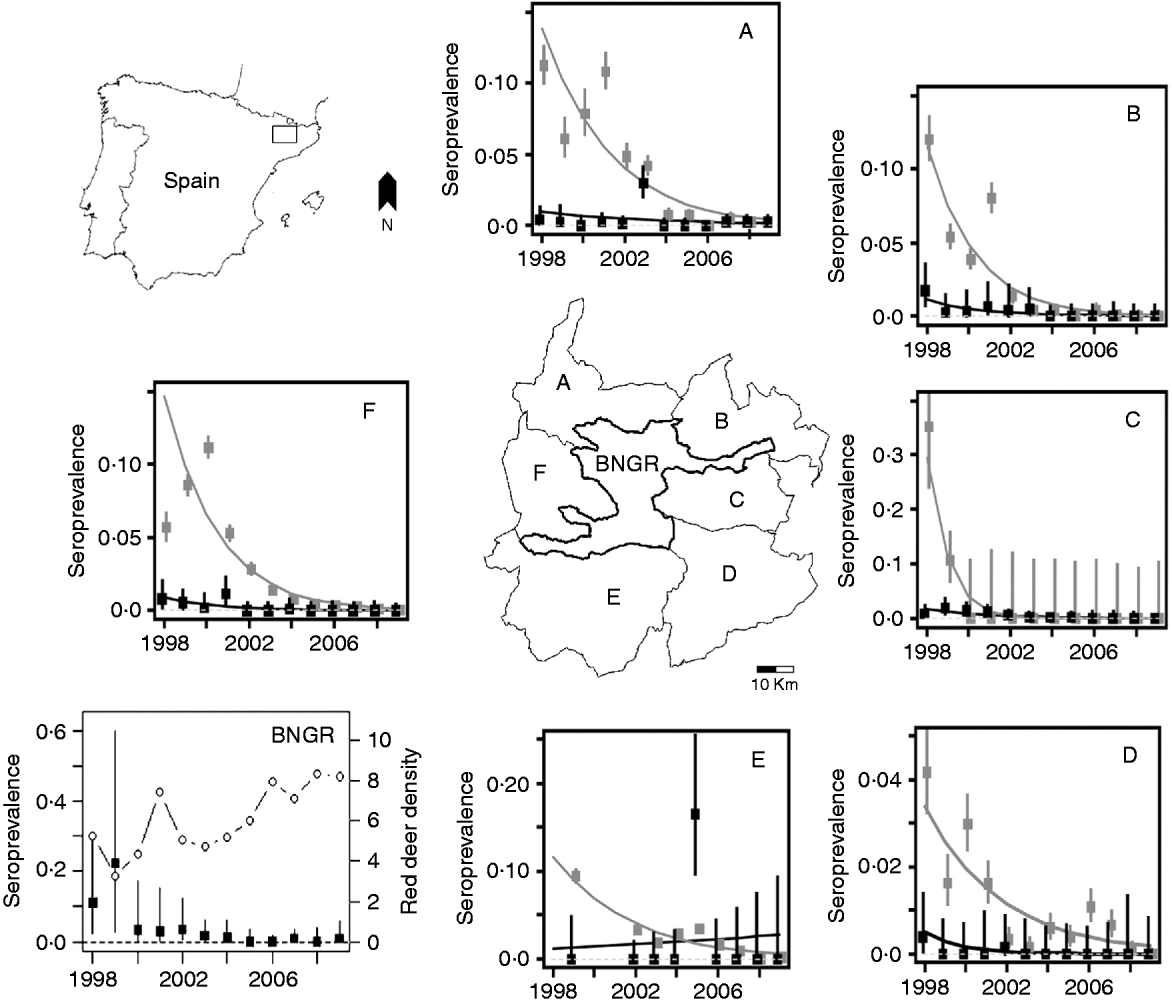
Fig. 1. Map of Spain with the Boumort area in the Catalan pre-Pyrenees enlarged. The Boumort area consists of the Boumort National Game Reserve (BNGR) and six surrounding municipalities: Baix Pallars (A), Vall d'Aguilar (B), Cabó (C), Coll de Nargó (D), Isona and Abella de la Conca (E) and Conca de Dalt and Pobla de Segur (F). Each plot shows individual cattle (black squares) and small ruminants (grey squares) infected with Brucella spp. In the BNGR plot, solid circles represent individual seroprevalence in red deer, whereas the open circles represent red deer density (deer/100 ha). Note that the y axes vary in each plot. Bars represent 95% confidence intervals.
Table 1. Number of cattle, sheep, goats and red deer tested for Brucella spp. in the period 1998–2009 in the Boumort area of Spain
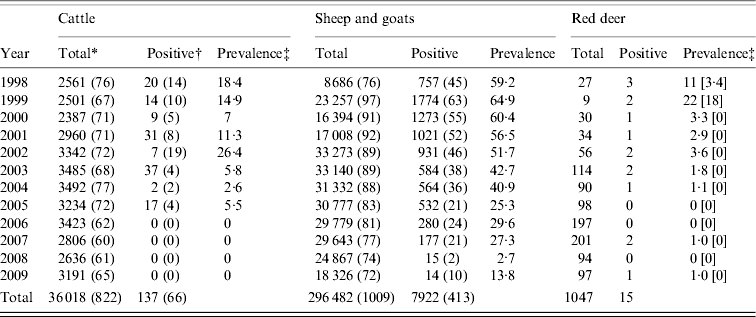
* Total number of animals tested and in parentheses the total number of herds tested.
† Number of animals testing positive and in parentheses the number herds with one or more positive tests.
‡ Apparent prevalence and in square brackets the ‘true’ prevalence using the sensitivity and specificity values given in the text.
To qualify the Boumort area (i.e. BNGR and its neighbouring municipalities; Fig. 1) as brucellosis-free (as defined by Spanish law 2611/1996 published in BOE no. 307 on 21 December 1996), a yearly ‘test-and-slaughter’ programme [7] was established from 1988 on cattle herds in the Boumort area. Although some sheep and goat herds were tested and removed prior to 2000, from this year all livestock (cattle and small ruminants) were included in the programme. This programme mandated the testing of all cattle, sheep and goats aged >1 year, and the slaughter of either all Brucella test-positive animals or flocks with prevalences >18%. This resulted in 360 180 tests, 63 698 on cattle (annual mean 3030 tests, range 2466; 3492 on approximately 67 herds per year) and 296 482 on small ruminants (sheep and goats; annual mean 27 100 tests, range 15 250; 33 270 tests on approximately 84 herds per year). All samples were tested for brucellosis using the complement fixation test (CFT) with 82·9% sensitivity and 91·4% specificity [Reference Alton8]. CFT does not differentiate between different species of Brucella, but the direct isolation, culture (e.g. Farrell's medium) and identification of the aetiological agent following standard procedures [9] showed that 100% of cattle that tested positive (n=7) were infected with B. melitensis (internal report, Departament d'Agricultura, Alimentació i Acció Rural, Generalitat de Catalunya, Spain).
For livestock we had access to herd- and individual-level prevalences, but not to which herds individual animals were from. In addition, herd-level prevalence analyses give equal weight to large and small herds, while individual-level analyses provide biased standard errors because samples within and between herds are not independent. Thus, for livestock we conducted a logistic regression analyses to assess whether brucellosis herd seroprevalence declined during the study period. However, for deer we performed the same statistical approach but used the individual prevalences. We considered ‘year’ as a continuous explanatory variable, but rescaled it so that 1998 was equal to zero. The movement of domestic livestock probably varied according to the municipality and so we included ‘region’ as a random effect that adjusted the intercept (i.e. seroprevalence in 1998) and the slope (i.e. time trend); simpler models were not well supported by Akaike's Information Criterion. We also combined data from Isona and Abella de la Conca due to the high rate of cattle movement between these two municipalities. We used a quasi-binomial analysis to account for overdispersion [Reference Zuur10]. All analyses were conducted in R version 2.12.1 [R Development Core Team (http://www.R-project.org)] with the lme4 package (http://CRAN.R-project.org/package=lme4).
Brucellosis seroprevalence generally declined in all regions at both herd and individual levels in all species in the period 1998–2009 (Table 1, Fig. 1). The overall time trend declined significantly for all three groups (cattle: βyear=−0·31±0·00015; sheep and goats: βyear=−0·49±0·0006; red deer: βyear=−0·38±0·10, all P values <0·001), although different municipalities varied both in their starting prevalences and their rates of decline (Fig. 1). The linear model was not a good fit for some municipalities, particularly for Isona and Abella de la Conca, both of which suffered a serious outbreak in 2005. However, we still prefer this simpler approach as a way of illustrating overall time trends. Our conclusions remained the same when only data after 2000 were used, i.e. when all herds in the study area were included in the ‘test-and-slaughter’ programme (results not shown).
Brucellosis was not detected in cattle from 2006 to 2009 (Table 1) nor in red deer in 2005, 2006 or 2008, and only one deer out of 97 tested positive in 2009 (Table 1, Fig. 1). The latest positive animals were three adult females (two in 2007 and one in 2009), thus rather than being a case of recent transmission, these animals may have been exposed to the disease at an earlier date when the prevalence in livestock was higher. The herd-level prevalence of brucellosis was higher in small ruminants than in cattle, but only 0·076% of small ruminants (i.e. individuals) tested positive in 2009 (Table 1).
Our study shows coincident declines in brucellosis seroprevalence in cattle, sheep, goats and red deer during a period of time when control measures were being implemented on domestic stock but not red deer. During this same time period red deer populations increased from 6 to 8 deer/100 ha (Fig. 1). This evidence is suggestive, but not conclusive, that brucellosis infections in red deer may have been driven, in large part, by spillover infections from domestic species.
Our results differ from those of Cross et al. [Reference Cross11], who found that elk densities in Wyoming were associated with a change in the ability of elk to serve as a reservoir of brucellosis infection. This difference could be due to many factors, including different disease agents (B. abortus vs. B. melintensis), herd sizes (the largest herds in Wyoming are composed of 1000 animals, whereas in Boumort herds are of <50 animals) or the lack of supplementary feeding of red deer in the Boumort area, a practice that seems to encourage both aggregation and disease transmission in Wyoming. Although red deer may not be a competent reservoir for Brucella spp., in BNGR we can conclude that in the absence of such controlled experiments the removal of infected livestock is an alternative method to draw inferences about the relative importance of different host species to the dynamic of brucellosis in free-ranging populations of red deer. On the other hand, since we did not undertake further studies on post-isolation of Brucella spp., we can not exclude the possibility of cross-species transmission between livestock and deer in the Boumort area. However, given the observed declines in brucellosis seroprevalence, we expect brucellosis to be eradicated from red deer, small ruminants and cattle over the next few years in this region.
ACKNOWLEDGEMENTS
E. Serrano is supported by the Juan de la Cierva postdoctoral programme of the MICINN, (Spain). Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. Government. The authors thank Dr Jorge Ramón López-Olvera and Dr Roser Velarde for their constructive comments on an earlier version of this work. This work was partially supported by the National Science Foundation and National Institutes of Health Ecology of Infectious Disease Grant DEB-1067129.
DECLARATION OF INTEREST
None.



